Abstract
High hydrostatic pressure (HHP) has been shown to induce immunogenic cell death of cancer cells, facilitating their uptake by dendritic cells (DC) and subsequent presentation of tumor antigens. In the present study, we demonstrated immunogenicity of the HHP-treated tumor cells in mice. HHP was able to induce immunogenic cell death of both TC-1 and TRAMP-C2 tumor cells, representing murine models for human papilloma virus-associated tumors and prostate cancer, respectively. HHP-treated cells induced stronger immune responses in mice immunized with these tumor cells, documented by higher spleen cell cytotoxicity and increased IFNγ production as compared to irradiated tumor cells, accompanied by suppression of tumor growth in vivo in the case of TC-1 tumors, but not TRAMP-C2 tumors. Furthermore, HHP-treated cells were used for DC-based vaccine antigen pulsing. DC co-cultured with HHP-treated tumor cells and matured by a TLR 9 agonist exhibited higher cell surface expression of maturation markers and production of IL-12 and other cytokines, as compared to the DC pulsed with irradiated tumor cells. Immunization with DC cell-based vaccines pulsed with HHP-treated tumor cells induced high immune responses, detected by increased spleen cell cytotoxicity and elevated IFNγ production. The DC-based vaccine pulsed with HHP-treated tumor cells combined with docetaxel chemotherapy significantly inhibited growth of both TC-1 and TRAMP-C2 tumors. Our results indicate that DC-based vaccines pulsed with HHP-inactivated tumor cells can be a suitable tool for chemoimmunotherapy, particularly with regard to the findings that poorly immunogenic TRAMP-C2 tumors were susceptible to this treatment modality.
Keywords: dendritic cells, docetaxel, high hydrostatic pressure, immunotherapy, cancer
Introduction
Cancer immunotherapy, especially when combined with other therapeutic modalities such as chemotherapy, is an attractive approach to cancer treatment. Synergistic effects of combinations of immunotherapy and chemotherapy have been demonstrated in a number of pre-clinical and clinical studies (1,2).
Dendritic cells (DCs) are key players in the immune response as they are able to capture antigens with their pattern-recognition receptors, to process and present them to naïve T-cells, inducing their activation (3), and thus building an essential bridge between innate and adaptive responses. The possibility of their generation in vitro enabled their use for immunotherapy of cancer (4), and a number of clinical trials have been performed in the last decade (5,6). Typically, an autologous dendritic cell-based vaccine represents in vitro cultured dendritic cells pulsed with tumor antigens that can be in the form of tumor cells with subsequent DC maturation. For DC pulsing, tumor cells can be inactivated by their lysis (ultrasonic treatment, repeated freeze-thaw), lethal irradiation or other methods before mixing them with DC. Selection of the optimal inactivation method can be crucial for DC vaccine optimization, together with selection of proper maturation-inducing agents.
Therefore, a significant effort has also been invested in increasing the immunogenicity of dying cancer cells used for vaccine production. Until now several chemotherapeutic agents [anthracyclines (7), oxaliplatin, platinum complexes (8), bortezomib (9)] and physical modalities [UV-C, irradiation (10), HHP] have been identified as inducers of immunogenic cell death (ICD). ICD is characterized by the cell-surface expression and release of damage associated molecular patterns (DAMPs). DAMPs found to be crucial for ICD include surface exposed chaperone protein calreticulin (CRT) and heat shock proteins 70 (HSP70) and 90 (HSP90), actively secreted ATP and passively released high-mobility group box 1 protein (HMGB1). These signals can activate innate immunity and, importantly, interact with phagocytosis-related receptors, purinergic receptors and pattern-recognition receptors expressed by DCs and thereby stimulate presentation of tumor antigens to T cells.
High hydrostatic pressure (HHP) has been demonstrated as a convenient tool for tumor cell inactivation preserving their immunogenic capacity (11,12). Recently, induction of ICD by HHP has been shown in several human tumor cell lines. HHP-treated cells were able to induce monocyte-derived DC maturation, and DC co-cultured with HHP-treated tumor cells were able to induce T cell activation in vitro. These encouraging results suggest that HHP can be an important tool for tumor cell inactivation before their use for DC pulsing or as cellular vaccines (13).
Chemotherapeutic drugs affect rapidly growing cells and, as a consequence, cause collateral damage to cells of the immune system. In this regard, they are considered immunosuppressive. However, there is increasing evidence that some cancer chemotherapies may actually aid the immunotherapy by activating the immune system rather than suppressing it (14,15). Chemotherapeutic drugs such as cyclophosphamide, doxorubicin, paclitaxel or docetaxel (16) were reported to possess immunomodulatory activities and appeared to be suitable for chemoimmunotherapy (17,18).
Docetaxel is a widely used chemotherapeutic drug and represents a first-line chemotherapy for metastatic castration-resistant prostate cancer (19,20). The autologous dendritic cell-based vaccines are intensively studied as an immunotherapy for prostate cancer, and the first cellular immunotherapy based on activated peripheral blood mononuclear cells, Sipuleucel T, has been FDA-approved (21). Collectively, combination chemoimmunotherapy based on docetaxel combined with the DC treatment represents an attractive modality for advanced prostate cancer therapy.
In the present study, we investigated, using murine tumor models, the immunogenicity of the HHP-inactivated tumor cells in vivo and, furthermore, the possibility to use HHP-treated tumor cells for preparation of DC-based vaccines. We have demonstrated the therapeutic capacity of the HHP cells-pulsed DC vaccines in combination with docetaxel treatments to inhibit growth of the TRAMP-C2 and TC-1 murine tumors. We have focused on the immunotherapy of poorly immunogenic TRAMP-C2 tumors, an animal model of prostate cancer treatment. For comparison, the study was completed with experiments using immunogenic TC-1 tumors representing a murine model for human papilloma virus 16-associated tumors, previously shown to be sensitive to the experimental DC treatments in various settings (22–24).
Materials and methods
Mice
C57BL/6 male mice, 6–8 weeks old, were obtained from AnLab Ltd., Prague, Czech Republic. Experimental protocols were approved by the Institutional Animal Care Committee of the Institute of Molecular Genetics, Prague.
Tumor cell lines
The TC-1 tumor cell line (obtained from the ATCC collection) was developed by co-transfection of murine C57BL/6 lung cells with HPV16 E6/E7 genes and activated (G12V) Ha-ras plasmid DNA (25). TRAMP-C2 tumor cells (obtained from the ATCC collection), MHC class I-deficient, were established from a heterogeneous 32-week tumor of the transgenic adenocarcinoma mouse prostate (TRAMP) model (26). TC-1 cells were maintained in RPMI-1640 medium (Sigma-Aldrich GmbH, Steinheim, Germany) supplemented with 10% FCS (PAN Biotech GmbH, Aidenbach, Germany), 2 mM L-glutamine and antibiotics; TRAMP-C2 cells were maintained in D-MEM medium (Sigma-Aldrich) supplemented with 5% FCS, Nu-Serum IV (5%; BD Biosciences, Bedford, MA, USA), 0.005 mg/ml human insulin (Sigma-Aldrich), dehydroisoandrosterone (DHEA, 10 nM; Sigma-Aldrich) and antibiotics. Both cell lines were cultured at 37ºC in a humidified atmosphere with 5% CO2 cells. In the in vivo experiments, 5×104 TC-1 cells and 1×106 TRAMP-C2 cells were administered for the challenge. In our hands, 5×104 TC-1 cells represent 5 TID50 doses and 1×106 TRAMP-C2 cells represent 3 TID50 doses.
