Abstract
Aims
Cardiac resynchronization therapy (CRT) reduces morbidity and mortality in heart failure. However, prediction of the outcome remains difficult. We aimed to investigate for echocardiographic predictors of ventricular arrhythmias and fatal outcome and to explore how myocardial function is changed by biventricular pacing in heart failure.
Methods and results
We prospectively included 170 heart failure patients (66 ± 10 years, New York Heart Association class 2.8 ± 0.5, 48% ischaemic cardiomyopathy) and recorded ventricular arrhythmias and fatal end point defined as death, heart transplantation, or left ventricular assist device implantation during 2 years. Two-dimensional echocardiography was performed before and 6 months after CRT implantation. CRT response was defined as ≥15% reduction in end-systolic volume at 6 months. Speckle-tracking technique was performed to assess longitudinal and circumferential left ventricular function, defined as global longitudinal (GLS) and circumferential strain (GCS), and to assess mechanical dyssynchrony, defined as mechanical dispersion. GLS before CRT was a predictor of fatal end point independently of CRT response [hazard ratio, HR 1.14 (1.02–1.27), P = 0.02]. Patients with GLS better than −8.3% showed event-free survival benefit (log rank, P < 0.001). Mechanical dispersion at 6 months was an independent predictor of ventricular arrhythmias [HR 1.20 (1.06–1.35), P = 0.005]. CRT responders (59%) had improvement of both GLS and GCS.
Conclusion
In heart failure patients with CRT, worse longitudinal function before CRT was an important predictor of fatal outcome during 2 years, independently of CRT response. Mechanical dispersion at 6 months was a strong predictor of ventricular arrhythmias. CRT response by reverse remodelling was dependent on improvement of both longitudinal and circumferential function.
Keywords: cardiac resynchronization therapy, speckle tracking echocardiography, heart failure, ventricular arrhythmia, left ventricular mechanics
Introduction
Cardiac resynchronization therapy (CRT) reduces morbidity and mortality and induces reverse remodelling in heart failure patients with both mild1,2 and severe symptoms.3,4 CRT treatment was introduced in the mid-1990s, but still the CRT responder rate remains low at 67% and substantial efforts have been invested to improve responder rate and to explore how myocardial mechanics are influenced by biventricular pacing. Furthermore, the impact of CRT on mortality and on ventricular arrhythmias in individual patients is not fully clarified. Some studies have shown no change5,6 or a reduction in the incidence of ventricular arrhythmias by CRT pacing,7,8 often linked to reverse remodelling.7 Recently, freedom from ventricular arrhythmias after CRT has been linked to improved mechanical dyssynchrony.9,10 Heart failure patients eligible for CRT have reduced myocardial function and most often mechanical dyssynchrony. Echocardiographic strain is a sensitive method to assess both myocardial function and dyssynchrony.11–13 We have previously explored the predictive value of strain echocardiography in patients after myocardial infarction and reported that both global longitudinal strain (GLS) and mechanical dispersion, a measure of dyssynchrony, predict ventricular arrhythmias and mortality.14
In this study, we aimed to explore for echocardiographic predictors of ventricular arrhythmias and death, heart transplantation, or left ventricular assist device (LVAD) implantation in heart failure patients eligible for CRT. We hypothesized that LV longitudinal function has prognostic value and that improvement of mechanical dispersion can predict freedom from arrhythmic events. A second aim was to investigate how myocardial left ventricular (LV) longitudinal and circumferential function changes by CRT in relation to reverse remodelling.
Methods
Patient population
This prospective clinical study included patients from two centres, Oslo University Hospital, Norway, and Centre Hospitalier Universitaire, Rennes, France. We consecutively included heart failure patients eligible for CRT implantation according to current CRT guidelines with QRS ≥ 120 ms, ejection fraction (EF) ≤ 35%, New York Heart Association (NYHA) functional Class II–IV and on optimal medical therapy.15 Atrial fibrillation was defined as permanent atrial fibrillation.
The patients successfully received a CRT device system without (CRT-P) or with (CRT-D) defibrillator function at the discretion of the implanting cardiologist. Device programming was set individually by the implanting cardiologist in agreement with general standards as deemed appropriate for clinical circumstance. Before CRT implantation and at 6 months after CRT implantation (CRT turned on), we performed echocardiography and patients reported NYHA functional status. Patients were followed for 2 years from CRT implantation or until a defined fatal end point. Patients with <90% biventricular pacing were excluded. CRT response was defined as reverse remodelling defined as ≥15% reduction in end-systolic volume (ESV) at 6 months by echocardiography.
All patients gave written informed consent. The study complied with the Declaration of Helsinki and was approved by the Regional Committees for Medical Research Ethics.
End points
Our pre-specified arrhythmic end point was first sustained ventricular arrhythmic event following CRT implantation defined as ventricular tachycardia with rate >120 bpm lasting >30 s, ventricular fibrillation, appropriate anti-tachycardia pacing therapy, appropriate defibrillator shock therapy, and documented sudden cardiac arrest. Arrhythmia detection was documented from device interrogation every 6 months and through home monitoring.
