Abstract
Purpose
Acute kidney injury (AKI) is common after cardiovascular surgery and is usually diagnosed on the basis of the serum creatinine (SCr) level and urinary output. However, SCr is of low sensitivity in patients with poor renal function. Because urinary liver-type fatty-acid-binding protein (L-FABP) reflects renal tubular injury, we evaluated whether perioperative changes in urinary L-FABP predict AKI in the context of abdominal aortic repair.
Methods
Study participants were 95 patients who underwent endovascular abdominal aortic aneurysm repair (EVAR) and 42 who underwent open repair. We obtained urine samples before surgery, after anesthesia induction, upon stent placement, before aortic cross-clamping (AXC), 1 and 2 h after AXC, at the end of surgery, 4 h after surgery, and on postoperative days (PODs) 1, 2, and 3, for measurement of L-FABP. We obtained serum samples before surgery, immediately after surgery, and on PODs 1, 2, and 3, for measurement of SCr. We also plotted receiver-operating characteristic (ROC) curves to identify cutoff laboratory values for predicting the onset of AKI.
Results
With EVAR, urinary L-FABP was significantly increased 4 h after the procedure (P = 0.014). With open repair, urinary L-FABP increased significantly to its maximum by 2 h after AXC (P = 0.007). With AKI, SCr significantly increased (P < 0.001, P = 0.001) by POD 2. ROC analysis showed urinary L-FABP to be more sensitive than SCr for early detection of AKI.
Conclusion
Urinary L-FABP appears to be a sensitive biomarker of AKI in patients undergoing abdominal aortic repair.
Keywords: Urinary liver-type fatty-acid-binding protein, Acute kidney injury, Abdominal aortic repair
Introduction
Acute kidney injury (AKI) is common after cardiovascular surgery, sometimes requiring postoperative hemodialysis, and is associated with increased morbidity and mortality [1]. AKI occurs after abdominal aortic aneurysm repair surgery in >30 % of cases and leads to a prolonged hospital stay [2]. Both open abdominal aortic aneurysm repair and infrarenal aortic cross-clamping (AXC) decrease renal blood flow and increase renal vascular resistance. These changes, which indicate diminished global perfusion with redistribution of renal blood flow toward the cortical compartment, persist for at least 1 h after release of the aortic clamp [3–5]. Infrarenal AXC produces profound and sustained alterations in renal hemodynamics and may be particularly harmful in patients with impaired renal function or when it is prolonged [3].
Endovascular abdominal aortic aneurysm repair (EVAR), performed with intra-arterial contrast enhancement, has become an important treatment for infrarenal abdominal aortic aneurysm. Although prospective studies have shown better renal outcomes with EVAR than with open repair, the long-term durability and safety of stent grafting remain unclear [6–8]. EVAR requires intra-arterial administration of contrast medium, which can impair renal function and even lead to end-stage renal disease [9–11]. Although the incidence of contrast-induced AKI is low (2 %) in the general population, it is high (5–10 %) in patients at risk for kidney disease, such as those with cardiovascular disease [12, 13]. Early diagnosis and treatment of AKI may be essential for improved perioperative renal outcomes after both EVAR and open repair. Biomarkers are used effectively in the diagnosis of AKI, with assay of serum creatinine (SCr) being the gold standard. However, SCr is of low sensitivity in patients with poor renal function or low muscle mass [14, 15]. Several new biomarkers of kidney injury have been investigated, both experimentally and clinically, with liver-type fatty-acid-binding protein (L-FABP) being a promising candidate [16]; it is recognized as the most useful alternative biomarker of kidney injury. Matsui et al. described urinary L-FABP as an early predictor of AKI after cardiac surgery [17]. In addition, Kamijo et al. showed urinary L-FABP to be an excellent biomarker for clinical prediction and monitoring of renal disease [18]. Several studies have shown the usefulness of urinary L-FABP for the detection of AKI after cardiac surgery [19] and contrast-induced nephropathy [20].
We conducted a prospective study with two aims: (1) to evaluate the perioperative changes in urinary L-FABP that occur with EVAR and open abdominal aortic aneurysm repair, and (2) to examine the usefulness of urinary L-FABP for predicting AKI after either type of abdominal aortic repair.
Methods
The study protocol was approved by the Institutional Review Board of St. Marianna University School of Medicine (No 1966), Kawasaki, Japan, and registered University Hospital Medical Information Network (UMIN) Clinical Data Registry (ID 000006584). Written informed consent was obtained from all patients enrolled in the study.
Study design
We conducted a two-part prospective study in a university hospital setting: one part to investigate AKI associated with EVAR and the other to investigate AKI associated with open repair. Consecutive patients scheduled for EVAR (n = 95) or open repair (n = 42) between October 2011 and June 2015 were enrolled. Each patient’s surgeon chose between EVAR and open repair by considering the patient’s age and the type of abdominal aortic aneurysm in the absence of any concern regarding the patient’s tolerance for study procedures. Excluded from the study were patients undergoing dialysis or requiring emergency surgery.
EVAR study protocol
Anesthesia
No premedication or epidural anesthesia was administered to any patient in the EVAR group. General anesthesia was induced with remifentanil and propofol. Tracheal intubation was facilitated with rocuronium, and general anesthesia was maintained with sevoflurane in an air–oxygen mixture and remifentanil.