High hydrostatic pressure and irradiation cell treatments
Tumor cells were treated by HHP (100, 150, 175 and 200 MPa) in the custom-made device (Resato International BV, Roden, the Netherlands) that is located in the GMP manufacturing facility, Sotio a.s. (Prague, Czech Republic). This device allows reliable treatment of the tumor cells by defined levels of HHP for specified periods of time (10 min in the case of 200 MPa) (13). Inactivation of tumor cells by irradiation (150 Gy) was performed as previously described (22).
Dendritic cell preparation
Dendritic cells (DC) were prepared from bone marrow precursors as described by Indrová et al (24) and Lutz et al (27) with slight modifications (28). Briefly, the bone marrow cells were cultured for 7 days in the complete RPMI-1640 medium supplemented with 2×10−5 M mercaptoethanol (Calbiochem, La Jolla, CA, USA), 10 ng/ml GM-CSF and IL-4 (R&D Systems, Minneapolis, MN, USA). On day 5, the DC were pulsed with HHP-treated or irradiated (IR-treated) tumor cells by 48-h incubation in the ratio of 2:1 (DC/tumor cells, 106 DC/ml). DC pulsed with the tumor cells were treated for 24 h with unmethylated CpG containing phosphorothioate-modified oligodeoxynucleotide CpG 1826 (5′-TCCATGACGTTCCTGACGTT-3′) (29) at a final concentration of 5 μg/ml (Generi Biotech, Hradec Králové, Czech Republic), were sulfur-modified in their backbone (phosphorothioate) and synthesized under endotoxin-free conditions. On day 7, non-adherent cells were harvested. These cells, designated as DC, contained ~60–70% CD11c+ cells. For mouse immunization experiments, DC were washed twice with PBS and injected subcutaneously (s.c.) in PBS, 300 μl/2×106 cells/mouse.
Immunization/challenge experiments with tumor cells
Mice were twice immunized with 5×106 irradiated tumor cells in a three-week interval (s.c., irradiation dose was 150 Gy, HHP dose was 200 MPa) (13,30,31). For in vivo studies, 10 days after the second immunization, mice were challenged s.c. with corresponding tumor cells (TC-1, 5×104; TRAMP-C2, 1×106 cells/mouse). Mice were observed twice weekly, and the numbers of tumor-bearing mice and the size of the tumors were recorded. Two perpendicular diameters of the tumors were measured with a caliper and the tumor size was expressed as the tumor area (cm2). For in vitro analyses of the immune response, three mice were sacrificed. Single-cell suspensions from the spleens were prepared by homogenization through a cell strainer (70 μm; BD Biosciences, San Jose, CA, USA). Erythrocytes were osmotically lysed using ammonium chloride-potassium lysis buffer, the cell suspension was washed three times in the RPMI-1640 medium and used for further analysis by FACS, chromium release assay, ELISA (IFNγ) and ELISPOT (IFNγ).
Immunization/challenge experiments with dendritic cells
Mice were twice immunized with 2×106 cells of DC-based vaccine in a two-week interval. For in vivo studies, 10 days after the second immunization, mice were challenged s.c. with corresponding tumor cells (TC-1, 5×104; TRAMP-C2, 1×106 cells/mouse). Mice were observed twice weekly, and the numbers of tumor-bearing mice and the size of the tumors were recorded. Two perpendicular diameters of the tumors were measured with a caliper and the tumor size was expressed as the tumor area (cm2). For in vitro analyses of the immune response, three mice were sacrificed. Single-cell suspensions from the spleens were prepared as mentioned above and used for further analysis by FACS, chromium release assay, ELISA (IFNγ) and ELISPOT (IFNγ).
Therapeutic experiments
The therapeutic schemes were designed for combined chemoimmunotherapy treatment of early growing tumors. TC-1 (5×104 cells) or TRAMP-C2 (1×106 cells) tumor cells were s.c. transplanted on day 0. Docetaxel, 30 mg/kg (Actavis, North Bruncwik, NJ, USA) was repeatedly administered on days 7, 21 and 35 intraperitoneally (i.p.). Dendritic cells were administered on days 14, 28 and 42 in the vicinity of the tumor cell challenge site or peritumorally when the growing tumors appeared. Mice were observed twice a week and the size of the tumors was recorded. Two perpendicular diameters of the tumors were measured with a caliper and the tumor size was expressed as the tumor area (cm2).
Flow cytometry
Cell surface expression of CRT, HSP90, MHC class I, CD54 and CD80 on the tumor cells was analyzed by flow cytometry. Tumor cells were collected from the cell culture 24 h after the HHP or IR treatment [106 cells/ml/well, 12-well plate (Nunc, Roskilde, Denmark)]. Cells (5×105/sample) were washed and labeled with primary antibodies for 25 min at 4ºC, followed by wash steps and alternatively labeled by incubation with Alexa 647- or DyLight 649-conjugated secondary antibody for 30 min at 4ºC. Apoptotic cells were determined by Annexin V apoptosis detection kit (eBiosciences) according to the manufacturer's instructions. Samples were kept in the dark and 10 min before the analysis, Hoechst 33258 was added at a final concentration of 2 μg/ml. Expression of cell surface molecules on the DC or spleen cells was analyzed by flow cytometry. Cell suspensions were washed and preincubated with anti-CD16/CD32 antibody to minimize non-specific binding for 15 min at 4ºC following washing step and incubation with labeled primary antibody for 30 min at 4ºC. Relevant isotype controls of irrelevant specificity were used. FACS buffer (PBS, 1% FBS, 0.1% NaN3) was used for all washing steps and analysis. The following antibodies were used for FACS analyses: BD: anti-MHC class I (PE anti-H-2Db clone KH95 and PE anti-H-2Kb clone AF6-88.5), FITC anti-I-Ab (Aβb) (AF6-120.1), PE anti-CD54 (3E2), PE anti-CD80 (16-10A1), PE anti-CD86 (GL1), PE anti-CD274 (MIH5); BioLegend, Inc. (San Diego, CA, USA): BV421 or APC anti-CD11c (HL3), APC-CD45 (30-F11), FITC anti-CD8α (LY-2), BV711 anti-CD4 (RM4-5), PE anti-CD44 (IM7), PE-Cy7 CD62L (MEL-14); R&D Systems (Basel, Switzerland): anti-HSP70 (242707); Abcam (Cambridge, UK): anti-CRT (ab2907); Enzo Life Sciences, Inc. (Farmingdale, NY, USA): anti-HSP90 (AC88). Secondary antibodies anti-mouse conjugated to DyLight 649 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) or anti-rabbit conjugated to Alexa 647 (Cell Signaling Technology, Danvers, MA, USA) were also used. FACS analysis was performed using an LSR II flow cytometer (BD Biosciences) and analyzed by FlowJo 7.6.5 software.
