Abstract
Aims
The current study was designed to test that vasa vasorum (VV) plays a role in the progression of cardiac allograft vasculopathy (CAV) in patients with heart transplantation (HTX).
Methods and results
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) were performed in the left anterior descending artery in 19 segments of 19 HTX patients (median 2.1 years from HTX). Each segment is composed of both the continuous lesions: (i) CAV area: intimal thickness >0.5 mm with 5 mm length and (ii) VV area: intimal thickness ≤0.5 mm with 5 mm length. The per cent VV volume (VV volume/vessel volume × 100, %VV) was evaluated in the VV area with OCT (in CAV area VV cannot be assessed because of limited penetration power of OCT). A year later, the association between the baseline %VV and the change in per cent plaque volume (plaque volume/vessel volume × 100, %PV) was evaluated with IVUS. To a normal distribution, Δ%PV (follow-up %PV–initial %PV) was undergone square root transformation. The correlations between the %VV at baseline study and square root-Δ%PV were significant both in the CAV area and in the VV area (r = 0.787, P < 0.001 and r = 0.701, P < 0.001, respectively). In multivariable analysis, only the %VV was significantly correlated with square root-Δ%PV in both areas.
Conclusion
The current study demonstrated a significant association between the VV volume and the progression of plaque volume in both the CAV area and the VV area. Thus, VV may be a potential predictor and possible therapeutic target to attenuate CAV.
Keywords: cardiac allograft vasculopathy, heart transplantation, vasa vasorum
Introduction
Cardiac allograft vasculopathy (CAV) is an accelerated form of intimal hyperplasia of the coronary artery after heart transplantation (HTX) characterized by diffuse progressive luminal narrowing.1 In some patients, CAV develops as early as 1 year after HTX and is the major cause of long-term morbidity and mortality after HTX.2,3
The vasa vasorum (VV) is a complex plexus of microvessels located in the vessel wall.4 Proliferation of the VV has been shown to play an important role in the progression and complications of atherosclerosis and typically precedes the development of intimal thickening of the coronary artery.5,6 CAV shares several common features with atherosclerosis and so we thought that assessment of the adventitial VV may assist in predicting the progression of CAV.7,8
Intravascular ultrasound (IVUS) is a well-established method for evaluating changes in coronary atherosclerotic plaque and has been used to quantify this process in CAV.9,10 On IVUS, CAV area could be defined as an intimal thickening of >0.5 mm.11 Optical coherence tomography (OCT) is an emerging tool to evaluate the coronary artery near histological levels including the morphology of adventitial VV.12 However, measurement of the adventitial VV under CAV area is limited by the penetration power of OCT.
We hypothesized that the quantitatively assessed VV density, measured adjacent to the CAV area, could represent the VV of the CAV area because of the diffuse disease pattern of the CAV and VV network.7,13 This study aimed to evaluate the association between the VV density and the changes in the intimal plaque volume in patients with CAV.
Methods
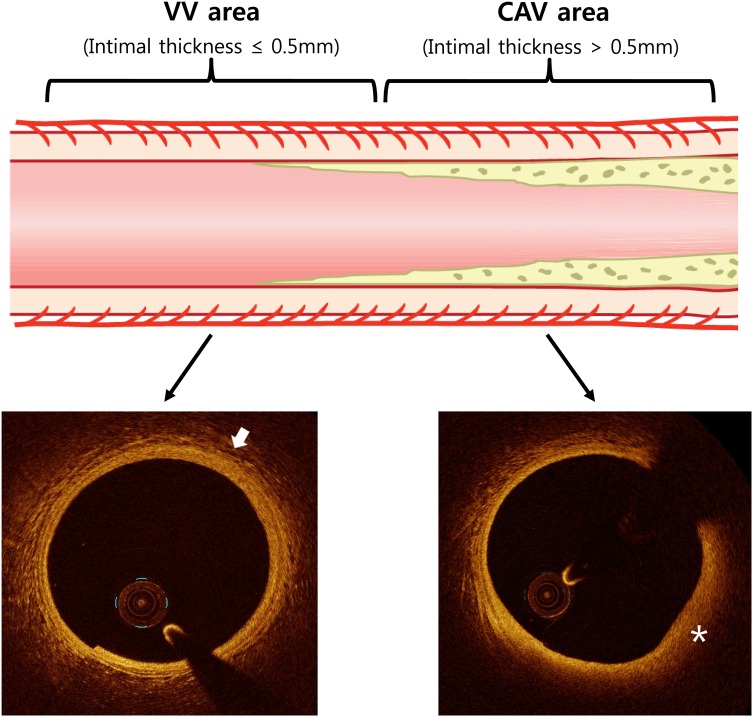
All HTX recipients with >18 years old were eligible for study unless they had chronic kidney disease ≥Stage IV (glomerular filtration rate <30 mL/min), active infection, active rejection, or were unable to participate in the study. From 14 September 2011 to 9 July 2012, there were 121 consecutive HTX patients who were referred for their annual coronary angiography. Among them, there were 27 patients who underwent both OCT and IVUS of the left anterior descending coronary artery. All of the 27 patients were evaluated follow-up IVUS 1 year later. The following continuous lesions were evaluated for our study: (i) VV area: a lesion consecutive to the proximal or distal part of the CAV area with an intimal thickening ≤0.5 mm and length >5 mm that would clearly evaluate VV with OCT, and (ii) CAV area: intimal thickness >0.5 mm with a lesion of >5 mm (Figure 1). For the homogenicity of the study, sample location was limited to the proximal portion of the upper one-third of mid-left anterior descending coronary artery. The scheme of analysis is described in Figure 2. The study protocols were approved by the institutional review board of the Mayo Clinic. All patients gave their written informed consents to participate in this study.