Sample collection
Urine samples (10 ml) were obtained before surgery, after anesthesia induction, upon stent placement, at the end of surgery, 4 h after surgery, and on postoperative days (PODs) 1, 2, and 3 for measurement of urinary L-FABP and urinary albumin. Urine samples were centrifuged at 1000 g for 5 min at 4 °C and stored at −80 °C until analysis. In addition, serum samples were obtained before surgery, immediately after surgery, and on PODs 1, 2, and 3 to measure serum creatinine (SCr).
Open-repair study protocol
Anesthesia
No premedication was administered to any patient in the open-repair group. All patients in this group received epidural anesthesia before the surgery. An epidural catheter was inserted via the Th9/10, Th10/11, or Th11/12 intervertebral space. General anesthesia was induced with remifentanil and propofol. Tracheal intubation was facilitated with rocuronium. Anesthesia was maintained with sevoflurane in an oxygen–air mixture and remifentanil. Levobupivacaine (0.125 or 0.25 %) was administered via epidural catheter during the surgery. Dopamine, prostaglandin E1, and carperitide were infused continuously during surgery.
Sample collection
Urine samples (10 ml) were obtained before surgery, after anesthesia induction, before aortic cross-clamping (AXC), 1 and 2 h after AXC, at the end of surgery, 4 h after surgery, and on PODs 1, 2, and 3 for measurement of urinary L-FABP and urinary albumin. These urine samples were prepared and stored as described above. In addition, serum samples were obtained before surgery, immediately after surgery, and on PODs 1, 2, and 3 to measure SCr.
Clinical monitoring of EVAR and open-repair patients
During the first 48 h postoperative period, we monitored patients for AKI as defined according to AKI network criteria [21]. We also monitored patients’ estimated glomerular filtration rate (eGFR) at the start of this study, which was calculated according to the Japanese-coefficient-modified Chronic Kidney Disease Epidemiology Collaboration equation: [22].
Assay of urinary L-FABP, urinary albumin, and SCr
Urinary L-FABP levels were determined by enzyme-linked immunosorbent assay (ELISA) with use of the human L-FABP ELISA kit (CMIC, Tokyo, Japan). Urinary albumin was measured by immunonephelometry. SCr was measured by an enzymatic method.
Statistical analyses
Study variables are expressed as median [interquartile range (IQR)]. Between-group (AKI vs. non-AKI; EVAR vs. open repair) differences were analyzed using the Mann–Whitney U test or chi-square test, as appropriate. One-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test, was used for multiple comparisons. Receiver-operating characteristic curves (ROCs) were plotted to identify cutoff laboratory values for predicting AKI onset. Univariate analysis was used to select the clinical risk factor for the occurrence of AKI and the characteristics showing a significant difference between AKI and non-AKI groups. Following univariate analysis, significant unadjusted predictors with P < 0.05 were used in a multivariate logistic regression analysis. Multivariate logistic regression analysis was performed using a forward selection method. All statistical analyses were performed with IBM SPSS Statistics, version 21.0 (IBM, Tokyo, Japan). P < 0.05 was considered significant for all analyses.
Results
EVAR study
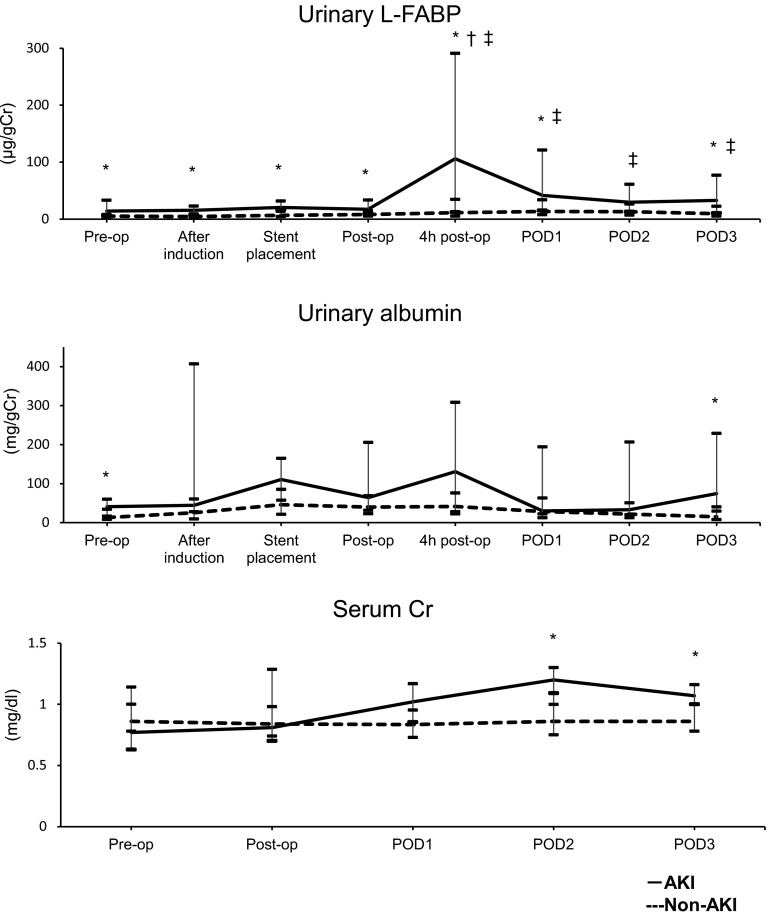
AKI developed postoperatively in nine (9.5 %) of the 95 patients enrolled in the EVAR study: stage 1 AKI in eight patients and stage 2 AKI in one patient. None required postoperative renal replacement therapy (RRT). Body weight (P = 0.03) and body mass index (BMI) (P = 0.001) were significantly lower, diabetes mellitus (P = 0.049) and nonsteroidal anti-inflammatory drug (NSAID) use (P = 0.002) were significantly more prevalent, and duration of anesthesia (P = 0.046) and length of hospital stay (P = 0.005) were significantly longer in the AKI group than in the non-AKI group (Table 1). Before surgery, after anesthesia induction, and upon stent placement, urinary L-FABP level was high and was significantly increased (P = 0.014) in the AKI group at 4 h after surgery; it decreased over the three PODs (Fig. 1).