Confocal microscopy
HHP-treated TC-1 and TRAMP-C2 cells were collected and washed twice with PBS. The cells were then incubated for 30 min with primary anti-CRT antibody (FMC 75; Enzo Life Sciences) diluted in PBS, followed by washing and incubation with the Alexa Fluor 488 goat anti-mouse secondary antibody (Molecular Probes). Cells were washed twice with PBS and fixed in 4% paraformaldehyde for 20 min and mounted on slides. Cells were examined under a DMI 6000 inverted Leica TCS AOBS SP5 tandem scanning confocal microscope with an AR (488 nm) laser and an ×63 oil immersion objective.
ELISA
For HMGB1 release, supernatants from the tumor cell culture were collected 24 h after HHP treatment (106 cell/ml/well, 12-well plate (Nunc) and analyzed by an ELISA kit (IBL International GmbH, Hamburg, Germany) according to the manufacturer's instructions. For IL-1β, IL-6, IFNγ and IL-12 production, supernatants from the DC culture were collected 24 h after the addition of CpG 1826 and analyzed by ELISA kits (BD Biosciences) according to the manufacturer's instructions. For IFNγ production, supernatants from the spleen single-cell suspension were collected after 48-h incubation [2×106 cell/ml/well, 12-well plate (Nunc)] and analyzed by an ELISA kit (BD Biosciences) according to the manufacturer's instructions.
ELISPOT
To determine the amount of IFNγ-secreting cells, an ELISPOT kit for detection of murine IFNγ (BD Biosciences, San Diego, CA, USA) was used. Spleen cells were cultured for 48 h and then placed into the wells of ELISPOT plates (concentration 5×105, 1×105 and 5×104 cells/well) for 24 h. The plates were then processed according to the manufacturer's instructions (BD Biosciences). Colored spots were counted with CTL Analyzer LLC (CTL, Cleveland, OH, USA) and analyzed using ImmunoSpot Image Analyzer software.
Chromium release microcytotoxicity assay
The cytolytic activity of effector cells was tested in 18-h 51Cr release assay, as previously described (32,33). Briefly, spleen cells from control and immunized mice that served as effector cells were treated with ammonium chloride-potassium lysing buffer (1 min) to deplete erythrocytes. The mixtures of effector cells with 51Cr-labeled tumor targets were incubated in selected target/effector cell ratios (1:25, 1:50, 1:100 and 1:200) in triplicate in 96-well round bottom microtiter plates (Nunc). The percentage of specific 51Cr release was expressed according to the formula: [cpm experimental release - cpm control release/cpm maximum release/cpm control release] × 100.
Statistical analyses
For statistical analyses of in vitro experiments, Student's t-test was used. For evaluation of in vivo experiments, analysis of variance (ANOVA) from the NCSS, Number Cruncher Statistical System (NCSS, LLC, Kaysville, UT, USA) statistical package was utilized. Standard deviations are indicated in the figures.
Results
HHP, but not IR, induces ICD markers on both TC-1 and TRAMP-C2 tumor cells
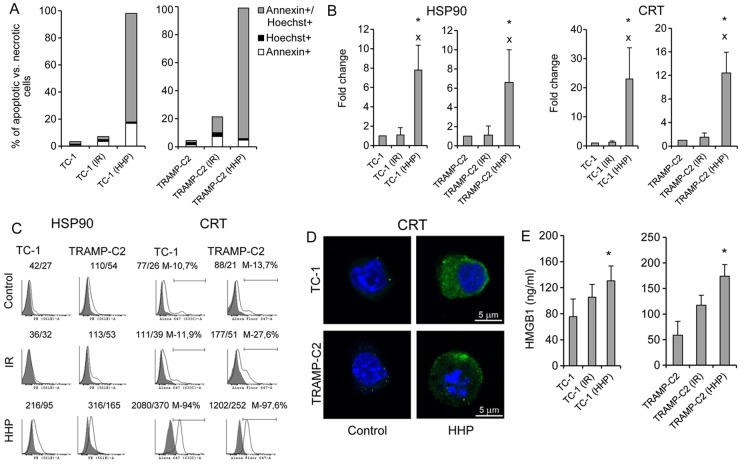
First, we determined the ability of HHP to induce ICD in murine TC-1 or TRAMP-C2 cells, and then we compared the effect of HHP to the effect of irradiation (150 Gy) that has been standardly used for treatment of cells during preparation of DC vaccines in our previous studies (22). Fig. 1A shows that the percentage of late apoptotic/dead tumor cells (Annexin V+/Hoechst+) after the treatment with 200 MPa HHP was >80% within 24 h. The presence of ICD markers HSP90 and CRT on the cell surface of the tested cells was also significantly increased (Fig. 2B and C). Fluorescence microscopy images of HHP-treated tumor cells stained for CRT confirmed increased expression of CRT after HHP treatment (Fig. 1D). Release of HMGB1, late-stage ICD marker, in the supernatant was further analyzed. Fig. 1E demonstrates a significant increase of HMGB1 in the tumor cell supernatants after HHP treatment. Induction of ICD by 200 MPa HHP was similar both in TC-1 and TRAMP-C2 tumor cells. No significant upregulation of ICD markers was detected after irradiation with 150 Gy. The treatment with HHP of 200 MPa was selected as it was the most effective in inducing apoptosis and expression of ICD markers and simultaneously arresting cell proliferation, as determined by colony-forming assay in experiments, in which the effects of different doses of HHP (100, 150, 175, 200 and 250 MPa) were compared (data not shown).
Figure 1.
Phenotype of mouse TC-1 and TRAMP-C2 tumor cells 24 h after the treatment with HHP or IR. TC-1 and TRAMP-C2 tumor cells were treated with 200 MPa for 10 min and compared with irradiated (150 Gy) tumor cells. (A) Annexin V/Hoechst staining made 24 h after HHP or IR treatment. (B and C) Expression of cell surface molecules HSP90 and CRT on TC-1 or TRAMP-C2 tumor cells (presented as a fold change of mean fluorescence intensity (MFI) or histograms with MFI). (D) Fluorescence microscopy images of HHP-treated TC-1 and TRAMP-C2 tumor cells stained for CRT expression. (E) Release of HMGB1 into the tumor cell supernatants after HHP or IR-treatment. Representative results from at least three independent experiments. *P<0.05 vs. untreated control, xP<0.05 vs. IR treated group, two-sided Student's t-test.
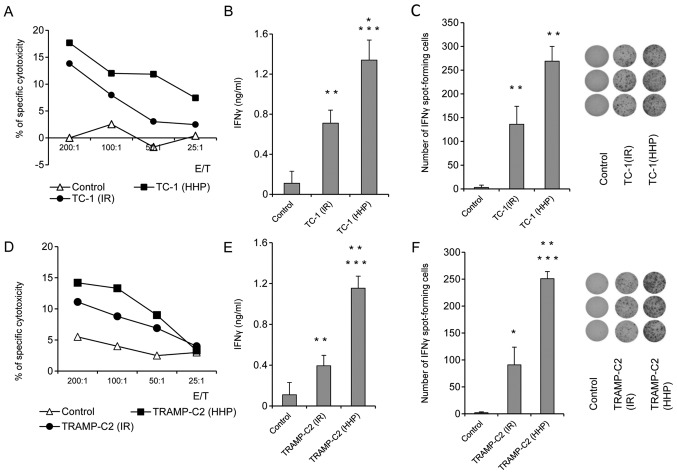
Figure 2.