Figure 1.
This illustration shows the study segment of the study. In the VV area, which is adjacent to the CAV area, the VV can be clearly evaluated with OCT (arrow). In the CAV area, the evaluation of the VV in plaque area (asterisk) is difficult because of the limited penetration power of the OCT. CAV, coronary allograft vasculopathy; VV, vasa vasorum.
Figure 2.
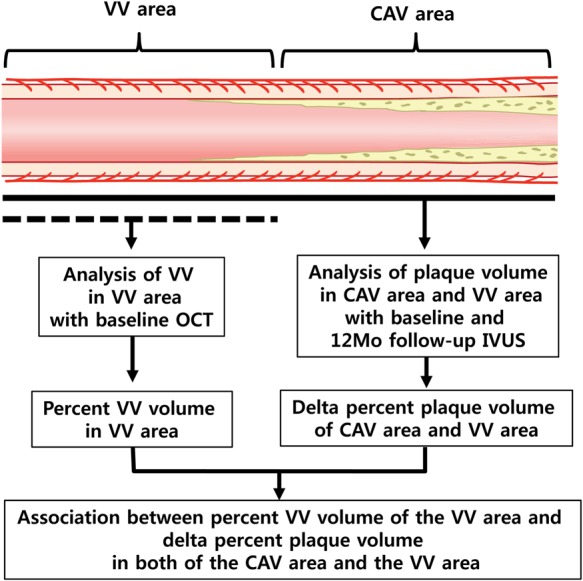
This is the scheme of analysis of the study. CAV, coronary allograft vasculopathy; OCT, optical coherence tomography; IVUS, intravascular ultrasound; VV, vasa vasorum.
IVUS image acquisition and analysis
The IVUS examination was performed as previously described.14 In brief, after intracoronary administration of 100–200 mg nitroglycerin, a 20 MHz IVUS catheter (Eagle Eye Gold, Volcano Corporation, Rancho Cordova, CA, USA) was advanced into the middle to distal left anterior descending coronary artery, and automatic pullback at 0.5 mm/s was done. Offline volumetric reconstruction analyses of IVUS were performed by one independent experienced examiner, who is blinded to the clinical data and the OCT findings, using the Volcano Image Analysis software V3.1 (Volcano Corporation). Quantitative volumetric analysis was performed in the 5-mm segment of both the CAV area and the VV area. The average vessel volume (mm3) was calculated by average vessel area (mm2) × 5-mm lesion length. The per cent plaque volume (%PV) was determined by (plaque volume/average vessel volume) * 100. The difference between the follow-up %PV and the initial %PV was expressed as the delta per cent plaque volume (Δ%PV). A total of 15 segments of IVUS were analysed by two independent examiners to evaluate inter-observer and intra-observer variabilities on plaque volume. Intra-observer agreement was calculated 10 days after the first study. The Lin's concordance correlation coefficient values for the inter-observer and intra-observer agreement were 0.947 and 0.971, respectively.
OCT image acquisition and analysis
Acquisition and analysis of OCT images were performed also as previously described.15 For the acquisition of OCT images, the C7-XR OCT intravascular imaging system (St Jude Medical, St Paul, MN, USA) was used. The imaging catheter (Dragonfly, St Jude Medical) was advanced into the mid-to-distal segment of the left anterior descending coronary artery, and automatic pullback of 50 mm at a speed of 20 mm/s (100 frames/s) was initiated in concordance with the blood clearance through the infusion of the radiocontrast media. All OCT images were digitally stored and analysed offline using LightLab imaging (St Jude Medical). We excluded segments that contained poor images to observe the VV in the adventitia of coronary artery because of the incomplete blood removal or plaque thickness >0.5 mm, because the light signal of the OCT could be attenuated in such segments. We also excluded segments with major branches that occupied >90 degrees of vessel wall, which obscure VV in the vessel adventitia.