Table 1.
Patient characteristics and clinical outcomes in EVAR study
| Non–AKI (n = 86) | AKI (n = 9) | P value | |
|---|---|---|---|
| Sex (M/F) | 70/16 | 8/1 | |
| Age (years) | 78 (73–83) | 78 (75–82) | 0.949 |
| Body weight (kg) | 60 (53–68) | 51 (48–61) | 0.030 |
| BMI (kg/m2) | 23 (21–25) | 20 (17–21) | 0.001 |
| ASA status (II/III) | 67/19 | 5/4 | 0.138 |
| Comorbidity, n (%) | |||
| Diabetes mellitus | 9 (10) | 3(33) | 0.049 |
| Hypertension | 69 (80) | 9 (100) | 0.141 |
| Ischemic heart disease | 33 (38) | 3 (33) | 0.787 |
| Chronic kidney disease | 18 (21) | 3 (33) | 0.394 |
| Concomitant medications, n (%) | |||
| Ca inhibitor | 51 (59) | 7 (78) | 0.297 |
| ACE inhibitor/ARB | 38 (44) | 4 (44) | 0.988 |
| Statin | 31 (36) | 2 (22) | 0.407 |
| Diuretic | 8 (9) | 1 (11) | 0.860 |
| NSAID | 0 (0) | 1 (11) | 0.002 |
| Smoking history | 41 (48) | 5 (56) | 0.802 |
| Preoperative urinary L-FABP (μg/g Cr) | 5 (3.3–7.9) | 14.1 (6.5–32.9) | 0.002 |
| Preoperative urinary albumin (mg/g Cr) | 13.0 (7.9–33.6) | 40.7 (16.4–59.9) | 0.016 |
| Preoperative SCr (mg/dl) | 0.86 (0.77–1.01) | 0.77 (0.63–1.14) | 0.644 |
| Preoperative eGFRa (ml/min) | 63.1 (51.1–69.1) | 57.5 (48.7–87.3) | 0.854 |
| General anesthesia | 86 | 9 | |
| General anesthesia with epidural | 0 | 0 | |
| Duration of anesthesia (min) | 208 (185–234) | 225 (220–290) | 0.046 |
| Duration of surgery (min) | 128 (105–155) | 145 (133–210) | 0.069 |
| Fluids infusion (ml) | 1500 (1213–1800) | 1650 (1300–2000) | 0.337 |
| Estimated blood loss (ml) | 78 (39–143) | 114 (76–148) | 0.230 |
| Contrast media (ml) | 118 (100–160) | 143 (116–226) | 0.138 |
| Operative and postoperative details | |||
| Mechanical ventilation, n (%) | 0 (0) | 0 (0) | |
| Length of hospital stay (days) | 13 (12–14) | 17 (14–23) | 0.005 |
| RRT required upon discharge | 0 (0) | 0 (0) | |
| In-hospital death | 0 (0) | 0 (0) | |
Data are expressed as median (interquartile range) or number (%)
EVAR endovascular aortic repair, AKI acute kidney injury, BMI body mass index, ASA American Society of Anesthesiologists, ACE inhibitor angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, NSAID nonsteroidal anti-inflammatory drug, Ca calcium channel, Cr creatinine, SCr serum creatinine, eGFR estimated glomerular filtration rate, RRT renal replacement therapy
aeGFR was calculated according to the Japanese coefficient-modified Chronic Kidney Disease Epidemiology Collaboration equation: eGFR = 194 × (creatinine)−1.094 × (age)−0.287 × (0.739 if female)22
Fig. 1.
Changes in urinary L-FABP, urinary albumin, and SCr in EVAR. Acute kidney injury (AKI; solid line) and non-AKI groups (dashed line). L-FABP liver-type fatty acid-binding protein, SCr serum creatinine, EVAR endovascular aneurysm repair, Pre-op preoperative value, After induction value after induction of anesthesia, Post-op immediate postoperative value, 4 h post-op value 4 h after the operation, POD postoperative day. *P < 0.05 vs. non-AKI group at the same time point; † P < 0.05 vs. respective preoperative level in the same (AKI) group; ‡ P < 0.05 vs. respective preoperative level in the same (non-AKI) group
SCr and urinary albumin did not change in the non-AKI group during the perioperative period; however, in the AKI group, it increased significantly on PODs 2 and 3 (P = 0.000 and P = 0.011, respectively; Fig. 1). Multivariate logistic regression analysis performed for both groups showed preoperative urinary L-FABP level to be a predictor of postoperative AKI [odds ratio (OR) 6.76; confidence interval (CI) 1.76–25.94, P = 0.005; Table 2]. BMI was also shown to be a predictor factor. The cutoff preoperative urinary L-FABP level was 9.0 μg/g Cr (Table 3).