In vitro immune response after immunization with HHP-treated or IR-treated TC-1 and TRAMP-C2 tumor cells. Mice were immunized two times in a 3-week interval with 5×106 HHP- or IR-treated TC-1 and TRAMP-C2 tumor cells. Ten days after the last immunization, pooled splenocytes of three mice were used for in vitro analysis. 51Cr microcytotoxicity assay of splenocytes from mice immunized with HHP or IR-treated TC-1 (A) or TRAMP-C2 tumor cells (D). (B and E) IFNγ production by splenocytes of immunized mice (ELISA). (C and F) The number of IFNγ-producing cells (ELISPOT assay). Statistical significances were determined by Student's t-test. (B) ***P<0.001 TC-1(HHP) vs. Control; **P<0.01 TC-1(IR) vs. Control; *P<0.05 TC-1(HHP) vs. TC-1(IR); (C) **P<0.001 TC-1(HHP) vs. Control; TC-1(IR) vs. Control; TC-1(HHP) vs. TC-1(IR); (E) ***P<0.001 TC-1(HHP) vs. Control; **P<0.01 TC-1(IR) vs. Control; TC-1(HHP) vs. TC-1(IR); (F) ***P<0.001 TC-1(IR) vs. Control; **P<0.01 TC-1(HHP) vs. TC-1(IR); *P<0.05 TC-1(HHP) vs. Control.
Prophylactic immunization with HHP-treated tumor cells induces higher immune responses in mice when compared with IR-treated tumor cells both in TC-1 and TRAMP-C2 tumor models
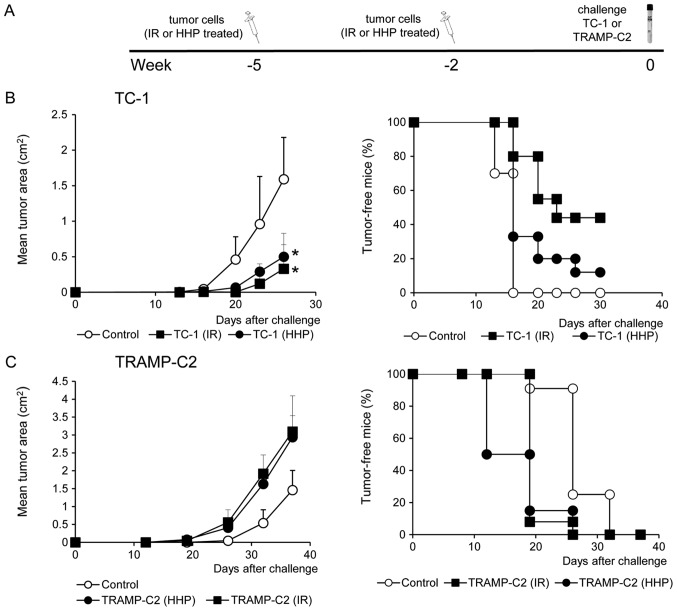
To study the ability of HHP and IR-treated tumor cells to induce immune response and their antitumor potency, mice were immunized twice at a three-week interval with 5×106 HHP- or IR-treated TC-1 or TRAMP-C2 tumor cells. Ten days after the second immunization, mice were challenged with relevant tumor cells in doses of 5×104 TC-1 or 106 TRAMP-C2. Three mice from each group were left without challenge and used for parallel in vitro analyses. In vitro analyses of the spleen effector cells prepared ten days after the second immunization with HHP-treated TC-1 or TRAMP-C2 tumor cells showed an increased cytotoxic effect of spleen effector cells on the corresponding targets. In the group of mice immunized with IR-treated tumor cells, a similar but slightly lower effect was observed (Fig. 2A and D). Despite the fact that the analysis of the spleen effector cells after immunization with HHP-treated tumor cells showed only moderately augmented cytotoxic effect when compared to immunization with IR-treated tumor cells, analysis of IFNγ production revealed significant differences. Compared to the IR-treated tumor cells, mice immunized with HHP-treated tumor cells displayed significantly increased IFNγ production by spleen cells measured by the ELISA assay (Fig. 2B and E) and significantly increased number of IFNγ-producing cells detected by the ELISPOT assay (Fig. 2C and F). These results were similar in both tumor models, immunogenic TC-1 and weakly immunogenic TRAMP-C2. However, after the challenge of immunized mice with the corresponding tumor cells, significant inhibition (P<0.05) of tumor growth was recorded only in the groups of mice immunized with the HHP or IR-treated TC-1 tumor cells and challenged with corresponding TC-1 cells (Fig. 3B). In contrast, mice immunized with HHP and IR-treated TRAMP-C2 cells did not exhibit any inhibition of tumor growth after the challenge with TRAMP-C2 cells (Fig. 3C).
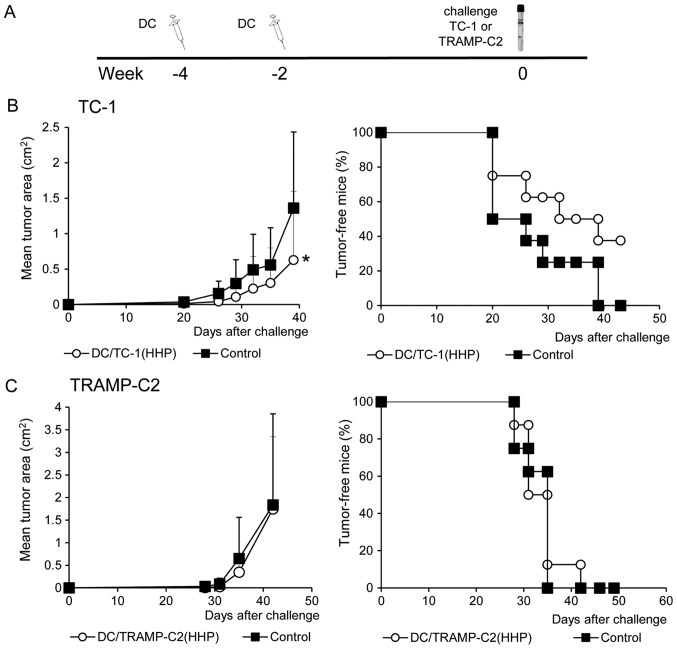
Figure 3.
The effect of immunization with HHP-treated or IR-treated TC-1 and TRAMP-C2 tumor cells. Mice (10 mice per group) were two times s.c. immunized in a 3-week interval with 5×106 HHP- or IR-treated TC-1 and TRAMP-C2 tumor cells (A). Ten days after the second immunization, mice were challenged with 5×104 TC-1 (B) or 106 TRAMP-C2 (C) tumor cells. Tumor growth (left panel) and the percentage of tumor-free mice (Kaplan-Maier plot) (right panel) are shown; TC-1, P<0.05 (untreated vs. HHP-treated, IR-treated). TRAMP-C2, not significant (analysis of variance). The experiments were repeated twice with similar results.