Our pre-specified fatal end point was a composite of all cause death, heart transplantation, and LVAD implantation. Only patients with limited anticipated survival undergo heart transplantation or LVAD implantation. Follow-up data on mortality were collected from the National Person Identification Registry.
End points were evaluated from CRT implantation throughout a 2-year follow-up period.
Echocardiography
Echocardiographic studies were performed using Vivid 7 and Vivid E9 system (GE Healthcare, Horten, Norway) and analysed with commercially available software (EchoPAC®, GE). Cardiac volumes were indexed by body surface area, and LVEF was obtained by Simpson's biplane method. By two-dimensional speckle-tracking echocardiography, longitudinal strain was obtained from three apical views, and circumferential strain was obtained from three short-axis planes of the LV (frame rate >50/s). Three cardiac cycles were recorded. Peak strain during the entire cardiac cycle, either negative or positive, was assessed in 18 LV segments. Apical lateral and septal segments' strains were averaged to obtain strain from the 16 LV segments16 which were then averaged to GLS and global circumferential strain (GCS), respectively.14,17,18 If >4/18 LV segments failed to track, the examination was excluded. The time interval from start Q/R on ECG to peak negative longitudinal strain during the whole cardiac cycle, i.e. including post-systolic strain, was assessed in each LV segment. Mechanical dispersion was defined as the standard deviation of time to peak negative longitudinal strain from the 16 LV segments.13,14,19 Echocardiographic recordings were analysed blinded to clinical data.
Statistical analyses
Continuous data were presented as mean ± standard deviation. Proportions were compared by χ2 test or Fisher's exact test. Comparisons of means were analysed by Student's unpaired and paired t-test (SPSS 21, SPSS Inc., Chicago, IL, USA). Cox regression analyses were performed to explore for predictors of arrhythmic and fatal end points. Significant echocardiographic parameters from the univariate model (P < 0.05) were included in multivariate models to determine independent predictors of arrhythmic and fatal end points. A restricted number of parameters were included in the multivariate models to avoid statistical model over-fitting. GLS and EF were not included together in the same multivariate analysis due to strong collinearity (R < −0.5). Receiver operating characteristic (ROC) curves were constructed for GLS before CRT and we reported the C statistic for the ability to discriminate between patients with and without fatal end point during 2-year follow-up. The ROC curve value closest to the upper left corner was defined to provide optimal sensitivity and specificity of discrimination. The difference in fatal end point according to this discriminating GLS value was compared by log-rank test and displayed in a Kaplan–Meier plot.
Reproducibility of strain analyses was expressed by intra-observer and inter-observer variability intra-class correlation coefficients. Two-sided P-values <0.05 were considered significant for all analyses.
Results
Patient characteristics
We included 170 heart failure patients (66 ± 10 years, 24% women) (Table 1). Eighty-one (48%) patients had ischaemic cardiomyopathy, while the 89 (52%) remaining had genetic, cytotoxic, post-myocarditis, or idiopathic dilated cardiomyopathy. Thirty-eight (22%) patients had right ventricular (RV) pacemaker or implantable cardioverter defibrillator (ICD) prior to CRT implantation. Left bundle branch block (LBBB) was present in 137 (81%), 17 (10%) had non-LBBB, and 16 (9%) patients had wide QRS with LBBB configuration due to RV pacing prior to CRT implantation (Table 1).
Table 1.
Baseline characteristics in all patients (left) and separated according to fatal end point during 2-year follow-up (right)
| All patients n = 170 |
Patients surviving n = 146 |
Death, heart transplantation, or LVAD n = 24 |
P-value* | |
|---|---|---|---|---|
| Age (years) | 66 ± 10 | 66 ± 9 | 64 ± 12 | 0.27 |
| Female/male (n) (%) | 40/130 (24/76) | 34/112 (33/77) | 6/18 (25/75) | 0.86 |
| BSA (m2) | 2.0 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.2 | 0.22 |
| HR (bpm) | 70 ± 14 | 69 ± 14 | 76 ± 16 | 0.06 |
| CRT-D/CRT-P (n) (%) | 139/31 (82/18) | 119/27 (82/18) | 20/4 (83/17) | 0.99 |
| QRS (ms) | 165 ± 22 | 165 ± 22 | 165 ± 23 | 0.91 |
| ICM/non-ICM) (n) (%) | 81/89 (48/52) | 68/78 (47/53) | 13/11 (54/46) | 0.49 |
| Atrial fibrillation, permanent (n) (%) | 30 (18) | 25 (17) | 5 (21) | 0.77 |
| LBBB/non-LBBB/RV-pacing (n) (%) | 137/17/16 (81/10/9) | 116/16/14 (80/11/9) | 21/1/2 (88/4/8) | 0.56 |
| NYHA class | 2.8 ± 0.5 | 2.8 ± 0.4 | 3.0 ± 0.5 | 0.06 |
| Medications (n) (%) | ||||
| β-Blocker | 157 (92) | |||
| ACE-I or ARB | 160 (94) | |||
| Aldosterone antagonist | 67 (39) | |||
| Diuretics | 142 (84) | |||
| Digitalis | 18 (11) | |||
| EF (%) | 26 ± 9 | 27 ± 9 | 22 ± 5 | 0.006 |
| ESV index (mL/m2) | 70 ± 30 | 68 ± 29 | 84 ± 31 | 0.01 |
| GLS (%) | −8.2 ± 3.9 | −8.6 ± 4.0 | −5.6 ± 3.1 | <0.001 |
| GCS (%) | −10.9 ± 3.3 | −11.1 ± 3.3 | −9.9 ± 2.9 | 0.12 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; bpm, beats per minute; BSA, body surface area; CRT-D, cardiac resynchronization therapy with implantable cardioverter defibrillator; CRT-P, cardiac resynchronization therapy without implantable cardioverter defibrillator; EF, ejection fraction; ESV, end-systolic volume; GCS, global circumferential strain; GLS, global longitudinal strain; HR, heart rate; ICM, ischaemic cardiomyopathy; LBBB, left bundle branch block; LVAD, left ventricular assist device; NYHA, New York Heart Association; RV, right ventricular.