Table 2.
Multivariate logistic regression analyses for AKI in the EVAR group
| Variable | Multivariate analysis | ||
|---|---|---|---|
| OR | 95 % CI | P value | |
| BMI | 0.51 | 0.31–0.84 | 0.008 |
| Diabetes mellitus | 1.46 | 0.89–2.39 | 0.138 |
| NSAID | 5.85 | 0.00–0.00 | 1.000 |
| Length of hospital stay | 1.13 | 0.76–1.67 | 0.553 |
| Urinary L-FABP pre-operation | 6.76 | 1.76–25.94 | 0.005 |
| Urinary L-FABP after induction | 0.65 | 0.05–9.19 | 0.746 |
| Urinary L-FABP stent placement | 10.6 | 0.59–190.9 | 0.109 |
| Urinary L-FABP 4 h postoperation | 0.96 | 0.24–3.83 | 0.957 |
| SCr POD2 | 115.9 | 0.72–18,548.3 | 0.066 |
AKI acute kidney injury, EVAR endovascular aneurysm repair, OR odds ratio, CI confidence interval, BMI body mass index, NSAID nonsteroidal anti-inflammatory drug, L-FABP liver-type fatty-acid-binding protein, SCr serum creatinine, POD postoperative day
Table 3.
Urinary L-FABP levels predictive of AKI in the EVAR study
| Time point | Cutoff value (μg/g Cr) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Preoperation | 9.0 | 0.67 | 0.82 | 0.63 | 0.85 |
| After anesthesia induction | 5.9 | 0.89 | 0.61 | 0.51 | 0.93 |
| At stent placement | 16.3 | 0.78 | 0.80 | 0.64 | 0.89 |
| Postoperation | 9.2 | 0.89 | 0.57 | 0.48 | 0.92 |
| 4 h postoperation | 87.8 | 0.56 | 0.92 | 0.76 | 0.82 |
| POD1 | 68.1 | 0.44 | 0.87 | 0.63 | 0.78 |
| POD2 | 28.1 | 0.56 | 0.74 | 0.48 | 0.79 |
| POD3 | 62.3 | 0.44 | 0.92 | 0.71 | 0.79 |
L-FABP liver-type fatty-acid-binding protein, AKI acute kidney injury, EVAR endovascular aneurysm repair, PPV positive predictive value, NPV negative predictive value, POD postoperative day, Cr creatinine
ROC analysis
The biomarker with the largest area under the curve (AUC) for predicting AKI onset was urinary L-FABP at the following time points: before surgery, after anesthesia induction, upon stent placement, and 4 h after surgery; AUCs were 0.83, 0.81, 0.79, and 0.75, respectively (Table 4). Urinary L-FABP cutoff values at different time points for EVAR are shown in Table 3.
Table 4.
AUC vs. time in the EVAR group
| Preoperation | After induction | Stent placement | Postoperation | 4-h postoperation | P0D1 | P0D2 | P0D3 | |
|---|---|---|---|---|---|---|---|---|
| Urinary l-FABP | 0.83 (0.69–0.96) | 0.81 (0.69–0.93) | 0.79 (0.64–0.95) | 0.72 (0.54–0.90) | 0.75 (0.57–0.94) | 0.70 (0.52–0.88) | 0.69 (0.50–0.87) | 0.71 (0.52–0.90) |
| Urinary albumin | 0.72 (0.57–0.87) | 0.67 (0.48–0.86) | 0.71 (0.52–0.90) | 0.63 (0.42–0.84) | 0.65 (0.42–0.88) | 0.62 (0.43–0.82) | 0.64 (0.45–0.84) | 0.76 (0.57–0.95) |
| SCr | 0.45 (0.18–0.72) | – | – | 0.54 (0.28–0.79) | – | 0.69 (0.50–0.88) | 0.84 (0.74–0.94) | 0.73 (0.57–0.89) |
Data are given as AUC (95% confidence interval)
AUC area under the curve, EVAR endovascular aortic repair, After induction after induction of anesthesia, Postoperation immediately after surgery, POD postoperative day, L-FABP liver-type fatty-acid-binding protein, SCr serum creatinine
Open-repair study
AKI developed postoperatively in 13 (31.0 %) of the 42 patients enrolled in the open-repair study and was stage 1 in all of them. No patient required postoperative RRT. Male sex (P = 0.000) was more prevalent, age (P = 0.025) was greater, and preoperative ischemic heart disease (P = 0.016) more prevalent among open-repair patients in the AKI than in the non-AKI group (Table 5).
Table 5.