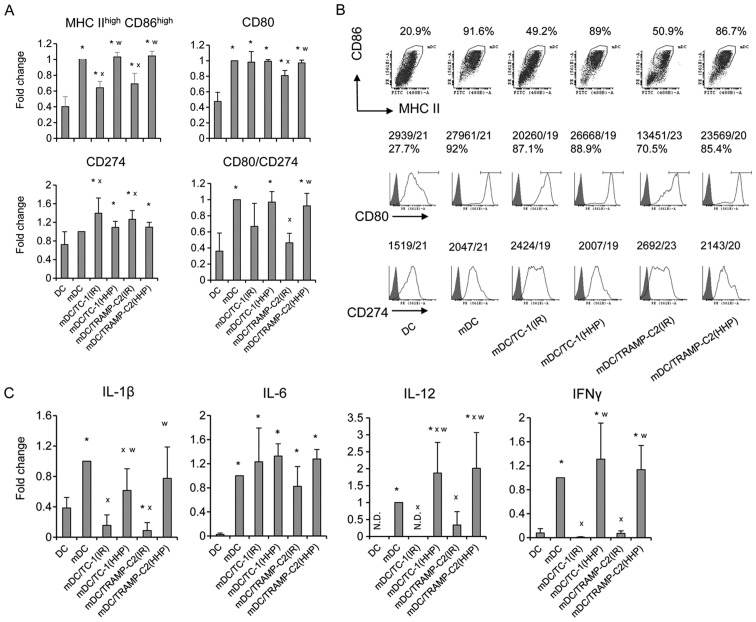
Pulsing with HHP-treated TC-1 or TRAMP-C2 tumor cells increased expression of maturation markers on DC and stimulated production of cytokines characteristic for matured DC
Next, the HHP-treated TC-1 and TRAMP-C2 cells were used for DC pulsing, and the phenotypes of matured DC vaccines, unpulsed or pulsed with the IR-treated tumor cells or HHP-treated tumor cells, were compared (Fig. 4). We did not see any significant differences between unpulsed cells and HHP-treated tumor cells-pulsed DC. In both cases, CpG ODN1826-mediated maturation increased the proportion of matured MHC class IIhigh/CD86high dendritic cells and increased CD80 and CD274 cell surface expression (Fig. 4A and B). The ratio between the CD80 and CD274 cell surface expressions (demonstrated by MFIvalues) was higher on matured cells compared to the immature controls (Fig. 4A). Both unpulsed cells and HHP-treated tumor cells-pulsed matured DC produced IL-12, as well as IL-1β, IL-6 and IFNγ (Fig. 4C). HHP-treated tumor cell-pulsed matured DC produced significantly higher amounts of IL-12 and IFNγ as compared to the unpulsed cells. No significant differences were observed between TRAMP-C2 and TC-1 cell co-culture. On the other hand, pulsing with the IR-treated tumor cells resulted in reduction of the proportion of matured MHC class IIhigh/CD86high dendritic cells in the DC populations, decreased the ratio between the CD80 and CD274 cell surface expression, and also significantly inhibited IL-12, IFNγ and IL-1β production, as compared to both unpulsed cells and HHP-treated tumor cell-pulsed matured DC (Fig. 4). These results indicate that DC co-culture with IR-treated, but not HHP-treated tumor cells, can impair DC maturation.
Figure 4.
Phenotype of mouse DC after the interaction with HHP- or IR-treated TC-1 or TRAMP-C2 tumor cells. DC were prepared from bone marrow precursors and pulsed with HHP- or IR-treated tumor cells by 48-h incubation in the ratio of 2:1. DC pulsed with the tumor cells were then treated for 24 h with CpG 1826 and analyzed by flow cytometry. (A and B) Expression of MHC class II and costimulatory molecules (as a fold change of MFI or as a dot plot/histograms with MFI). (C) Production of cytokines by DC culture (as a fold change relative to mature DC designated as mDC). mDC produced IL-1β in the range of 233–749 pg/ml, IFNγ in the range of 299–749 pg/ml, IL-6 in the range of 18.2–49.2 ng/ml, and IL-12 in the range of 760–2235 pg/ml. Representative results from at least four independent experiments. *P<0.05 vs. DC, xP<0.05 vs. mDC, wP<0.05 vs. DC pulsed with IR-treated tumor cells, Student's t-test.
Prophylactic immunization with DC-based vaccine pulsed with HHP-treated TC-1 or TRAMP-C2 tumor cells induces strong immune response, but inhibits growth of TC-1 tumors only
In the next series of experiments, HHP-treated tumor cell-pulsed matured DC were investigated in vivo. HHP-treated tumor cell-pulsed matured DC were selected for further in vivo experiments as pulsing of DC with IR-treated tumor cells negatively affected DC maturation in terms of expression of costimulatory molecules and production of selected cytokines. Mice were immunized twice in a 2-week interval with 2×106 HHP-treated tumor cell-pulsed matured DC. Ten days after the second immunization, mice were challenged with relevant tumor cells, in doses of 5×104 TC-1 or 106 TRAMP-C2 tumor cells. Three mice from each group were left without challenge and used for parallel in vitro analyses. Both HHP-treated TRAMP-C2 and TC-1 cells pulsed DC vaccines induced strong immune responses, as determined by spleen cell analysis performed ten days after the second immunization (Fig. 5). Immunization with HHP-treated TC-1 or TRAMP-C2 pulsed DC vaccines showed a significantly increased cytotoxic effect of spleen effector cells on the corresponding targets (Fig. 5A). As compared with control mice, mice immunized with both HHP-treated TRAMP-C2 and TC-1 cells pulsed DC vaccines displayed significantly increased numbers of IFNγ-producing cells detected by ELISPOT assay (Fig. 5B) and significantly increased IFNγ production by spleen cells measured by ELISA assay (Fig. 5C). A significant increase was also found in the percentage of CD4+ and CD8+ CD44+ CD62L− T lymphocytes (Fig. 5D and E). These results were similar in both tumor models, immunogenic TC-1 and weakly immunogenic TRAMP-C2. Contrary to the results in vitro, in vivo analysis showed significant inhibition (P<0.05) of the tumor growth only in the group of mice immunized with the HHP-treated TC-1 tumor cell-pulsed matured DC and challenged with corresponding TC-1 cells (Fig. 6B). Mice immunized with the HHP-treated TRAMP-C2 tumor cell-pulsed matured DC did not exhibit any inhibition of tumor growth after the challenge with TRAMP-C2 cells (Fig. 6C). In both experiments, the percentage of tumor-free mice are shown in the right panel.