*Survival vs. fatal end point.
Two patients were excluded due to <90% biventricular pacing.
End points
Fatal end point occurred in 14% (24/170) during an average follow-up period of 2.0 ± 0.1 years. Of these, 6 patients were heart transplanted, 2 patients received an LVAD, and 16 patients died. An arrhythmic end point was documented in 18/170 (11%) patients during an average follow-up period of 1.9 ± 0.3 years.
Prediction of death, heart transplantation, and LVAD implantation
GLS, EF, and ESV index before CRT were markers of fatal end point (Table 1) (Figure 1). In multivariate analysis, GLS before CRT was an independent predictor of fatal end point (Table 2). CRT response at 6 months was also a strong marker of fatal end point (Table 2). In separate multivariate Cox regression analysis, GLS before CRT and CRT responder status were both independent predictors of fatal end point [HRGLS before CRT 1.14 (1.02–1.27), P = 0.02, and HRCRT response 0.16 (0.05–0.56), P = 0.004].
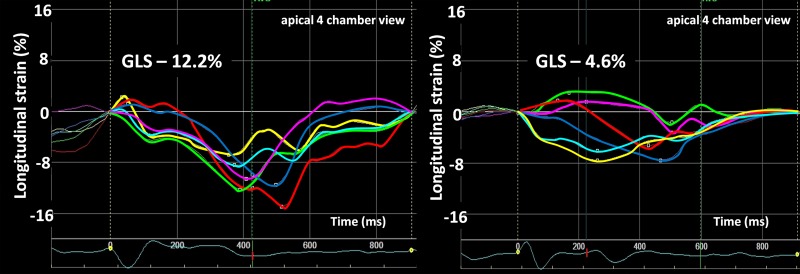
Figure 1.
Longitudinal strain curves from apical four-chamber view displaying 6 of the 18 LV segments used to calculate GLS before CRT. Left panel: patient with moderately impaired longitudinal function (GLS, −12.2%) before CRT and alive at 2 years. Right panel: patient with severely impaired longitudinal function (GLS, −4.6%) before CRT and dead at 2 years.
Table 2.
Cox regression analyses for predictors of ventricular arrhythmias (left) and fatal end point (death, heart transplantation, or left ventricular assist device implantation) (right) during 2 years from CRT implantation
| Ventricular arrhythmia (18/170) |
Death, heart transplantation, or LVAD (24/170) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Before CRT | ||||||||
| Age (years) | 1.02 (0.97–1.07) | 0.54 | 0.98 (0.94–1.02) | 0.29 | ||||
| Female | 0.18 (0.02–1.38) | 0.10 | 1.08 (0.43–2.7) | 0.87 | ||||
| HR (bpm) | 1.02 (0.99–1.05) | 0.22 | 1.02 (1.0–1.05) | 0.06 | ||||
| QRS (ms) | 1.00 (0.98–1.02) | 0.83 | 1.00 (0.98–1.02) | 0.98 | ||||
| ICM (n) | 4.29 (1.41–13.03) | 0.01 | 1.29 (0.58–2.89) | 0.53 | ||||
| NYHA class | 3.53 (0.97–12.80) | 0.06 | 2.97 (0.99–8.95) | 0.05 | ||||
| EF (%) | 0.99 (0.94–1.04) | 0.56 | 0.94 (0.90–0.98) | <0.001 | ||||
| ESV index (mL/m2) | 1.02 (1.00–1.03) | 0.01 | 1.01 (1.00–1.03) | 0.01 | 1.01 (0.99–1.02) | 0.27 | ||
| GLS (%) | 1.11 (0.99–1.24) | 0.07 | 1.18 (1.07–1.30) | 0.001 | 1.16 (1.05–1.30) | 0.006 | ||
| MD (per 10 ms) | 1.00 (0.92–1.10) | 0.96 | 1.04 (0.97–1.12) | 0.27 | ||||
| GCS (%) | 1.14 (0.97–1.34) | 0.12 | 1.12 (0.97–1.29) | 0.12 | ||||
| 6 months with CRT | ||||||||
| HR (bpm) | 1.02 (0.96–1.07) | 0.58 | 1.03 (0.98–1.08) | 0.27 | ||||
| NYHA class | 1.26 (0.41–3.93) | 0.69 | 5.08 (1.85–13.9) | 0.002 | ||||
| EF (%) | 0.95 (0.90–0.99) | 0.03 | 0.95 (0.90–1.01) | 0.08 | 0.90 (0.86–0.95) | <0.001 | ||
| CRT response | 0.38 (0.12–1.15) | 0.09 | 0.15 (0.04–0.51) | 0.003 | ||||
| GLS (%) | 1.15 (1.04–1.26) | 0.01 | 1.11 (1.01–1.22) | 0.03 | ||||
| MD (per 10 ms) | 1.21 (1.08–1.36) | 0.001 | 1.20 (1.06–1.35) | 0.005 | 1.02 (0.88–1.18) | 0.81 | ||
| GCS (%) | 1.12 (0.96–1.29) | 0.14 | 1.19 (1.037–1.37) | 0.01 | ||||
Multivariate analyses were performed among significant parameters at 6 months to predict ventricular arrhythmias and among significant parameters before CRT to predict fatal end point.