Patient characteristics and clinical outcomes in the open-repair study
| Non–AKI group (n = 29) | AKI group (n = 13) | P value | |
|---|---|---|---|
| Sex (M/F) | 26/3 | 12/1 | <0.001 |
| Age (years) | 67 (64–72) | 72 (69–78) | 0.025 |
| Body weight (kg) | 61 (56–74) | 68 (64–69) | 0.187 |
| BMI (kg/m2) | 23 (21–25) | 24 (24–26) | 0.094 |
| ASA status (II/III) | 21/8 | 7/6 | 0.486 |
| Comorbidity, n (%) | |||
| Diabetes mellitus | 2 (7) | 3 (23) | 0.134 |
| Hypertension | 24 (92) | 12 (92) | 0.414 |
| Ischemic heart disease | 13 (45) | 11 (85) | 0.016 |
| Chronic kidney disease | 7 (24) | 5 (38) | 0.342 |
| Concomitant medications, n (%) | |||
| Ca inhibitor | 17 (59) | 8 (62) | 0.859 |
| ACE inhibitor/ARB | 17 (59) | 8 (62) | 0.859 |
| Statin | 14 (48) | 5 (38) | 0.555 |
| Diuretic | 2 (7) | 2 (15) | 0.386 |
| NSAID | 1 (3) | 0 (0) | 0.498 |
| Smoking history | 16 (55) | 7 (54) | 0.936 |
| Preoperative urinary L-FABP (μg/g Cr) | 4.1 (2.3–7.0) | 4.4 (2.9–11.9) | 0.863 |
| Preoperative urinary albumin (mg/g Cr) | 9.9 (6.4–13.8) | 20 (8.3–69.3) | 0.128 |
| Preoperative SCr (mg/dl) | 0.85 (0.75–1.06) | 0.92 (0.85–1.31) | 0.268 |
| Preoperative eGFRa (ml/min) | 67.3 (52.7–77.9) | 62.3 (44.8–68.7) | 0.196 |
| General anesthesia | 0 | 0 | |
| General anesthesia with epidural | 29 | 13 | |
| Duration of anesthesia (min) | 470 (410–585) | 580 (500–640) | 0.084 |
| Duration of surgery (min) | 345 (261–445) | 443 (350–499) | 0.077 |
| Duration of AXC (min) | 66 (55–88) | 58 (46–95) | 0.624 |
| Fluids infusion (ml) | 5560 (4300–6750) | 5560 (4100–6890) | 0.924 |
| Estimated blood loss (ml) | 2620 (1620–3926) | 2343 (2179–3835) | 0.765 |
| Operative and postoperative details | |||
| Mechanical ventilation, n (%) | 0 (0) | 2 (29) | |
| Length of hospital stay (days) | 19 (16–28) | 20 (17–24) | 0.917 |
| RRT required upon discharge | 0 (0) | 0 (0) | |
| In-hospital death | 0 (0) | 0 (0) | |
Data are expressed as median (interquartile range) or number (%)
AKI acute kidney injury, BMI body mass index, ASA American Society of Anesthesiologists, ACE inhibitor angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, NSAIDs nonsteroidal anti-inflammatory drugs, Cr creatinine, SCr serum creatinine, eGFR estimated glomerular filtration rate, RRT renal replacement therapy, AXC aortic cross-clamping
aeGFR was calculated according to the Japanese coefficient-modified Chronic Kidney Disease Epidemiology Collaboration equation: eGFR = 194 x (creatinine)−1.094 × (age)−0.287 × (0.739 if female)22
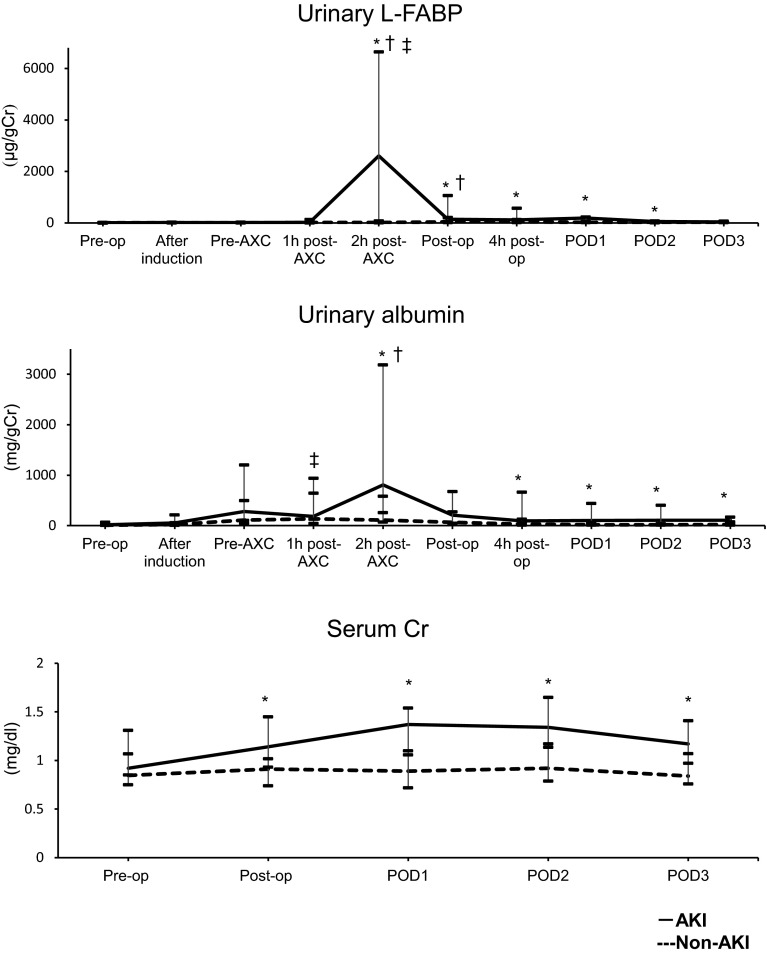
In open-repair patients in whom AKI developed, urinary L-FABP levels were significantly increased to their maximum by 2 h after AXC (P = 0.007). They decreased gradually thereafter to POD 3 (Fig. 2). SCr levels were significantly increased immediately after surgery and on PODs 1, 2, and 3 (P = 0.010, 0.001, 0.001, and 0.002, respectively; Fig. 2). Urinary albumin levels were significantly increased 2 h after AXC, 4 h after surgery, and on PODs 1, 2, and 3 (P = 0.009, 0.008, 0.002, 0.002, and 0.010, respectively; Fig. 2). Results of multivariate logistic regression analysis for both AKI and non-AKI groups showed urinary L-FABP at 2 h post-AXC and SCr at POD2 to be predictors of postoperative AKI (OR 1.58, CI 1.13–2.21, P = 0.007; OR 64.0, CI 4.03–1016.2, P = 0.003; Table 6). The cutoff urinary L-FABP level at 2 h post-AXC was 173.0 μg/g Cr (Table 7).
