Abstract
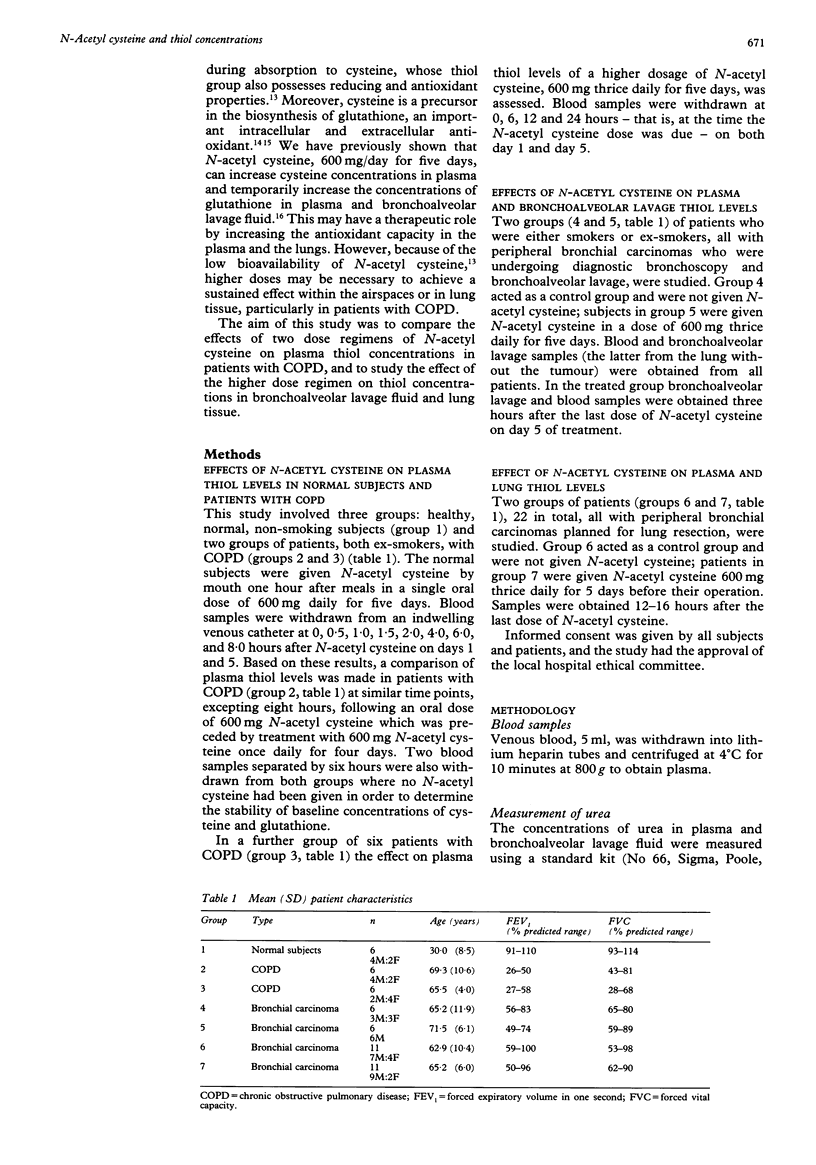
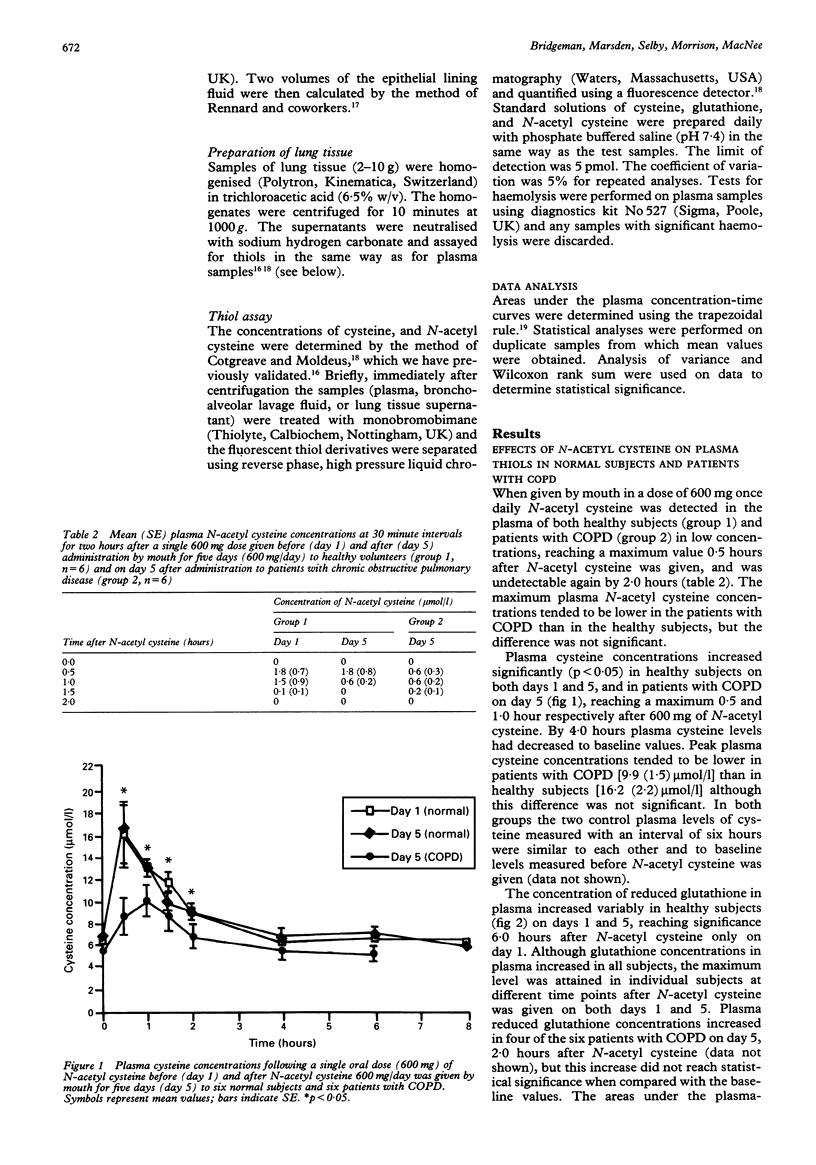
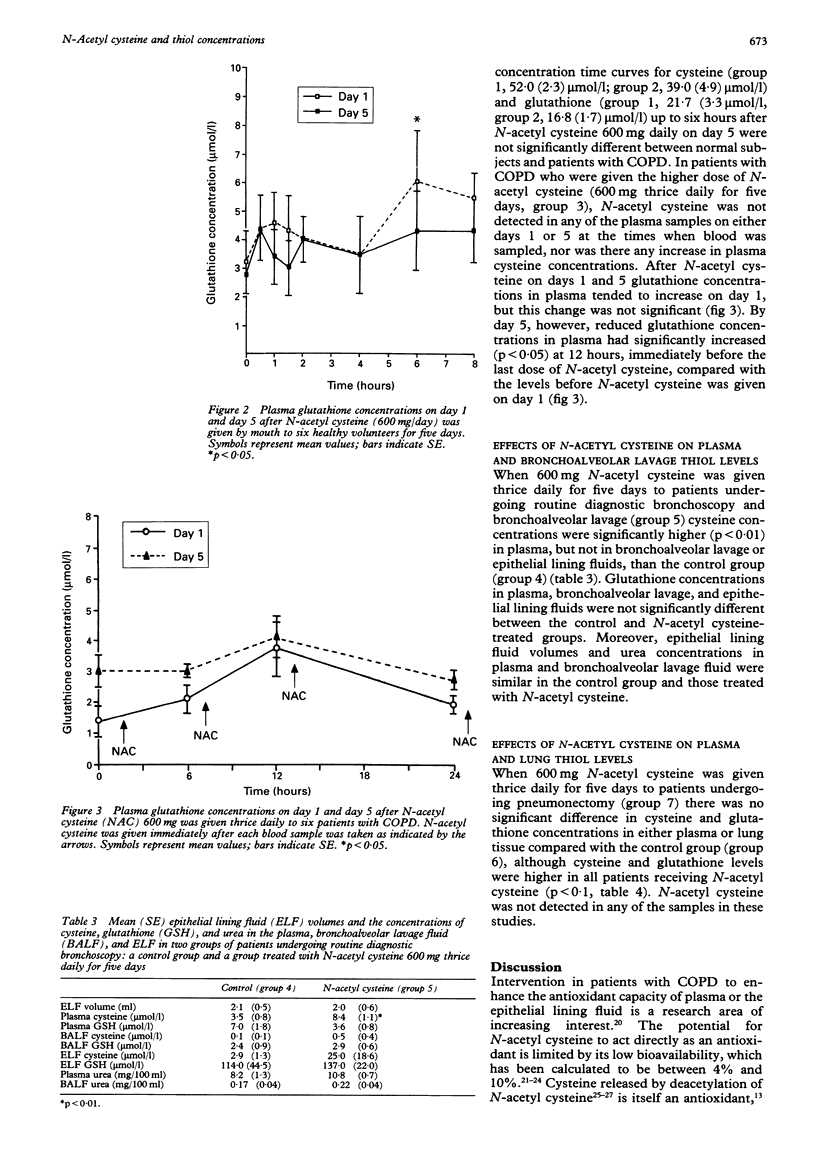
BACKGROUND--Oxidant/antioxidant imbalance may occur in the lungs of patients with chronic obstructive pulmonary disease (COPD). Glutathione is an important extracellular and intracellular thiol oxidant in the lungs. These studies were carried out to determine the effect of N-acetyl cysteine on thiol concentrations in plasma, bronchoalveolar lavage fluid, and lung tissue. METHODS--Studies were carried out on normal subjects, patients with COPD, and those undergoing lung resection. In the first study N-acetyl cysteine was given to three groups; healthy subjects (600 mg once daily by mouth) and two groups of patients with COPD. In the first group of patients with COPD the dose was 600 mg once daily and in the second 600 mg thrice daily, all for five days. The latter dosage regimen was also given to six patients before bronchoscopy and to 11 patients before lung resection. Lung glutathione (GSH) levels in bronchoalveolar lavage fluid or lung tissue were compared with the same numbers of patients who did not receive N-acetyl cysteine. RESULTS--N-acetyl cysteine was detected in plasma after a single 600 mg dose in normal subjects and patients with COPD up to 1.5 hours after the drug was given. Plasma cysteine concentrations increased in normal subjects on both days 1 and 5, and in patients with COPD on day 5. Glutathione concentrations in plasma increased on day 1 in normal subjects but not in patients with COPD given 600 mg N-acetyl cysteine daily. With the higher dose of 600 mg thrice daily, however, there was a sustained elevation of GSH concentrations in plasma in patients with COPD. In patients undergoing routine diagnostic bronchoscopy and bronchoalveolar lavage those who were given N-acetyl cysteine (600 mg) thrice daily for five days had higher concentrations