bpm, beats per minute; CI, confidence interval; EF, ejection fraction; ESV, end-systolic volume; GCS, global circumferential strain; GLS, global longitudinal strain; HR, heart rate; ICM, ischaemic cardiomyopathy; MD, mechanical dispersion; NYHA, New York Heart Association; SR, sinus rhythm; VA, ventricular arrhythmia.
By ROC analysis, a GLS before CRT worse than or equal to −8.3% detected patients with fatal end point with a sensitivity of 88% (95% CI 68–97%) and specificity of 55% (95% CI 47–64%) [C-statistics 0.73 (95% CI 0.64–0.82)]. Patients with GLS before CRT better than −8.3% had better survival free from fatal end point compared with those with GLS worse than or equal to −8.3% (log-rank, P < 0.001) (Figure 2).
Figure 2.
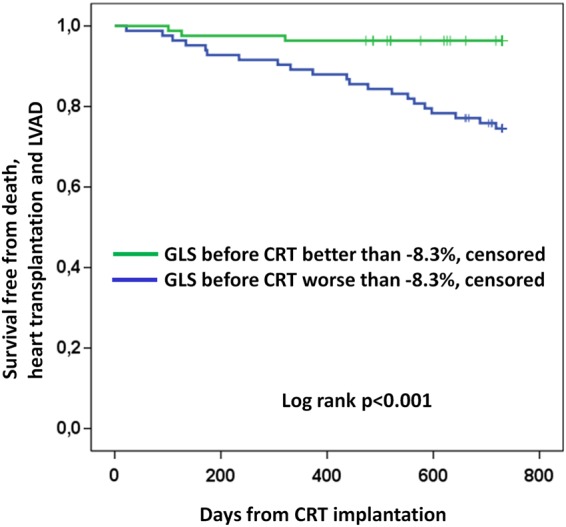
Survival plot showing 2-year survival free from death, heart transplantation, or LVAD implantation according to GLS before CRT implantation.
Additionally, in the sub-group of CRT non-responders separately (n = 63), worse GLS before CRT was a marker of fatal end point (−8.9 ± 4.4 vs. −5.9 ± 3.3%, P = 0.02). CRT non-responders with a GLS before CRT better than −8.3% had a survival benefit compared with CRT non-responders with GLS worse than −8.3% (P = 0.02).
Prediction of ventricular arrhythmia
Mechanical dispersion at 6 months was more pronounced in patients with ventricular arrhythmias than in those without (112 ± 47 vs. 80 ± 30 ms, P = 0.001) (Figure 3). Mechanical dispersion at 6 months was a predictor of ventricular arrhythmias independently of EF at 6 months (Table 2). Mechanical dispersion improved at 6 months in patients without ventricular arrhythmia during the 2-year follow-up while it remained unchanged in patients experiencing ventricular arrhythmias (Figure 4).
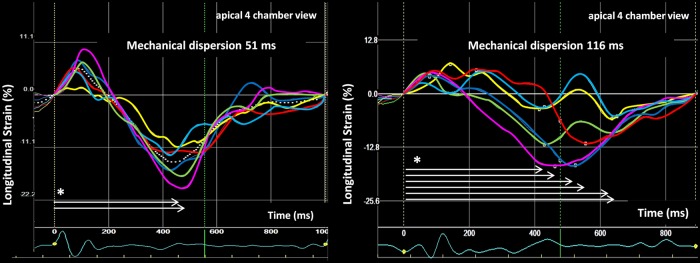
Figure 3.
Longitudinal strain curves from apical four-chamber view displaying 6 of the 18 LV segments used to calculate mechanical dispersion at 6 months with CRT. Left panel: patient with mechanical dispersion 51 ms. The patient did not have ventricular arrhythmias during 2-year follow-up. Right panel: patient with mechanical dispersion 116 ms, i.e. severe mechanical dyssynchrony. The patient experienced several sustained ventricular tachycardias. *White arrows indicate time to peak strain (ms).