Fig. 2.
Changes in urinary L-FABP, urinary albumin and SCr in OR. Acute kidney injury (AKI; solid line) and non-AKI groups (dashed line). Pre-op preoperative value, After induction value after induction of anesthesia, AXC value after aorta cross-clamping, Post-op immediate postoperative value, 4 h post-op value 4 h after operation, POD postoperative day, L-FABP liver-type fatty-acid-binding protein, SCr serum creatinine, OR open repair *P < 0.05 vs. the non-AKI group at the same time point; † P < 0.05 vs. the respective preoperative level in the same (AKI) group; ‡ P < 0.05 vs. the respective preoperative level in the same (non-AKI) group
Table 6.
Results of multivariate logistic regression analyses for AKI in the open-repair group
| Variable | Multivariate analysis | ||
|---|---|---|---|
| OR | 95 % CI | P value | |
| Sex | 1.13 | 0.00–969.6 | 0.971 |
| Age | 1.51 | 0.95–2.42 | 0.085 |
| Ischemic heart disease | 0.78 | 0.44–1.38 | 0.389 |
| Urinary L-FABP 2 h post-AXC | 1.58 | 1.13–2.21 | 0.007 |
| Urinary L-FABP postoperation | 0.56 | 0.12–2.63 | 0.103 |
| Urinary L-FABP 4 h postoperation | 0.50 | 0.06–3.97 | 0.515 |
| SCr postoperation | 0.00 | 0.00–87.0 | 0.103 |
| SCr POD1 | 13.4 | 0.00–1.15 | 0.824 |
| SCr POD2 | 64.0 | 4.03–1016.2 | 0.003 |
| Urinary albumin (10 mg/g Cr) 2 h postAXC | 1.00 | 1.00–1.00 | 0.402 |
AKI acute kidney injury, OR odds ratio, CI confidence interval, L-FABP liver-type fatty-acid-binding protein, Cr creatinine, SCr serum creatinine, POD postoperative day
Table 7.
Urinary L-FABP levels predictive of AKI in the open-repair study
| Time point | Cutoff value (μg/g Cr) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Preoperation | 12.7 | 0.25 | 0.81 | 0.12 | 0.91 |
| After anesthesia induction | 9.9 | 0.42 | 0.69 | 0.12 | 0.92 |
| Pre-AXC | 5.6 | 0.83 | 0.42 | 0.13 | 0.96 |
| 1 h post-AXC | 37.2 | 0.50 | 0.79 | 0.20 | 0.94 |
| 2 h post-AXC | 173.0 | 0.67 | 0.83 | 0.30 | 0.96 |
| Postoperation | 32.3 | 0.92 | 0.54 | 0.17 | 0.98 |
| 4 h postoperation | 348.5 | 0.50 | 0.92 | 0.40 | 0.95 |
| POD1 | 112.1 | 0.62 | 0.83 | 0.28 | 0.95 |
| POD2 | 48.6 | 0.54 | 0.83 | 0.25 | 0.95 |
| POD3 | 19.5 | 0.85 | 0.61 | 0.19 | 0.97 |
L-FABP liver-type fatty-acid-binding protein, AKI acute kidney injury, AXC aorta cross-clamping, PPV positive predictive value, NPV negative predictive value, POD postoperative day, Cr creatinine
Suprarenal AXC was applied in eight patients who underwent open repair; AKI developed in five (63 %), and peak urinary L-FABP concentration was 8410 μg/g Cr (6050–10,995 μg/g Cr). Infrarenal AXC was applied in the other five AKI patients (15 %) who underwent open repair; peak urinary L-FABP concentration in these patients was 90 μg/g Cr (25–212 μg/g Cr).
ROC analysis
The biomarker with the largest AUC for predicting AKI onset was urinary L-FABP at 2 h after AXC, at the end of surgery, and 4 h after surgery; AUCs were 0.77, 0.75, and 0.76, respectively (Table 8). We determined urinary L-FABP cutoff values at different time points for EVAR and open repair, as shown in Table 7.