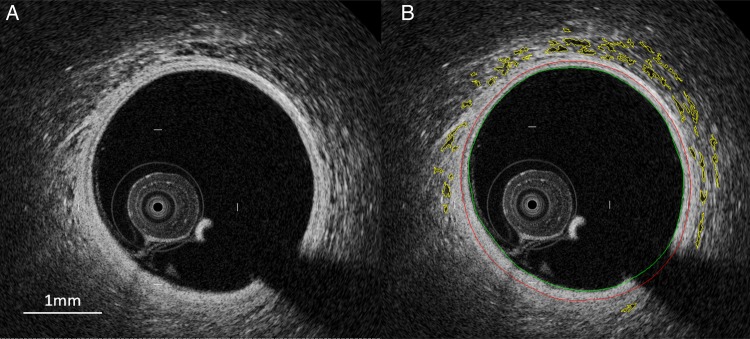
Volumetric analysis of the VV and its matched vessel in the VV area was performed using the ANALYZE software 11.0 (Biomedical Imaging Resource, Rochester, MN, USA) which was demonstrated as the useful modality of 3D volumetric generation and analysis of OCT images as previously described.16 First, the data of the OCT images were converted in to the DICOM file. On the ANALYZE program, the DICOM file of the OCT images was loaded as red channel data with an 8-bit matrix of 20 × 20 × 200 µm cubic voxels. The 5-mm length of the VV area was traced in every slice of the cross-sectional OCT image by a distance of 200 µm. Adventitial VV was defined as signal-voiding tubular structures with major diameters of 50–300 µm, observed in at least two consecutive slices, and located within 1 mm from the lumen-intima border.16 After determining and tracing each VV in all of separate slice (Figure 3), we set the range of slice number that would be analysed. The analysis of VV volume rendering process was done by the voxel number × cubic voxel size of VVs according to the continuous frame. The vessel volume was defined as the reconstructed volume surrounded by the boundaries between the media and the adventitia of coronary artery. The VV volume fraction (%VV) was expressed as (VV volume/vessel volume) × 100. Image analysis was performed by one examiner who is well-trained to analyse OCT images and blinded to the clinical data and IVUS findings. To evaluate inter-observer and intra-observer variabilities on volumetric analysis of VV with OCT, a total of 100 image slices were analysed by two independent assessors on VV volume. Intra-observer agreement was calculated 14 days after the first study. The Lin's concordance correlation coefficient values for the inter-observer and intra-observer agreement were 0.905 and 0.919, respectively.
Figure 3.
After storage with the DICOM file, OCT image converted to red channel image data (A). The same image with boundaries of the VV is shown in (B). Yellow lines indicate the areas of VV.
To match the study lesion location between OCT and IVUS, anatomical landmarks such as side branches and calcium were used. The coronary angiography was also used to get the information of the distribution and location of the side branches. The distance from the anatomical landmark to each study segment point was determined using the longitudinal reconstructed OCT and IVUS image sets to ensure that distance measurements were the same between the both methods. All matching between OCT and IVUS images was done by an independent assessor who is blinded to the volumetric analysis of IVUS and OCT. Another independent observer confirmed that the OCT, baseline IVUS, and follow-up IVUS locations were same for all study segments.
Statistical analysis
Continuous variables are denoted as the mean ± standard deviation or median and inter-quartile range (25 and 75% quartiles) as appropriate. Discrete variables are presented as frequency (percentage). Comparisons between index and follow-up measures were tested with Wilcoxon signed rank test. Comparisons between independent groups were conducted with Mann–Whitney U test. Correlation between variables was evaluated with Pearson's or Spearman's rank correlation as appropriate. As the Δ%PV (follow-up %PV − initial %PV) in the CAV area and the VV area showed a non-normal distribution, a square root transformation was applied to the Δ%PV for normal distribution (W = 0.975, P = 0.870). Multivariable linear regression analysis was performed to study the independent correlates of the change in the %PV. Potential collinearity was considered acceptable, and the regression model was stable at a variance inflation factor of <4. All statistical tests were two-sided, and a P-value of <0.05 was considered statistically significant. Statistical analysis was performed using JMP 10.0 (SAS Institute, Cary, NC, USA).
Results
Among the 27 patients, 3 patients had poor OCT image and 5 patients did not have a suitable lesion segment for the study because of the diffuse CAV lesions. Finally, 25 segments in 19 patients were registered for analysis. Because six of them also had a study lesion at the lower mid or distal left anterior descending coronary artery, they were excluded from analysis, leaving 19 samples.
The baseline clinical characteristics are shown in Table 1. The follow-up study was conducted after 12.5 ± 1.2 months. OCT analysis in VV area showed that the vessel volume was 60.5 ± 18.1 mm3 and VV volume was 2.1 ± 0.6 mm3, and thus, the %VV was 3.6 ± 0.9%. The mean total VV count was 835 ± 266. The initial and follow-up volumetric analysis of the grey-scale IVUS is shown in Table 2. The average lumen area, vessel area, and vessel volume were not different between the initial and the follow-up IVUS studies in either the CAV area or the VV area. However, compared with the initial study, the follow-up plaque volume and the %PV were significantly increased both in the CAV area and in the VV area. Between the %VV and the initial %PV, there was significant correlation of the CAV area and marginal correlation of the VV area (r = 0.597, P = 0.007 and r = 0.416, P = 0.077, respectively). Table 3 shows the correlation between the square root-Δ%PV and the %VV and other risk factors of CAV. In both the CAV area and the VV area, %VV was significantly correlated with the square root-Δ%PV (r = 0.787, P < 0.001 and r = 0.701, P < 0.001, respectively, Figure 4). Among the other risk factors, only years after HTX was significantly correlated with the square root-Δ%PV in the CAV area (r = 0.678, P = 0.001). In the post hoc analysis, which included six segments of the lower two-thirds, the result also showed a significant correlation between the %VV and the square root-Δ%PV both in the CAV area and in the VV area (r < 0.776, P < 0.001 and r < 0.756, P < 0.001, respectively).