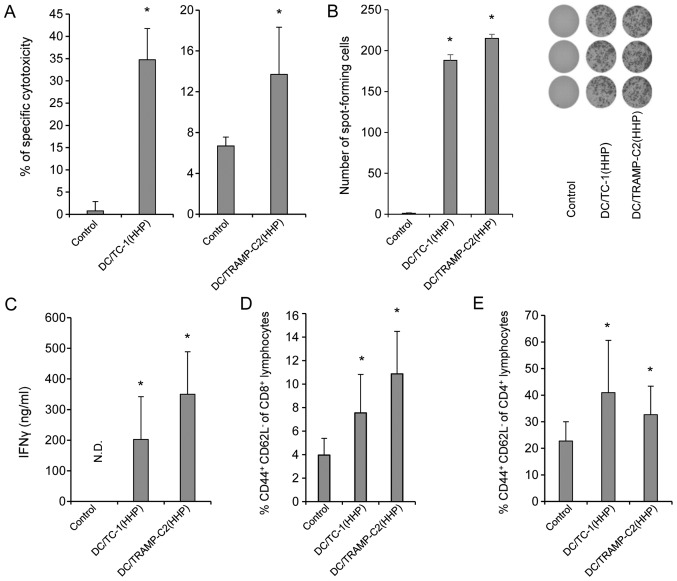
Figure 5.
In vitro immune response after immunization with DC-based vaccines pulsed with HHP-treated TC-1 or TRAMP-C2 tumor cells. Mice were immunized two times in a 2-week interval with 2×106 DC pulsed with HHP-treated TC-1 or TRAMP-C2 tumor cells. Ten days after the last immunization, pooled splenocytes of three mice were used for in vitro analysis. 51Cr microcytotoxicity assay of splenocytes from mice immunized with HHP- and IR-treated TC-1 tumor cells or TRAMP-C2 tumor cells (A). (B) The number of IFNγ-producing cells (ELISPOT assay). (C) IFNγ production by splenocytes of immunized mice (ELISA). N.D. means that IFNγ production was under detection limit and for Student's t-test was considered as 0. (D) Percentage of CD44+ CD62L− of CD8+ lymphocytes. (E) Percentage of CD44+ CD62L− of CD4+ lymphocytes. Statistical significance was determined by Student's t-test; *P<0.05 vs. control.
Figure 6.
The effect of immunization with DC-based vaccines pulsed with HHP-treated TC-1 or TRAMP-C2 tumor cells. Mice (8 mice per group) were two times s.c. immunized in a 2-week interval with 2×106 DC pulsed with HHP-treated TC-1 or TRAMP-C2 tumor cells (A). Ten days after the second immunization, mice were challenged with 5×104 TC-1 (B) or 1×106 TRAMP-C2 (C) tumor cells. Tumor growth (left panel) and the percentage of tumor-free mice (Kaplan-Maier plot) (right panel) are shown; *P<0.05 vs. control (analysis of variance). The experiments were repeated twice with similar results.
Combined chemoimmunotherapy of TC-1 and TRAMP-C2 tumors with docetaxel and DC-based vaccine significantly inhibits growth of subcutaneous tumors
The therapeutic efficacy of HHP-treated tumor cell-pulsed matured DC was then tested in the therapeutic setting when a combination of chemotherapy and immunotherapy with DC-based vaccine was employed. TC-1 (5×104 cells) or TRAMP-C2 (106 cells) tumor cells were s.c. transplanted on day 0 and treated with three doses of docetaxel chemotherapy in a 2-week interval. Dendritic cells were administered at regular intervals between the docetaxel chemotherapy. As shown in Fig. 7B, the growth of immunogenic TC-1 tumors was significantly inhibited by the treatment with DC alone or with the combination of docetaxel and DC vaccine [DC/TC-1(HHP)] (P<0.05 vs. control). Docetaxel alone delayed the growth of tumors, but no significant difference was evident. A representative experiment of two independent ones is given in Fig. 7B. When the incidences of tumors in mice from two performed experiments were merged [Control 19/19, docetaxel 18/20, docetaxel + DC/TC-1(HHP) 14/19, DC/TC-1-HHP 6/10], the only significant difference was found between the control group and the group of combined chemoimmunotherapy (χ2; docetaxel +DC/TC-1(HHP) vs. Control P<0.01). These results indicate the beneficial effect of the combination of chemotherapy with immunotherapy. The same therapeutic setting was also used for the treatment of poorly immunogenic TRAMP-C2 tumors. As shown in Fig. 7C, monotherapies with docetaxel alone or DC/TRAMP-C2(HHP) vaccine alone significantly inhibited growth of TRAMP-C2 tumors. However, when these monotherapies were combined, the therapeutic effect was even stronger. Significant inhibition of tumor growth was found between docetaxel alone or DC/TRAMP-C2(HHP) alone groups and the group treated with a combination of docetaxel and DC/TRAMP-C2(HHP) vaccine (P<0.05). The tumor-inhibitory effect was noted as reduction of the size of growing tumors; there was no difference between the incidences of tumors when two independent experiments were merged [Control 25/26, docetaxel 22/22, docetaxel + DC/TRAMP-C2(HHP) 22/22, DC/TRAMP-C2(HHP) 21/22].
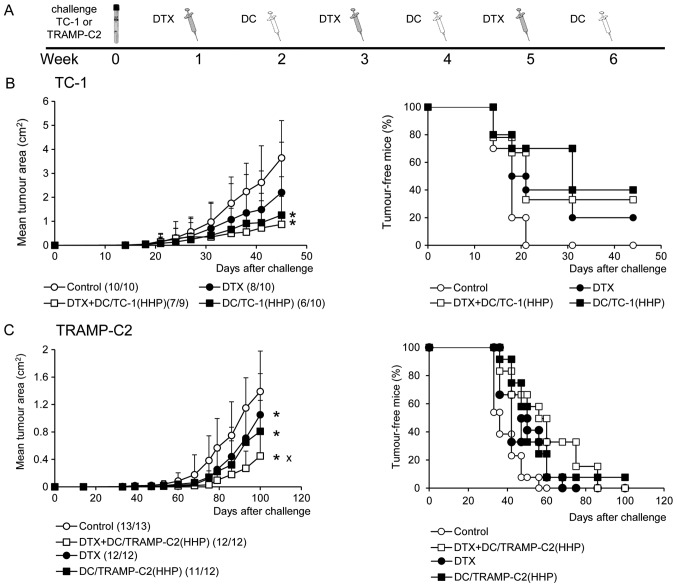
Figure 7.
Combined chemoimmunotherapy of TC-1 or TRAMP-C2 tumors. (A) TC-1 (5×104 cells) or TRAMP-C2 (106 cells) tumor cells were s.c. transplanted on day 0. Docetaxel (DTX) was i.p. administered in a dose of 30 μg/kg, on days 7, 21 and 35. Dendritic cells (2×106 cells) were s.c. administered peritumorally on days 14, 28 and 42. (B) Tumor growth (left panel) of TC-1 tumors and the percentage of tumor-free mice (Kaplan-Maier plot) (right panel). (C) Tumor growth (left panel) of TRAMP-C2 tumors and the percentage of tumor-free mice (right panel) (Kaplan-Maier plot). Results are representative of two independent experiments. *P<0.05 vs. control, xP<0.05 vs. DC/TRAMP-C2(HHP), DTX (analysis of variance).