Figure 4.
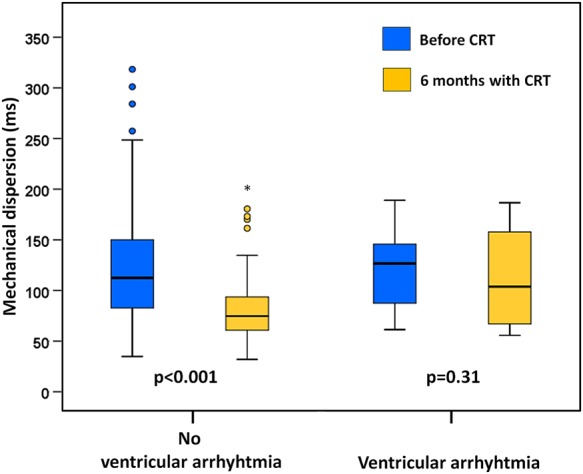
Patients without ventricular arrhythmias during the 2-year follow-up showed resynchronization by improvement of mechanical dispersion after 6 months with biventricular pacing. Patients with ventricular arrhythmias during follow-up (n = 13) did not show resynchronization.
For parameters before CRT, both ischaemic cardiomyopathy and ESV index were independent predictors of ventricular arrhythmia [HRICM 4.20 (1.38–12.80), P = 0.01, and HRESV index 1.02 (1.01–1.03), P = 0.01].
Changes in myocardial function
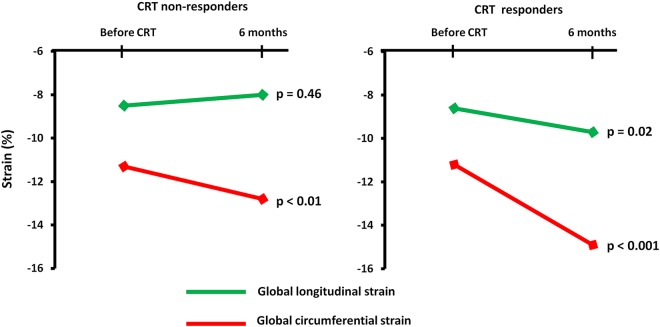
In the total population, EF (27 ± 9 to 35 ± 12%, P < 0.001) and GCS (−11.2 ± 3.4 to −14.1 ± 4.4%, P < 0.001) improved 6 months after CRT, while GLS did not change (−8.5 ± 3.9 to −8.8 ± 4.6%, P = 0.29). CRT responder rate by reverse remodelling was 59% (89/152). In CRT responders, GLS improved while there was no improvement of GLS in non-responders (Table 3) (Figure 5). GCS improved in both CRT responders and non-responders.
Table 3.
Changes in functional class and echocardiographic parameters in CRT non-responders (left) and CRT responders (right)
| CRT non-responders (n = 63) |
CRT responders (n = 89) |
|||||
|---|---|---|---|---|---|---|
| Before CRT | 6 months with CRT | P-value | Before CRT | 6 months with CRT | P-value | |
| NYHA class | 2.8 ± 0.4 | 2.2 ± 0.5 | <0.001 | 2.8 ± 0.5 | 2.1 ± 0.5 | <0.001 |
| EF (%) | 27 ± 9 | 28 ± 10 | 0.37 | 27 ± 9 | 40 ± 12 | <0.001 |
| ESV index (mL/m2) | 68 ± 32 | 70 ± 32 | 0.08 | 71 ± 30 | 42 ± 23 | <0.001 |
| GLS (%) | −8.3 ± 4.3 | −7.9 ± 4.2 | 0.46 | −8.6 ± 3.6 | −9.7 ± 4.8 | 0.02 |
| GCS (%) | −11.3 ± 3.8 | −12.8 ± 4.4 | 0.004 | −11.2 ± 3.1 | −14.9 ± 4.3 | <0.001 |
| MD (ms) | 115 ± 44 | 87 ± 34 | <0.001 | 125 ± 55 | 80 ± 32 | <0.001 |
CRT, cardiac resynchronization therapy; EF, ejection fraction; ESV, end-systolic volume; GCS, global circumferential strain; GLS, global longitudinal strain; MD, mechanical dispersion; NYHA, New York Heart Association.
Figure 5.
Changes in global longitudinal strain and global circumferential strain in CRT non-responders (left) and CRT responders (right).
There were more patients with non-ischaemic (60%) than with ischaemic cardiomyopathy (40%) among CRT responders (P = 0.03). The improvement of GLS in CRT responders was mainly driven by non-ischaemic cardiomyopathy patients (−9.1 ± 3.6 to −10.8 ± 4.1%, P = 0.002), since there was no improvement of GLS in CRT responders with ischaemic cardiomyopathy as a group (−7.9 ± 3.7 to −8.0 ± 5.2%, P = 0.88). In contrast, GCS and EF improved in CRT responders with both non-ischaemic (GCS: −11.2 ± 3.3 to −15.4 ± 4.6%, P < 0.001, and EF: 28 ± 9 to 41 ± 12%, P < 0.001) and ischaemic cardiomyopathy (GCS: −11.2 ± 2.6 to −14.1 ± 3.5%, P < 0.001, and EF: 25 ± 8 to 38 ± 11%, P < 0.001).