of cysteine in the plasma, but no significant differences in cysteine concentrations in bronchoalveolar lavage or epithelial lining fluid compared with a control group; nor were there any differences in reduced glutathione concentrations in plasma, bronchoalveolar lavage or epithelial lining fluids between the control and treated groups. Moreover, in patients undergoing lung resection those treated with N-acetyl cysteine (600 mg thrice daily for five days) had similar concentrations of cysteine and glutathione in both plasma and lung tissue when compared with a control untreated group. CONCLUSIONS--These data suggest that, even when given in high oral doses, N-acetyl cysteine does not produce a sustained increase in glutathione levels sufficient to increase the antioxidant capacity of the lungs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berggren M., Dawson J., Moldéus P. Glutathione biosynthesis in the isolated perfused rat lung: utilization of extracellular glutathione. FEBS Lett. 1984 Oct 15;176(1):189–192. doi: 10.1016/0014-5793(84)80938-2. [DOI] [PubMed] [Google Scholar]
- Boman G., Bäcker U., Larsson S., Melander B., Wåhlander L. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis. 1983 Aug;64(6):405–415. [PubMed] [Google Scholar]
- Borgström L., Kågedal B., Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31(2):217–222. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- Bridgeman M. M., Marsden M., MacNee W., Flenley D. C., Ryle A. P. Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine. Thorax. 1991 Jan;46(1):39–42. doi: 10.1136/thx.46.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R. D., Hackney J. D., Clark K., Posin C. Ozone and human blood. Arch Environ Health. 1975 Jan;30(1):40–43. doi: 10.1080/00039896.1975.10666631. [DOI] [PubMed] [Google Scholar]