Table 1.
Baseline clinical characteristics
| Variables | Values (n = 19) |
|---|---|
| Age, median (Q1, Q3) | 54 (46, 65) |
| Male sex, n (%) | 12 (63.2) |
| Current smoking, n (%) | 0 (0.0) |
| Diabetes, n (%) | 6 (31.6) |
| Hypertension, n (%) | 12 (63.2) |
| Dyslipidaemia, n (%) | 11 (57.9) |
| Renal insufficiency, n (%) | 11 (57.9) |
| Body mass index, kg/m2 | 27.8 ± 4.8 |
| Systolic blood pressure, mmHg | 109 ± 9 |
| Diastolic blood pressure, mmHg | 70 ± 8 |
| Heart rate, bpm | 80 ± 11 |
| Reason for HTX | |
| Ischaemic CMP, n (%) | 3 (15.8) |
| Non-ischaemic CMP, n (%) | 16 (84.2) |
| Years after HTX, median (Q1, Q3) | 2.1 (1.0, 8.0) |
| Donor age, median (Q1, Q3) | 30 (21, 41) |
| Cold ischaemic time, min | 191 ± 34 |
| CMV infection, n (%) | 6 (31.6) |
| LVEF (%) | 65.3 ± 4.5 |
| ISHLT grade | |
| CAV0, n (%) | 7 (36.8) |
| CAV1, n (%) | 10 (52.6) |
| CAV2, n (%) | 1 (5.2) |
| CAV3, n (%) | 1 (5.2) |
| Laboratory data | |
| Total cholesterol (mg/dL) | 200 ± 42 |
| Triglyceride (mg/dL) | 186 ± 106 |
| HDL (mg/dL) | 57 ± 17 |
| LDL (mg/dL) | 107 ± 27 |
| Creatinine (mg/dL) | 1.2 ± 0.3 |
| Fasting blood sugar (mg/dL) | 112.8 ± 61.4 |
| Medication | |
| Aspirin, n (%) | 3 (15.8) |
| Statin, n (%) | 18 (94.7) |
| Steroid, n (%) | 10 (47.4) |
| Azathioprine, n (%) | 3 (15.8) |
| Cyclosporin, n (%) | 3 (15.8) |
| Mycophenolate mofetil, n (%) | 13 (68.4) |
| Sirolimus, n (%) | 13 (68.4) |
| Tacrolimus, n (%) | 3 (15.8) |
BMI, body mass index; CAV, cardiac allograft vasculopathy; CMP, cardiomyopathy; CMV, cytomegalovirus; HDL, high-density lipoprotein; HTX, heart transplantation; ISHLT, International Society for Heart and Lung Transplantation; LDL, low-density lipoprotein.
Table 2.
Grey-scale data of the intravascular ultrasound
| Initial study (n = 19) | FU study (n = 19) | P-valuea | |
|---|---|---|---|
| CAV area (5 mm) | |||
| Average lumen area (mm2) | 10.3 ± 2.9 | 9.4 ± 3.6 | 0.133 |
| Average vessel area (mm2) | 13.3 ± 3.1 | 13.4 ± 3.9 | 0.852 |
| Average vessel volume (mm3) | 66.7 ± 15.1 | 67.1 ± 19.5 | 0.852 |
| Plaque volume (mm3) | 15.4 ± 3.9 | 20.8 ± 6.5 | <0.001 |
| Per cent plaque volume (%) | 23.9 ± 7.1 | 32.8 ± 11.2 | <0.001 |
| Vasa vasorum area (5 mm) | |||
| Average lumen area (mm2) | 9.9 ± 2.5 | 9.2 ± 3.4 | 0.215 |
| Average vessel area (mm2) | 11.9 ± 2.7 | 11.8 ± 3.6 | 0.859 |
| Average vessel volume (mm3) | 59.5 ± 13.5 | 59.0 ± 18.1 | 0.859 |
| Plaque volume (mm3) | 10.1 ± 2.8 | 12.8 ± 3.4 | <0.001 |
| Per cent plaque volume (%)b | 17.4 ± 5.0 | 23.2 ± 7.6 | <0.001 |
CAV, cardiac allograft vasculopathy; FU, follow-up.
aAll P-values are from the Wilcoxon singed rank test.
b(Plaque volume/average vessel volume) × 100.
Table 3.