Table 8.
AUC vs. time in the open-repair group
| Pre-operation | After induction | Pre-AXC | 1 h post-AXC | 2 h post-AXC | Postoperative | 4 h postoperative | POD1 | POD2 | POD3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Urinary L-FABP | 0.48 (0.29–0.68) | 0.53 (0.34–0.72) | 0.55 (0.36–0.73) | 0.58 (0.36–0.79) | 0.77 (0.58–0.95) | 0.75 (0.59–0.91) | 0.76 (0.60–0.92) | 0.73 (0.54–0.91) | 0.72 (0.53–0.90) | 0.69 (0.50–0.88) |
| Urinary albumin | 0.64 (0.45–0.86) | 0.71 (0.53–0.88) | 0.66 (0.48–0.84) | 0.57 (0.38–0.77) | 0.78 (0.61–0.95) | 0.59 (0.38–0.80) | 0.76 (0.60–0.91) | 0.81 (0.68–0.94) | 0.80 (0.66–0.94) | 0.75 (0.60–0.90) |
| SCr | 0.61 (0.41–0.81) | – | – | – | – | 0.75 (0.58–0.93) | - | 0.82 (0.68–0.95) | 0.82 (0.66–0.97) | 0.81 (0.66–0.96) |
Data are 95% confidence intervals)
AUC area under the curve, After induction after induction of anesthesia, Postoperative immediately after surgery, POD postoperative day, AXC aorta cross-clamping, L-FABP liver-type fatty-acid-binding protein, SCr serum creatinine
Differences in patient characteristics and surgical outcomes between EVAR and open repair
Median age of the open-repair patients was 69 years (IQR 65–75 years) and that of the EVAR patients was 78 years (IQR 74–83 years). The median hospital stay of the open-repair patients was 19 days (IQR 16–25 days) and 13 days (IQR 12–15 days) for EVAR patients. Preoperative ischemic heart disease was significantly more prevalent in the open-repair than in the EVAR group (P = 0.030). The incidence of AKI was greater in the open-repair than in the EVAR group (31.0 vs. 9.5 %, respectively), increase in urinary L-FABP in open-repair patients was greater than in EVAR patients, and peak urinary L-FABP level occurred earlier in the open-repair group than in the EVAR group. Hospital stays were significantly longer for open-repair patients than for EVAR patients (P < 0.001).
Discussion
The most important results of this study were that preoperative urinary L-FABP was a good predictor of AKI after EVAR, and 2 h post-AXC urinary L-FABP was a good predictor of AKI after open repair. Our data show that urinary L-FABP is a useful biomarker for early AKI detection after abdominal aortic aneurysm repair, whether EVAR or open repair. In patients in whom AKI developed after either procedure, the rise in urinary L-FABP occurred earlier than the rise in SCr. Ueta et al. reported AKI-associated increases in SCr, neutrophil gelatinase-associated lipocalin (NGAL), and L-FABP 2–6 h after EVAR [23]. They also found urinary Cr-corrected NGAL (NGAL/Cr) to be the best predictive marker for AKI [23]. Because AKI occurred only in their thoracic EVAR (TEVAR) group, the TEVAR procedure did not appear to affect renal blood flow differently from the EVAR procedure. Because our study used EVAR, we postulated that the cutoff urinary L-FABP level would be lower in the Ueta et al. study than in our study. Mori et al. observed that low levels of urinary L-FABP during hypothermic surgery for thoracic aortic aneurysm repair preceded the development of AKI [24]. Although urinary L-FABP was 62.1 ng/mg Cr in their AKI group, it was 1130 ng/mg Cr in their non-AKI group after termination of deep hypothermic circulatory arrest. They proposed that urinary L-FABP plays a role in kidney protection [24]. However, while urinary L-FABP levels were higher in their non-AKI group than in their AKI group, levels ranged more widely in the latter group. In addition, if Mori et al. had measured urinary L-FABP at several time points after surgery rather than only before and after cardiopulmonary bypass, their study results may have been different. Recently, Parr et al. reported that urinary L-FABP, but not urinary NGAL or urinary kidney-injury-molecule 1 (KIM-1), predicted poor AKI outcomes [25]. Our finding that urinary L-FABP increases earlier than SCr after AKI agrees with this latter report [25].
Hypoxic events, such as ischemic–reperfusion injury, cause the release of L-FABP from proximal tubular epithelial cells, correlating with the severity of renal injury; hence, urinary L-FABP level increases immediately after tubular damage. Because of low reuptake, L-FABP from the proximal renal tubules is the main source of urinary L-FABP; serum L-FABP does not increase after injury [26, 27]. Furthermore, Nakamura et al. demonstrated that serum L-FABP levels do not reflect urinary L-FABP levels in patients with sepsis [27].
Mori et al. reported that the difference in the patterns of urinary NGAL increase and urinary L-FABP increase after cardiac surgery results from differences in the mechanism of urinary secretion [28]. NGAL is filtered by glomeruli and reabsorbed by proximal tubules, with only 0.1–0.2 % remaining in the urine [28]. In the AKI setting, various stresses increase NGAL in the circulation through neutrophil activation, and the increased amount of NGAL is filtered in glomeruli. Some NGAL molecules are reabsorbed by the damaged proximal tubules, whereas others are excreted. Therefore, increased urinary NGAL is due mainly to impaired renal reabsorption, [29], and it takes longer for the NGAL levels than for urinary L-FABP levels to increase.