When dividing patients according to end points, all echocardiographic parameters improved in patients without arrhythmic end point and without fatal end point, except from GLS that did not improve in patients without arrhythmic or fatal end point (see Supplementary data online, Table S1).
Intra-observer and inter-observer variability intra-class correlation coefficients were 0.94 and 0.92, respectively, for longitudinal strain and 0.90 and 0.87, respectively, for circumferential strain.
Discussion
This study provides novel data showing that longitudinal myocardial function before CRT implantation was a strong predictor of all-cause death, heart transplantation, and LVAD during 2 years follow-up in heart failure patients. Importantly, this was found independently of CRT response. Furthermore, resynchronization from CRT pacing predicted freedom from ventricular arrhythmias.
Our results provide mechanistic insight on CRT's impact on myocardial function. LV longitudinal function improved in CRT responders while not in CRT non-responders. Our findings suggest that CRT response by reverse remodelling is dependent on improvement of longitudinal function in addition to the relatively larger improvement of circumferential function.
Death, heart transplantation, and LVAD implantation
This study is the first showing that longitudinal LV function by GLS before CRT was a strong predictor of fatal end point independently of CRT response. Knappe et al.20 showed in a MADIT-CRT sub-study that GLS was a marker of heart failure events or mortality as a combined end point different from ours. Additionally, they only studied patients in mild heart failure, while in our prospective study the majority of patients had severely symptomatic heart failure.
Our findings in CRT patients are in accordance with studies showing that longitudinal LV function has prognostic impact.13,21 A GLS of −8.3% before CRT had good ability to identify fatal end point 2 years after CRT. Interestingly, this critical strain value is in agreement with longitudinal strain values showing ability to identify transmural scars of 51–100% on cardiac magnetic resonance imaging in patients after myocardial infarction.22,23 Scar and fibrosis lead to poor global LV performance which again increases mortality and which may be the mechanism for our finding of worse strain as a predictor of the fatal outcome.
GLS before CRT and CRT response by reverse remodelling at 6 months were both predictors of fatal end point, independently of each other. Also others have shown that lack of CRT-induced reverse remodelling is related to heart failure events and death.24,25 We provide novel data demonstrating that preserved myocardial longitudinal function before CRT implantation shows additional value for predicting survival. Moreover, this is the first study showing that among CRT non-responders, GLS was able to identify patients surviving despite CRT non-response. This suggests that CRT non-responders are inhomogeneous as a group and that GLS as a sensitive measure can help discriminate those CRT non-responders with better from those with worse prognosis.
Ventricular arrhythmias
Lack of myocardial resynchronization at 6 months with CRT was a strong marker of life-threatening ventricular arrhythmias. Currently, EF is used to assess risk of ventricular arrhythmia and indication for primary prevention ICD in heart failure patients.15 EF at 6 months was a marker of ventricular arrhythmias, in line with a recent MADIT-CRT sub-study.26 However, mechanical dispersion predicted arrhythmias independently of EF at 6 months follow-up. Previous work from our research group have shown strong evidence that mechanical dispersion predicts ventricular arrhythmia in several different cardiomyopathies,13,14,19 also confirmed by others.27 This present study widens the application for mechanical dispersion, by being the first to show that mechanical dispersion after CRT-induced changes in mechanical dyssynchrony provides a measure of risk of ventricular arrhythmias. Our results for mechanical dispersion are in line with two recent studies using transverse and radial strain from 12 and 2 LV segments, respectively, for assessing mechanical dyssynchrony and with a different heart failure population.9,10 We believe that mechanical dispersion is a more robust measure and better reflects global cardiac arrhythmic risk as it derives from longitudinal strain with high reproducibility and includes all 16 LV segments. Nevertheless, the consistency across these studies, despite differences in strain methods, strengthens the common concept that resynchronization after CRT is a marker of freedom from arrhythmias. The aim of biventricular pacing is to synchronize electrical activation of the LV. Synchronous myocardial electrical activation, which we speculate may be reflected in lower mechanical dispersion, might reduce unfortunate slow conduction related to fibrosis and therefore reduce both generation of re-entry circuits and electrical dispersion leading to arrhythmias.
CRT's impact on myocardial function
In CRT responders, there were improvements in both longitudinal and circumferential function (Table 3). We propose that improved circumferential function contributes relatively more than longitudinal function to volumetric CRT response. In CRT non-responders, there was only a slight improvement of circumferential function, accompanied by a non-significant worsening of longitudinal function.