Relations of square root-Δ%PV with the clinical parameters for cardiac allograft vasculopathy
| Correlation coefficient | P-valuea | |
|---|---|---|
| CAV area (5 mm) | ||
| %VV | 0.787 | <0.001 |
| Patient age | −0.086 | 0.726 |
| Donor age | 0.199 | 0.413 |
| Years after HTX | 0.678 | 0.001 |
| Body mass index | 0.075 | 0.759 |
| Ischaemic time | 0.142 | 0.562 |
| Vasa vasorum area (5 mm) | ||
| %VV | 0.701 | <0.001 |
| Patient age | 0.044 | 0.855 |
| Donor age | 0.277 | 0.236 |
| Years after HTX | 0.242 | 0.304 |
| Body mass index | 0.300 | 0.199 |
| Ischaemic time | 0.381 | 0.097 |
CAV, cardiac allograft vasculopathy; HTX, heart transplantation; %VV, per cent vasa vasorum volume; Δ%PV, delta per cent plaque volume.
aAll P-values are from the Spearman's correlation except P-value of %VV from the Pearson's correlation.
Figure 4.
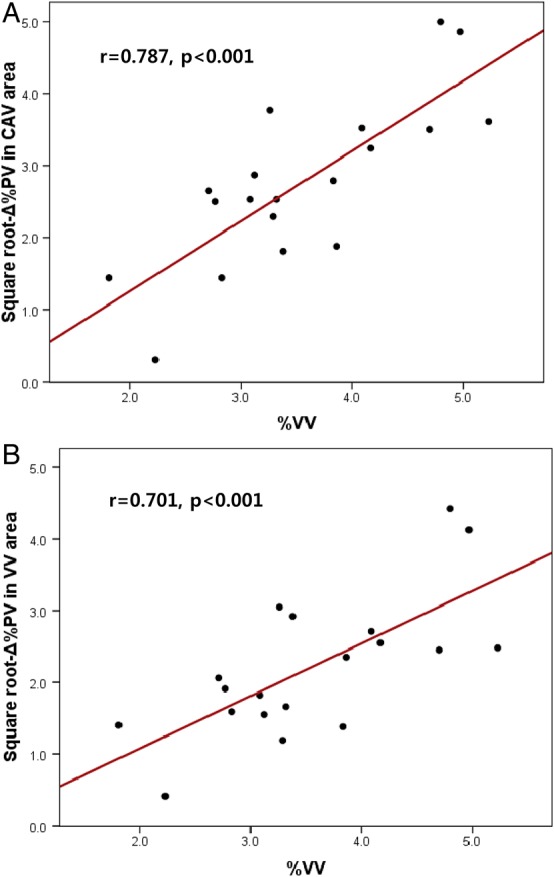
In both the CAV area (A) and the VV area (B), the %VV is significantly correlated with the square root-Δ%PV. Square root-Δ%PV, square root delta per cent plaque volume; %VV, per cent vasa vasorum volume; CAV, cardiac allograft vasculopathy; VV, vasa vasorum.
Although some of the univariate results were not statistically significant, multivariable analysis was performed using all the variables, because known clinical relevance as risk factors for progression of CAV. The multivariable linear regression showed that only the %VV and the square root-Δ%PV were independently correlated in both the CAV area and the VV area (Table 4). Table 5 shows progression of %PV within risk factor subgroups (defined by sex, years after HTX, donor age, and other possible risk factors for CAV and the progression of atherosclerosis). The result showed that the group divided by years after HTX >2 years (median year in this study) significantly increased in terms of the Δ%PV compared with the group <2 years after HTX (12.8 ± 6.3 vs. 3.6 ± 2.3%, P = 0.001).
Table 4.
Results of the multivariable linear regression to assess the independent correlates of the square root-Δ%PV
| SD | SC-β | 95% CI for SC-β | P-value | VIF | |
|---|---|---|---|---|---|
| CAV area (Model F ratio = 6.504, P = 0.003) | |||||
| %VV | 0.93 | 0.657 | 0.319 to 1.194 | 0.003 | 1.554 |
| Years after HTX | 3.57 | 0.332 | −0.071 to 0.843 | 0.092 | 1.679 |
| Patient age | 12.20 | 0.086 | −0.281 to 0.488 | 0.585 | 1.202 |
| Donor age | 10.72 | 0.212 | −0.151 to 0.654 | 0.211 | 1.314 |
| Body mass index | 4.51 | −0.033 | −0.469 to 0.392 | 0.849 | 1.490 |
| Ischaemic time | 33.61 | 0.141 | −0.202 to 0.538 | 0.362 | 1.125 |
| Vasa vasorum area (Model F ratio = 3.812, P = 0.021) | |||||
| %VV | 0.93 | 0.597 | 0.160 to 0.969 | 0.010 | 1.406 |
| Years after HTX | 3.57 | 0.241 | −0.214 to 0.693 | 0.275 | 1.607 |
| Patient age | 12.20 | 0.074 | −0.317 to 0.464 | 0.685 | 1.156 |
| Donor age | 10.72 | 0.224 | −0.182 to 0.622 | 0.254 | 1.264 |
| Body mass index | 4.51 | 0.077 | −0.347 to 0.541 | 0.706 | 1.412 |
| Ischaemic time | 33.61 | 0.182 | −0.202 to 0.571 | 0.316 | 1.092 |
CAV, cardiac allograft vasculopathy; CI, confidence interval; HTX, heart transplantation; %VV, per cent vasa vasorum volume; SD, sample standard deviation; Δ%PV, delta per cent plaque volume; SC-β, standardized coefficients β; VIF, variance inflation factor.
Table 5.