Discussion
HHP has been previously shown to induce endoplasmic reticulum stress and consequently ICD in both murine and human cell lineages (11,13,34). This suggests that HHP, along with other modalities, such as irradiation, photodynamic therapy using hypericin, hyperthermia or treatments with selected chemotherapeutic and cytotoxic agents, can be used for preparation of tumor cells capable of inducing effective antitumor immunity (35). HHP could also be used for tumor cell inactivation before their use as cellular vaccines or as antigen donors in DC-based vaccines.
In the first part of the study, our aim was to demonstrate the capability of HHP-treated tumor cells to induce immune responses in mice, in comparison with irradiated tumor cells. Lethal irradiation represents a standard procedure used for tumor cell inactivation before their usage for immunization or for DC pulsing, and HHP treatment can serve as an attractive alternative for this procedure. Before performing the in vivo experiments, we optimized the HHP treatments of TC-1 and TRAMP-C2 cell lines used for the studies and we demonstrated, in comparative experiments, that HHP induced higher levels of CRT and HSP90 expression on tumor cells, as well as HMGB1 production, as compared to irradiation. The immunogenicity of irradiated and HHP-treated cells was further monitored in vivo and we noted higher IFNγ production by spleen cells upon immunization with the HHP-treated compared to irradiated cells. However, we did not observe significantly higher cytotoxicity of the spleen cells from the animals immunized with HHP-treated cells and this finding was in agreement with the results of immunization-challenge experiments. We did not see any significant differences between vaccination with HHP- or IR-treated tumor cells; both vaccinations inhibited TC-1 tumor growth, as expected and previously observed for the animals immunized with IR-treated cells (30,31) while the TRAMP-C2 tumor growth was not blocked by both of the vaccination protocols. It has been previously shown that TRAMP-C2 tumor cells are not immunogenic, unless their immunogenicity was increased by IFNγ treatment, inducing MHC class I cell surface expression (36). Thus, it seems that HHP treatment, which induces ICD but not MHC class I and co-stimulatory molecule cell surface expression, does not induce protective immunity effective against TRAMP-C2 cells. It is of note that in the case of TC-1 tumors, which are apparently more sensitive to immune responses, effective immunity was induced by vaccination with both irradiated and HHP-treated TC-1 cells.
Furthermore, in order to assess the suitability of HHP as a tool for tumor cell preparation in the DC-based vaccine preparation protocols, we prepared a DC-based vaccine by co-culture of immature DC with HHP-treated TC-1 or TRAMP-C2 tumor cells and subsequent DC maturation with CpG ODN 1826. CpG ODN 1826, an agonist of Toll-like receptor 9, is a potent maturation agent for murine DC (37), and the capability of DC pulsed by co-cultivation with irradiated tumor cells and matured by CpG ODN 1826 to inhibit the TC-1 tumor growth has also been demonstrated in our laboratory (38). We have compared the phenotype of matured DC vaccines, unpulsed or pulsed with the IR- or HHP-treated tumor cells. The results suggest that DC co-culture with irradiated, but not HHP-treated tumor cells, interferes with their subsequent CpG ODN-driven maturation, since the matured DC culture of the cells pulsed with IR-treated cells displayed lower proportion of matured DC (defined as MHC class IIhigh/CD86high), and lower ratio between the expression of positive costimulatory molecule CD80 (B7.1) vs. negative costimulatory molecule CD274 (B7-H1). This ratio can be considered as an important marker suggesting the DC capability to transmit positive signaling to T cells (39). Selected cytokine expression levels, including that of IL-12, were lower in DC pulsed with IR-treated tumor cells, as compared to the unpulsed controls. Notably, this was not observed when the HHP-treated tumor cells DC were compared to the unpulsed controls. These results suggest that immature DC co-culture with HHP-treated cells represents a convenient protocol for the DC-based vaccine preparation and corroborates previous findings of Fucikova et al (13).
The next step was therefore to perform in vivo experiments and to evaluate the immunogenicity of the matured DC pulsed with the HHP-treated tumor cells. As expected, DC vaccines induced much higher IFNγ production by spleen cells as compared to immunization with tumor cells. This, together with further parameters investigated in the spleens (chromium release assay, effector memory CD4 and CD8 cell proportion), suggested that DC vaccines induced strong immunity against TC-1 or TRAMP-C2 tumors, respectively. However, as determined in the immunization-challenge experiments, DC vaccination in a prophylactic setting induced protection against TC-1, but not TRAMP-C2 tumor growth. This was in agreement with IR- and HHP-treated tumor cell immunization, confirming different immunogenicity or sensitivity of the TC-1 and TRAMP-C2 tumors to the immune response induced by prophylactic immunization.
Next, we tested the vaccine efficacy in a therapeutic setting in combination with docetaxel chemotherapy, which is clinically relevant especially for prostate cancer treatment (19,20). DC-based vaccines are in general intended to be used rather for tumor immunotherapy in a multimodal setting than for immunization. In our experiments, unlike in prophylactic use, the DC treatments of both immunogenic TC-1 and poorly immunogenic/treatable TRAMP-C2 tumors resulted in significant inhibition of the tumor growth, albeit the effect on the TRAMP-C2 appeared to be weaker as compared to the TC-1 tumors. The difference was observed for the therapeutic protocol using docetaxel and DC combination. This treatment led to the highest therapeutic effect, as compared to the chemotherapy or immunotherapy only treatments, in the case of the TRAMP-C2 prostate cancer model. In this model, both chemo- and immunotherapy, when used as monotherapies, displayed only moderate antitumor effects, and additive/synergistic effects were observed when these treatments were used in combination. On the contrary, synergistic effects of the combination therapy were not seen for the TC-1 therapy. We can speculate that TC-1 tumors are much more vulnerable to immunotherapy, as compared to the TRAMP-C2 tumors, and that it may be difficult to boost it. Moreover, DTX treatment can increase the TRAMP-C2 tumor cell sensitivity to the immune responses.
In conclusion, in the present study we demonstrated that HHP-treatment induced ICD in the cells of TRAMP-C2 and TC-1 murine tumor cell lines. Furthermore, our results show that DC-based vaccines pulsed with HHP-treated cells is an effective instrument for immunotherapy, mainly when combined with chemotherapy, as has been demonstrated in the prostate cancer TRAMP-C2 model, which is poorly immunogenic and difficult to treat.
Acknowledgements
The present study was supported by research grant provided by SOTIO a.s., and in part by MEYS (LM2011032), the Academy of Sciences of the Czech Republic (RVO 68378050), the project ‘BIOCEV-Biotechnology and Biomedicine Centre of the Academy of Sciences and Charles University’ (CZ.1.05/1.1.00/02.0109) from the European Regional Development Fund. The authors are grateful to Mrs. Renáta Turečková for skillful technical assistance and to Dr Šárka Takáčová for editorial help. Conflict of interest: J. Bartůňková and R. Špišek are employees and shareholders of SOTIO a.s.