There are conflicting results regarding CRT-induced changes in myocardial function. In line with us, it has been found that circumferential function is the relatively most important contributor to CRT response.28 However, others, like us, have shown that also longitudinal function improves in CRT responders.29,30 CRT response by reverse remodelling indicates a reduction in end-systolic LV volumes. We suggest that volumetric CRT response is more likely if there is additional contribution from improved longitudinal function than if improved circumferential function is contributing to LV volume reduction alone.
The relative improvements in GCS and mechanical dispersion were much smaller in CRT non-responders than in CRT responders, which may explain why CRT non-responders did not have volumetric CRT response (Table 3).
Only patients with non-ischaemic cardiomyopathy showed improvement of longitudinal function by GLS, while ischaemic patients did not. The fibre direction of the subendocardial myocardial layer is mainly longitudinally oriented, while the fibre direction in the midmyocardial layer is mainly circular, although all are helically ordered.31,32 Loss of longitudinal function from the subendocardial fibres is typical for ischaemic damage and may explain why longitudinal function by GLS did not improve in patients with ischaemic cardiomyopathy. Circumferential function from midmyocardial layers is often less affected by ischaemia and improved by CRT in patients with both non-ischaemic and ischaemic cardiomyopathy. We suggest that a longitudinal functional reserve is present in non-ischaemic patients, providing better chances for CRT response and explaining the better CRT response rate known to exist in non-ischaemic compared with ischaemic cardiomyopathy patients.24
Clinical implications
The reduction in mortality by CRT in heart failure is well documented,1–4 but prediction of survival remains difficult. We found prognostic importance in longitudinal function before CRT, and this novel finding may suggest that CRT may be less beneficial in heart failure patients with the poorest longitudinal function. However, we cannot conclude that CRT treatment will not improve survival in patients with poorest longitudinal function due to the predefined design of our study.
Our findings suggest that assessment of mechanical dispersion is important in risk prediction of ventricular arrhythmias and should be performed in CRT patients. Lack of resynchronization after CRT may indicate high arrhythmic risk and should initiate measures to optimize anti-arrhythmic treatment and surveillance, including upgrade a CRT-P with an ICD if clinically and ethically justified.
Limitations
Our findings should be confirmed in larger study populations. Due to small sample size and few end points, we had a restricted number of covariates in the multivariate Cox analyses to avoid statistical model over-fitting.
Eighteen (11%) patients were lost to follow up for a second echocardiography which is a potential bias.
Patients with ischaemic cardiomyopathy and myocardial scars have high risk of ventricular arrhythmia, as also shown in this study. Myocardial scar burden and location and its relation to CRT lead positions determine regional mechanical function and impact arrhythmic risk. The lack of this information is an important limitation to the strength of our findings.
This was a clinically uncontrolled trial of heart failure patients of different aetiologies, reflecting the population fulfilling current CRT indications. Heart failure patients of different aetiologies clearly show different prognosis, arrhythmic risk, and response to CRT.
Conclusions
In heart failure patients fulfilling CRT indications, longitudinal function by GLS before CRT implantation was an important predictor of survival free from death, heart transplantation, or LVAD implantation during 2 years with CRT, including among CRT non-responders. For the ventricular arrhythmia-free outcome however, improvement of mechanical dispersion was the most important predictor. CRT response by reverse remodelling, more common in non-ischaemic cardiomyopathy, was dependent on improvement of longitudinal function in addition to circumferential function.
Conflict of interest: None declared.
Funding
This work was supported by the Research Council of Norway funding the Center for Cardiological Innovation (203489 to N.E.H. and T.E.) and the South-Eastern Norway Regional Health Authority (2011094 to K.H.H.). Funding to pay the Open Access publication charges for this article was provided by the Research Council of Norway.
Supplementary data
Supplementary data are available at European Journal of Echocardiography online.