Change in the %PV in the cardiac allograft vasculopathy area according to clinical factors
| Initial %PV | Follow-up %PV | Δ %PVa | P-value of within factorb | P-value of between factorc | |
|---|---|---|---|---|---|
| Sex | 0.566 | ||||
| Male (n = 12) | 22.4 ± 5.3 | 30.6 ± 10.9 | 8.2 ± 7.2 | 0.016 | |
| Female (n = 7) | 26.3 ± 9.4 | 36.5 ± 11.4 | 10.1 ± 6.3 | <0.001 | |
| Years after HTX | 0.001 | ||||
| ≤2 years (n = 8) | 20.4 ± 3.3 | 24.0 ± 4.9 | 3.6 ± 2.2 | 0.008 | |
| >2 years (n = 11) | 26.4 ± 8.2 | 39.1 ± 10.0 | 12.8 ± 63 | 0.001 | |
| Donor age | 0.186 | ||||
| ≤30 years (n = 10) | 22.2 ± 5.43 | 29.2 ± 9.6 | 7.0 ± 6.7 | 0.002 | |
| >30 years (n = 9) | 25.7 ± 8.6 | 36.81 ± 1.9 | 11.1 ± 6.5 | 0.004 | |
| Diabetes | 0.550 | ||||
| Yes (n = 6) | 20.3 ± 3.8 | 27.8 ± 3.7 | 7.5 ± 4.3 | 0.031 | |
| No (n = 13) | 25.5 ± 7.8 | 35.1 ± 12.7 | 9.6 ± 7.7 | <0.001 | |
| Dyslipidaemia | 0.299 | ||||
| Yes (n = 11) | 25.4 ± 7.4 | 35.8 ± 11.6 | 10.3 ± 7.6 | 0.001 | |
| No (n = 8) | 21.7 ± 6.4 | 28.7 ± 9.5 | 7.0 ± 5.2 | 0.008 | |
| Renal insufficiency | 0.547 | ||||
| Yes (n = 11) | 24.7 ± 8.0 | 34.5 ± 12.0 | 9.7 ± 6.4 | 0.001 | |
| No (n = 8) | 22.6 ± 5.9 | 30.4 ± 10.2 | 7.8 ± 4.5 | 0.002 | |
| CMV infection | 0.511 | ||||
| Yes (n = 6) | 22.9 ± 3.2 | 29.8 ± 6.8 | 6.9 ± 4.1 | 0.031 | |
| No (n = 13) | 24.3 ± 8.4 | 34.1 ± 12.6 | 9.9 ± 7.6 | <0.001 | |
| ISHLT rejection grade ≥2 | 0.118 | ||||
| Yes (n = 7) | 23.7 ± 6.7 | 35.2 ± 11.2 | 11.5 ± 7.1 | 0.016 | |
| No (n = 12) | 23.9 ± 7.6 | 31.4 ± 11.3 | 7.4 ± 6.4 | <0.001 | |
| Underlying disease | 0.336 | ||||
| Ischaemic CMP (n = 3) | 21.1 ± 3.3 | 26.5 ± 7.4 | 5.4 ± 4.5 | 0.250 | |
| Non-ischaemic CMP (n = 16) | 24.4 ± 7.6 | 33.9 ± 11.5 | 9.6 ± 7.0 | <0.001 | |
| Maintenance regimen | 0.895 | ||||
| Sirolimus (n = 13) | 23.2 ± 5.1 | 32.5 ± 10.0 | 9.3 ± 7.4 | <0.001 | |
| Calcineurin inhibitors (n = 6) | 25.3 ± 10.8 | 33.5 ± 14.3 | 8.2 ± 5.7 | 0.031 |
CMV, cytomegalovirus; CMP, cardiomyopathy; HTX, heart transplantation; ISHLT, International Society for Heart and Lung Transplantation; %PV, per cent plaque volume.
aDifference between follow-up %PV and initial %PV.
bP-values are from the Wilcoxon signed rank test.
cP-values are from comparing the Δ %PV between factors with the Mann–Whitney U test.
Discussion
In this study, we showed that VV volume is one of the major determinant factors of the intimal plaque progression in patients with CAV. To the best of our knowledge, this report is the first to demonstrate the progression of plaque volume associated with VV volume using volumetric analysis.
CAV is an atheromatous disease and shares common features with atherosclerosis. In the early phase, both diseases are characterized by endothelial dysfunction followed by increased cell adhesion, leucocyte infiltration, intracellular lipids accumulation, and smooth muscle proliferation.7,8,17 Compared with native atherosclerosis, CAV has a tendency to undergo rapid and accelerated development.7 The prevalence of CAV is high even in the first year after HTX.2 Moreover, the progression of intimal thickness in the first year after HTX has been reported to be a significant predictor of future cardiac events.3 Therefore, prevention and early detection of CAV remain an important goal to improve the prognosis of the patients with HTX.