- Burgunder J. M., Varriale A., Lauterburg B. H. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36(2):127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- Cotgreave I. A., Berggren M., Jones T. W., Dawson J., Moldéus P. Gastrointestinal metabolism of N-acetylcysteine in the rat, including an assay for sulfite in biological systems. Biopharm Drug Dispos. 1987 Jul-Aug;8(4):377–386. doi: 10.1002/bdd.2510080408. [DOI] [PubMed] [Google Scholar]
- Cotgreave I. A., Eklund A., Larsson K., Moldéus P. W. No penetration of orally administered N-acetylcysteine into bronchoalveolar lavage fluid. Eur J Respir Dis. 1987 Feb;70(2):73–77. [PubMed] [Google Scholar]
- Cotgreave I. A., Moldéus P. Methodologies for the analysis of reduced and oxidized N-acetylcysteine in biological systems. Biopharm Drug Dispos. 1987 Jul-Aug;8(4):365–375. doi: 10.1002/bdd.2510080407. [DOI] [PubMed] [Google Scholar]
- Cotgreave I. A., Moldéus P. Methodologies for the application of monobromobimane to the simultaneous analysis of soluble and protein thiol components of biological systems. J Biochem Biophys Methods. 1986 Nov;13(4-5):231–249. doi: 10.1016/0165-022x(86)90102-8. [DOI] [PubMed] [Google Scholar]
- Doskocil M., Med M., Vimmer T. Musculus rectus femoris--zacátecní slacha, její funkcní význam. Acta Chir Orthop Traumatol Cech. 1987 Apr;54(2):99–107. [PubMed] [Google Scholar]
- Heffner J. E., Repine J. E. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989 Aug;140(2):531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kågedal B., Källberg M., Mårtensson J. Determination of non-protein-bound N-acetylcysteine in plasma by high-performance liquid chromatography. J Chromatogr. 1984 Nov 9;311(1):170–175. doi: 10.1016/s0378-4347(00)84705-2. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Taylor J. C. Abnormal interaction of alpha 1-antitrypsin and leukocyte elastolytic activity in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1979 Aug;120(2):411–419. doi: 10.1164/arrd.1979.120.2.411. [DOI] [PubMed] [Google Scholar]