References
- 1.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 2008;52:1834–43. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP et al. . Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T et al. . Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–50. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L et al. . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 5.Bradley DJ, Bradley EA, Baughman KL, Berger RD, Calkins H, Goodman SN et al. . Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA 2003;289:730–40. [DOI] [PubMed] [Google Scholar]
- 6.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B et al. . Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003;289:2685–94. [DOI] [PubMed] [Google Scholar]
- 7.Barsheshet A, Wang PJ, Moss AJ, Solomon SD, Al-Ahmad A, McNitt S et al. . Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy). J Am Coll Cardiol 2011;57:2416–23. [DOI] [PubMed] [Google Scholar]
- 8.Di Biase L, Gasparini M, Lunati M, Santini M, Landolina M, Boriani G et al. . Antiarrhythmic effect of reverse ventricular remodeling induced by cardiac resynchronization therapy: the InSync ICD (Implantable Cardioverter-Defibrillator) Italian Registry. J Am Coll Cardiol 2008;52:1442–9. [DOI] [PubMed] [Google Scholar]
- 9.Haugaa KH, Marek JJ, Ahmed M, Ryo K, Adelstein EC, Schwartzman D et al. . Mechanical dyssynchrony after cardiac resynchronization therapy for severely symptomatic heart failure is associated with risk for ventricular arrhythmias. J Am Soc Echocardiogr 2014;27:872–9. [DOI] [PubMed] [Google Scholar]
- 10.Kutyifa V, Pouleur AC, Knappe D, Al-Ahmad A, Gibinski M, Wang PJ et al. . Dyssynchrony and the risk of ventricular arrhythmias. JACC Cardiovasc Imaging 2013;6:432–44. [DOI] [PubMed] [Google Scholar]
- 11.Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation 2002;106:50–6. [DOI] [PubMed] [Google Scholar]
- 12.Delgado V, Bax JJ. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is clinically useful. Circulation 2011;123:640–55. [DOI] [PubMed] [Google Scholar]
- 13.Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A et al. . Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr 2012;25:667–73. [DOI] [PubMed] [Google Scholar]
- 14.Haugaa KH, Grenne BL, Eek CH, Ersboll M, Valeur N, Svendsen JH et al. . Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 2013;6:841–50. [DOI] [PubMed] [Google Scholar]
- 15.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K et al. . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–69. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al. . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 17.Hasselberg NE, Haugaa KH, Sarvari SI, Gullestad L, Andreassen AK, Smiseth OA et al. . Left ventricular global longitudinal strain is associated with exercise capacity in failing hearts with preserved and reduced ejection fraction. Eur Heart J Cardiovasc Imaging 2015;16:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R et al. . Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1–11. [DOI] [PubMed] [Google Scholar]
- 19.Haugaa KH, Hasselberg NE, Edvardsen T. Mechanical dispersion by strain echocardiography: a predictor of ventricular arrhythmias in subjects with lamin A/C mutations. JACC Cardiovasc Imaging 2015;8:104–6. [DOI] [PubMed] [Google Scholar]
- 20.Knappe D, Pouleur AC, Shah AM, Cheng S, Uno H, Hall WJ et al. . Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Fail 2011;4:433–40. [DOI] [PubMed] [Google Scholar]
- 21.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–64. [DOI] [PubMed] [Google Scholar]
- 22.Rost C, Rost MC, Breithardt OA, Schmid M, Klinghammer L, Stumpf C et al. . Relation of functional echocardiographic parameters to infarct scar transmurality by magnetic resonance imaging. J Am Soc Echocardiogr 2014;27:767–74. [DOI] [PubMed] [Google Scholar]
- 23.Sjoli B, Orn S, Grenne B, Ihlen H, Edvardsen T, Brunvand H. Diagnostic capability and reproducibility of strain by Doppler and by speckle tracking in patients with acute myocardial infarction. JACC Cardiovasc Imaging 2009;2:24–33. [DOI] [PubMed] [Google Scholar]
- 24.Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW et al. . Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation 2010;122:985–92. [DOI] [PubMed] [Google Scholar]
- 25.Bertini M, Hoke U, van Bommel RJ, Ng AC, Shanks M, Nucifora G et al. . Impact of clinical and echocardiographic response to cardiac resynchronization therapy on long-term survival. Eur Heart J Cardiovasc Imaging 2013;14:774–81. [DOI] [PubMed] [Google Scholar]
- 26.Ruwald MH, Solomon SD, Foster E, Kutyifa V, Ruwald AC, Sherazi S et al. . Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the Multicenter Automatic Defibrillator Implantation Trial |with Cardiac Resynchronization Therapy (MADIT-CRT) Trial. Circulation 2014;130:2278–86. [DOI] [PubMed] [Google Scholar]
- 27.Leong DP, Hoogslag GE, Piers SR, Hoke U, Thijssen J, Marsan NA et al. . The relationship between time from myocardial infarction, left ventricular dyssynchrony, and the risk for ventricular arrhythmia: speckle-tracking echocardiographic analysis. J Am Soc Echocardiogr 2015;28:470–7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Fung JW, Yip GW, Chan JY, Lee AP, Lam YY et al. . Improvement of left ventricular myocardial short-axis, but not long-axis function or torsion after cardiac resynchronisation therapy: an assessment by two-dimensional speckle tracking. Heart 2008;94:1464–71. [DOI] [PubMed] [Google Scholar]
- 29.Delgado V, Ypenburg C, Zhang Q, Mollema SA, Fung JW, Schalij MJ et al. . Changes in global left ventricular function by multidirectional strain assessment in heart failure patients undergoing cardiac resynchronization therapy. J Am Soc Echocardiogr 2009;22:688–94. [DOI] [PubMed] [Google Scholar]
- 30.Klimusina J, De Boeck BW, Leenders GE, Faletra FF, Prinzen F, Averaimo M et al. . Redistribution of left ventricular strain by cardiac resynchronization therapy in heart failure patients. Eur J Heart Fail 2011;13:186–94. [DOI] [PubMed] [Google Scholar]
- 31.Sengupta PP, Korinek J, Belohlavek M, Narula J, Vannan MA, Jahangir A et al. . Left ventricular structure and function: basic science for cardiac imaging. J Am Coll Cardiol 2006;48:1988–2001. [DOI] [PubMed] [Google Scholar]
- 32.Streeter DD Jr, Spotnitz HM, Patel DP, Ross J Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 1969;24:339–47. [DOI] [PubMed] [Google Scholar]