Previous studies have shown that neovascularization of the VV plays a significant role in both early and advanced phases of atherosclerosis.6,18 The close proximity of the VV to the intima makes it susceptible to infiltration of inflammatory cells and expression of the adhesion molecule. This susceptibility suggests that the VV serves as a conduit for the entry of pro-inflammatory and pro-atherosclerotic components into the wall of the coronary artery.19 Neovascularization of the VV appears to be a key feature even prior to the development of atherosclerosis. In a swine model, adventitial neovascularization of the VV is present early after a hypercholesterolaemic diet, preceding endothelial dysfunction and development of atherosclerosis.6 Previous human autopsy studies using microcomputed tomography have also shown that the VV density correlates well with the amount of advanced atherosclerotic plaque. Moreover, the VV density of the non-stenotic segment was higher than the VV density of the normal segment.5 In the present study, the %VV of the VV area significantly correlated with the progression of the intimal plaque volume both in the CAV area and in the VV area. These observations suggest that the measurement of the VV volume may enable the prediction of the initiation and progression of CAV in patients following HTX. Our observations suggest that controlling the neovascularization of the VV may provide a potential therapeutic target, a concept that is supported by previous observations. In a swine model of atherosclerosis induced by a high cholesterol diet, anti-angiogenic treatment targeting neovascularization of the VV leads to preservation of the VV density, inhibition of the vascular endothelial growth factor expression, and atherosclerosis progression.20,21
Compared with coronary angiography, IVUS provides not only accurate information on luminal stenosis but also changes in vessel size. In patients with HTX, a 5-year serial IVUS study revealed that changes in the external elastic membrane area showed a biphasic response that involved expansion in early phase and constriction in the late period.9 In this study, the post hoc analysis showed that the follow-up vessel area showed a decreasing trend compared with the initial vessel area (9.9 ± 2.8 vs. 11.1 ± 2.4 mm2, P = 0.125) in patients with >5 years after HTX, consistent with the previous report. There was no significant correlation between %VV and the delta average vessel volume in the CAV area and in the VV area (r = 0.001, P = 0.983 and r = −0.209, P = 0.391, respectively). In IVUS system, specific histologic plaque components can be identified with virtual histology analysis.10 In this study, changes of the fibrotic and fibro-fatty components of the CAV area were significantly correlated with %VV in terms of absolute volume and per cent absolute volume (see Supplementary data online, Table S1 and S2). Therefore, the quantitative histologic characteristics of plaque associated with the control VV could be another potential target in the treatment of CAV.
OCT is a high-resolution (10 µm), light-based, and in vivo imaging modality capable of detecting near-microscopic histological findings. A recent study showed that human adventitial VV could be evaluated with OCT in vivo.12 Moreover, we demonstrated the quantitative measurement of the adventitial VV volume with OCT and the significant correlation between the %VV and the %PV among patients in the early period after HTX.16 In our previous study, we used the ANALYZE software, which could calculate the VV volume from conventional intravascular OCT. Compared with the micro-CT, which is an established tool for the imaging of VV in vitro, there were significant correlations between the count and volume of the VV between both modalities. In addition, we analysed OCT imaging with same software in eight recipients for the evaluation of CAV. There was a significant positive correlation between the VV volume and plaque volume. However, our previous study was a cross-sectional study. Going one step further, we demonstrated that significant correlation between the VV volume and the progression of CAV in this study.
Analyses of risk factors in patients with CAV have identified both immune-mediated injury and non-immunologic factors.1 Immune-mediated injury includes HLA mismatch, the episode and grade of rejection, etc. Non-immunologic risk factors include hyperlipidaemia, donor's age, sex, diabetes mellitus, renal insufficiency, cytomegalovirus infection, and ischaemic time. In addition, recent studies have shown that sirolimus-based maintenance therapy attenuated plaque progression compared with calcineurin inhibitors after HTX.22 In this study, we compared the group of patients according to their CAV risk factors. The analysis showed that the %PV significantly progressed more in the group with patients >2 years after HTX than with those <2 years after HTX. Thus, although conventional risk factors of CAV and cardiovascular risk factors were not significantly affected in this study, duration of exposure to multiple risk factors, such as insulin resistance, renal dysfunction, and various effects, could play a role in the ongoing immunosuppressive therapy.
Limitations
This study had several potential limitations. First, the lengths of the VV area and the CAV area evaluated for plaque progression were relatively short, and the study analysis was limited to the proximal and the upper one-third of mid-left anterior descending coronary artery. Thus, generalizing our result to all the territories of the coronary artery after HTX is difficult. Second, several segments were excluded because of low image quality due to incomplete flushing with the radiocontrast media. This exclusion could introduce a selection bias. Moreover, on account of the limited penetration power of the OCT, we evaluated VV within 1 mm from the lumen. Therefore, the VV could have been underestimated in the study. Third, even though all matching between OCT and IVUS images were done according to the anatomical landmarks, considering the inherent differences in both techniques, this general matching process will be somewhat inaccurate to provide the same exact location. Fourth, in terms of the number of patients, there was a small sample size, and the various periods after HTX were mixed. It remains unclear whether the results could be confirmed in a larger patient sample with specific period. Fifth, the mean follow-up period was only 12 months. Therefore, further studies with a specific period and a larger number of patients with long-term follow-up are needed to confirm this observation.