References
- 1.Ramakrishnan R, Antonia S, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: A new perspective. Cancer Immunol Immunother. 2008;57:1523–1529. doi: 10.1007/s00262-008-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: Improving vaccines? Adv Drug Deliv Rev. 2006;58:975–990. doi: 10.1016/j.addr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1:1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, Sochorova K, Hromadkova H, Kayserova J, Vavrova K, Lastovicka J, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6:18192–18205. doi: 10.18632/oncotarget.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spísek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 8.Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: The immunogenic effects of platinum-based chemo-therapeutics. Clin Cancer Res. 2014;20:2831–2837. doi: 10.1158/1078-0432.CCR-13-3141. [DOI] [PubMed] [Google Scholar]
- 9.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: Therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology. 2013;2:e26536. doi: 10.4161/onci.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss EM, Frey B, Rödel F, Herrmann M, Schlücker E, Voll RE, Fietkau R, Gaipl US. Ex vivo- and in vivo-induced dead tumor cells as modulators of antitumor responses. Ann NY Acad Sci. 2010;1209:109–117. doi: 10.1111/j.1749-6632.2010.05743.x. [DOI] [PubMed] [Google Scholar]
- 12.Frey B, Rubner Y, Kulzer L, Werthmöller N, Weiss EM, Fietkau R, Gaipl US. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63:29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, Fialova A, Sojka L, Cartron PF, Houska M, et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer. 2014;135:1165–1177. doi: 10.1002/ijc.28766. [DOI] [PubMed] [Google Scholar]
- 14.Emens LA. Chemoimmunotherapy. Cancer J. 2010;16:295–303. doi: 10.1097/PPO.0b013e3181eb5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ménard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: Not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57:1579–1587. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dodewaard-de Jong JM, Verheul HM, Bloemendal HJ, de Klerk JM, Carducci MA, van den Eertwegh AJ. New treatment options for patients with metastatic prostate cancer: What is the optimal sequence? Clin Genitourin Cancer. 2015;13:271–279. doi: 10.1016/j.clgc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 18.Malvicini M, Rizzo M, Alaniz L, Piñero F, García M, Atorrasagasti C, Aquino JB, Rozados V, Scharovsky OG, Matar P, et al. A novel synergistic combination of cyclophosphamide and gene transfer of interleukin-12 eradicates colorectal carcinoma in mice. Clin Cancer Res. 2009;15:7256–7265. doi: 10.1158/1078-0432.CCR-09-1861. [DOI] [PubMed] [Google Scholar]
- 19.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 20.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 21.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 22.Reinis M, Indrová M, Mendoza L, Mikysková R, Bieblová J, Bubeník J, Símová J. HPV16-associated tumours: Therapy of surgical minimal residual disease with dendritic cell-based vaccines. Int J Oncol. 2004;25:1165–1170. [PubMed] [Google Scholar]
- 23.Reinis M, Stepanek I, Simova J, Bieblova J, Pribylova H, Indrova M, Bubenik J. Induction of protective immunity against MHC class I-deficient, HPV16-associated tumours with peptide and dendritic cell-based vaccines. Int J Oncol. 2010;36:545–551. doi: 10.3892/ijo_00000528. [DOI] [PubMed] [Google Scholar]
- 24.Indrová M, Reinis M, Bubeník J, Jandlová T, Bieblová J, Vonka V, Velek J. Immunogenicity of dendritic cell-based HPV16 E6/E7 peptide vaccines: CTL activation and protective effects. Folia Biol (Praha) 2004;50:184–193. [PubMed] [Google Scholar]
- 25.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 26.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 27.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 28.Stepanek I, Indrova M, Bieblova J, Fucikova J, Spisek R, Bubenik J, Reinis M. Effects of 5-azacytidine and trichostatin A on dendritic cell maturation. J Biol Regul Homeost Agents. 2011;25:517–529. [PubMed] [Google Scholar]
- 29.Yi AK, Krieg AM. CpG DNA rescue from anti-IgM-induced WEHI-231 B lymphoma apoptosis via modulation of I kappa B alpha and I kappa B beta and sustained activation of nuclear factor-kappa B/c-Rel. J Immunol. 1998;160:1240–1245. [PubMed] [Google Scholar]
- 30.Reinis M, Símová J, Indrová M, Bieblová J, Pribylová H, Moravcová S, Jandlová T, Bubeník J. Immunization with MHC class I-negative but not -positive HPV16-associated tumour cells inhibits growth of MHC class I-negative tumours. Int J Oncol. 2007;30:1011–1017. [PubMed] [Google Scholar]
- 31.Indrová M, Símová J, Bieblová J, Bubeník J, Reinis M. NK1.1+ cells are important for the development of protective immunity against MHC I-deficient, HPV16-associated tumours. Oncol Rep. 2011;25:281–288. [PubMed] [Google Scholar]
- 32.Bubenik J, Zeuthen J, Indrova M, Bubenikova D, Simova J. Kinetics and function of peritoneal-exudate cells during local IL-2 gene-therapy of cancer. Int J Oncol. 1994;4:13–16. doi: 10.3892/ijo.4.1.13. [DOI] [PubMed] [Google Scholar]
- 33.Indrová M, Mikysková R, Jandlová T, Vonka V, Bubeník J, Bieblová J. Adjuvant cytokine treatment of minimal residual disease after surgical therapy in mice carrying HPV16-associated tumours: Cytolytic activity of spleen cells from tumour regressors. Folia Biol (Praha) 2003;49:217–222. [PubMed] [Google Scholar]
- 34.Frey B, Janko C, Ebel N, Meister S, Schlücker E, Meyer-Pittroff R, Fietkau R, Herrmann M, Gaipl US. Cells under pressure - treatment of eukaryotic cells with high hydrostatic pressure, from physiologic aspects to pressure induced cell death. Curr Med Chem. 2008;15:2329–2336. doi: 10.2174/092986708785909166. [DOI] [PubMed] [Google Scholar]
- 35.Adkins I, Fucikova J, Garg AD, Agostinis P, Špíšek R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology. 2014;3:e968434. doi: 10.4161/21624011.2014.968434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martini M, Testi MG, Pasetto M, Picchio MC, Innamorati G, Mazzocco M, Ugel S, Cingarlini S, Bronte V, Zanovello P, et al. IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine. 2010;28:3548–3557. doi: 10.1016/j.vaccine.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto M, Sato M. Toll-like receptor signaling in anti-cancer immunity. J Med Invest. 2003;50:9–24. [PubMed] [Google Scholar]
- 38.Reinis M, Símová J, Indrová M, Bieblová J, Bubeník J. CpG oligodeoxynucleotides are effective in therapy of minimal residual tumour disease after chemotherapy or surgery in a murine model of MHC class I-deficient, HPV16-associated tumours. Int J Oncol. 2007;30:1247–1251. [PubMed] [Google Scholar]
- 39.Spranger S, Javorovic M, Bürdek M, Wilde S, Mosetter B, Tippmer S, Bigalke I, Geiger C, Schendel DJ, Frankenberger B. Generation of Th1-polarizing dendritic cells using the TLR7/8 agonist CL075. J Immunol. 2010;185:738–747. doi: 10.4049/jimmunol.1000060. [DOI] [PubMed] [Google Scholar]