- Morrison W. L., Gibson J. N., Scrimgeour C., Rennie M. J. Muscle wasting in emphysema. Clin Sci (Lond) 1988 Oct;75(4):415–420. doi: 10.1042/cs0750415. [DOI] [PubMed] [Google Scholar]
- Olsson B., Johansson M., Gabrielsson J., Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- Rasmussen J. B., Glennow C. Reduction in days of illness after long-term treatment with N-acetylcysteine controlled-release tablets in patients with chronic bronchitis. Eur Respir J. 1988 Apr;1(4):351–355. [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Riley D. J., Kerr J. S. Oxidant injury of the extracellular matrix: potential role in the pathogenesis of pulmonary emphysema. Lung. 1985;163(1):1–13. doi: 10.1007/BF02713801. [DOI] [PubMed] [Google Scholar]
- Sheffner A. L., Medler E. M., Bailey K. R., Gallo D. G., Mueller A. J., Sarett H. P. Metabolic studies with acetylcysteine. Biochem Pharmacol. 1966 Oct;15(10):1523–1535. doi: 10.1016/0006-2952(66)90197-3. [DOI] [PubMed] [Google Scholar]
- Taylor J. C., Madison R., Kosinska D. Is antioxidant deficiency related to chronic obstructive pulmonary disease? Am Rev Respir Dis. 1986 Aug;134(2):285–289. doi: 10.1164/arrd.1986.134.2.285. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Danis E. H., Del Vecchio P. J., Rosano C. L. Enhancement of intracellular glutathione protects endothelial cells against oxidant damage. Biochem Biophys Res Commun. 1985 Feb 28;127(1):270–276. doi: 10.1016/s0006-291x(85)80154-6. [DOI] [PubMed] [Google Scholar]
- Weitz J. I., Crowley K. A., Landman S. L., Lipman B. I., Yu J. Increased neutrophil elastase activity in cigarette smokers. Ann Intern Med. 1987 Nov;107(5):680–682. doi: 10.7326/0003-4819-107-5-680. [DOI] [PubMed] [Google Scholar]
- Ziment I. Acetylcysteine: a drug with an interesting past and a fascinating future. Respiration. 1986;50 (Suppl 1):26–30. doi: 10.1159/000195085. [DOI] [PubMed] [Google Scholar]