Conclusions
The study demonstrated the strong correlation between the VV volume and the progression of the plaque volume in both the CAV area and the VV area. In a multivariable model, the VV volume was the only factor significantly associated with plaque progression. This result supports the association between the extent of VV and the development of CAV. Therefore, VV could be a potential therapeutic target to prevent CAV after HTX.
Supplementary data
Supplementary data are available at European Heart Journal—Cardiovascular Imaging online.
Conflict of interest: none declared.
Funding
The research was supported by the National Institute of Health (NIH Grants HL-92954 and AG-3170) and the Mayo Foundation.
References
- 1.Weis M, von Scheidt W. Cardiac allograft vasculopathy: a review. Circulation 1997;96:2069–77. [DOI] [PubMed] [Google Scholar]
- 2.Rickenbacher PR, Pinto FJ, Chenzbraun A, Botas J, Lewis NP, Alderman EL et al. . Incidence and severity of transplant coronary artery disease early and up to 15 years after transplantation as detected by intravascular ultrasound. J Am Coll Cardiol 1995;25:171–7. [DOI] [PubMed] [Google Scholar]
- 3.Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA et al. . Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol 2005;45:1532–7. [DOI] [PubMed] [Google Scholar]
- 4.Williams JK, Heistad DD. Structure and function of vasa vasorum. Trends Cardiovasc Med 1996;6:53–7. [DOI] [PubMed] [Google Scholar]
- 5.Gossl M, Versari D, Hildebrandt HA, Bajanowski T, Sangiorgi G, Erbel R et al. . Segmental heterogeneity of vasa vasorum neovascularization in human coronary atherosclerosis. JACC Cardiovasc Imaging 2010;3:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR Jr, Schwartz RS et al. . Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest 1998;101:1551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res 2006;99:801–15. [DOI] [PubMed] [Google Scholar]
- 8.Colvin-Adams M, Harcourt N, Duprez D. Endothelial dysfunction and cardiac allograft vasculopathy. J Cardiovasc Transl Res 2013;6:263–77. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsui H, Ziada KM, Schoenhagen P, Iyisoy A, Magyar WA, Crowe TD et al. . Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: results from a 5-year serial intravascular ultrasound study. Circulation 2001;104:653–7. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez JM, de Prada JA, Burgos V, Sainz Laso F, Valls MF, Vilchez FG et al. . Virtual histology intravascular ultrasound assessment of cardiac allograft vasculopathy from 1 to 20 years after heart transplantation. J Heart Lung Transplant 2009;28:156–62. [DOI] [PubMed] [Google Scholar]
- 11.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ et al. . American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001;37:1478–92. [DOI] [PubMed] [Google Scholar]
- 12.Nishimiya K, Matsumoto Y, Takahashi J, Uzuka H, Odaka Y, Nihei T et al. . In vivo visualization of adventitial vasa vasorum of the human coronary artery on optical frequency domain imaging. Validation study. Circ J 2014;78:2516–8. [DOI] [PubMed] [Google Scholar]
- 13.Hildebrandt HA, Gossl M, Mannheim D, Versari D, Herrmann J, Spendlove D et al. . Differential distribution of vasa vasorum in different vascular beds in humans. Atherosclerosis 2008;199:47–54. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura RA, Lerman A, Chesebro JH, Ilstrup DM, Hodge DO, Higano ST et al. . Epicardial vasomotor responses to acetylcholine are not predicted by coronary atherosclerosis as assessed by intracoronary ultrasound. J Am Coll Cardiol 1995;26:41–9. [DOI] [PubMed] [Google Scholar]
- 15.Cassar A, Matsuo Y, Herrmann J, Li J, Lennon RJ, Gulati R et al. . Coronary atherosclerosis with vulnerable plaque and complicated lesions in transplant recipients: new insight into cardiac allograft vasculopathy by optical coherence tomography. Eur Heart J 2013;34:2610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki T, Rodriguez-Porcel M, Matsuo Y, Cassar A, Kwon TG, Franchi F et al. . Evaluation of coronary adventitial vasa vasorum using 3D optical coherence tomography—animal and human studies. Atherosclerosis 2015;239:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McManus BM, Horley KJ, Wilson JE, Malcom GT, Kendall TJ, Miles RR et al. . Prominence of coronary arterial wall lipids in human heart allografts. Implications for pathogenesis of allograft arteriopathy. Am J Pathol 1995;147:293–308. [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK et al. . Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–25. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation 1996;93:672–82. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SH, Herrmann J, Lerman LO, Holmes DR Jr, Napoli C, Ritman EL et al. . Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation 2002;105:415–8. [DOI] [PubMed] [Google Scholar]
- 21.Gossl M, Herrmann J, Tang H, Versari D, Galili O, Mannheim D et al. . Prevention of vasa vasorum neovascularization attenuates early neointima formation in experimental hypercholesterolemia. Basic Res Cardiol 2009;104:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo Y, Cassar A, Yoshino S, Flammer AJ, Li J, Gulati R et al. . Attenuation of cardiac allograft vasculopathy by sirolimus: Relationship to time interval after heart transplantation. J Heart Lung Transplant 2013;32:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]