Furthermore, in patients with urinary tract infection (UTI), median urinary angiotensinogen (AGT) levels were significantly increased, but urinary proteins NGAL, L-FABP, N-acetyl-beta-D glucosaminidase (NAG) beta 2-microglobulin (BMG), serum AGT, and creatinine levels did not differ significantly between groups [30]. This report showed that urinary L-FABP did not increase in UTIs. To the contrary, urinary NGAL was significantly increased in patients with UTI compared with that in healthy controls. Increased urinary NGAL indicates the presence of inflammatory processes in the urinary tract of adults [31] and is not a specific biomarker of AKI.
Urinary L-FABP increased earlier in our patients who underwent open repair than in those who underwent EVAR. This difference can be attributed to differences in renal tubular insult associated with EVAR and open repair. Abdominal AXC in open repair decreases and causes a shift in renal blood flow [3, 4], which may in turn cause a significant reduction in proximal tubular blood flow and lead to tubular epithelial cell hypoxia. Such hypoxia promotes L-FABP secretion into the urine. The decrease in renal blood flow and shift in the distribution of intrarenal blood flow occurs quickly after AXC. Proximal tubular ischemic–reperfusion injury causes AKI during the period of open repair. AXC decreases renal blood flow, and renal ischemia induces endothelial dysfunction and decreases production of vasodilatory substances such as nitric oxide. After ischemia, blood flow decreased to 60 % of preischemic levels in superficial cortex and to 16 % in the outer medulla, but it increased to 125 % of control values in the inner medulla [5]. This decrease in blood flow to the outer medulla diminishes oxygen and nutrient delivery to tubules in this region, thus increasing the risk of cell injury [5]. Contrast-induced nephropathy (CIN) is the main cause of AKI in the context of EVAR [32]. Itoh et al. reported that contrast medium causes apoptosis of renal tubular cells [33]; Geenen et al. speculated that contrast medium led to tubular necrosis in CIN [13].
In addition, the EVAR procedure may induce thrombosis and embolism, leading to AKI. Urinary L-FABP increased earlier in our patients who underwent open repair than in patients who underwent EVAR. Furthermore, the increase in urinary L-FABP was less with EVAR than with open repair, indicating that the degree of injury in EVAR was less. The hemodynamic change seems to be greater during open repair than during EVAR, and this change causes further deterioration of tubular blood flow and leads to severe renal damage. The increase in urinary L-FABP in open repair involving suprarenal AXC was greater than that in open repair involving infrarenal AXC. This difference indicates that suprarenal AXC causes more severe renal damage than that caused by infrarenal AXC.
Results of our study should be considered in light of its limitations. For instance, the observed AKI was not severe in most cases; it was stage 2 in all but one patient. Moreover, the elevation in urinary L-FABP was transient; it did not persist after surgery, leaving some doubt as to the degree of kidney injury that had occurred. We also did not take into account any differences in anesthesia. Patients who underwent open repair received sevoflurane with remifentanil and epidural anesthesia; patients who underwent EVAR received sevoflurane with remifentanil but not epidural anesthesia. Furthermore, various vasoactive drugs were administered during open repair but not during EVAR. It is possible that either of the anesthesia methods affected the renal circulation. However, Peyton et al. [34] reported no significant effect of epidural anesthesia on renal complications after major abdominal surgery. Therefore, we believe that our anesthesia methods had little, if any, effect on our study outcomes.
In conclusion, our study highlights urinary L-FABP as a sensitive biomarker of AKI in patients treated with abdominal aortic aneurysm repair. Preoperative urinary L-FABP can predict postoperative AKI, especially in patients treated with EVAR. Urinary L-FABP at 2 h post-AXC can predict postoperative AKI, in patients treated with open repair. In light of these results, we can expect perioperative monitoring of urinary L-FABP to become standard practice for AKI detection in patients treated with abdominal aortic aneurysm repair.
Acknowledgments
We would like to thank Prof. Takeshi Miyairi, Prof. Hiroshi Nishimaki, and Dr. Takashi Yasuda for their help in conducting the study. We thank Dr. Takahiko Ueno for assistance with the statistical analyses and Prof. Soichiro Inoue, Department of Anesthesiology at St. Marianna University School of Medicine, for his helpful advice. We also thank Ms. Tina Tajima for her help in reviewing and editing the manuscript.
Compliance with ethical standards
Conflict of interest
None
Contributor Information
Yumi Obata, Email: y2obata@marianna-u.ac.jp.
Atsuko Kamijo-Ikemori, Email: a2kamijo@marianna-u.ac.jp.
Daisuke Ichikawa, Email: ichikawa6008@gmail.com.
Takeshi Sugaya, Email: takeshi-sugaya@marianna-u.ac.jp.
Kenjiro Kimura, Email: kimura@marianna-u.ac.jp.
Yugo Shibagaki, Email: shibagaki@marianna-u.ac.jp.
Takeshi Tateda, Phone: 81-44-977-8111, Email: t2tateda@marianna-u.ac.jp.
References
- 1.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.ASN.0000130340.93930.DD. [DOI] [PubMed] [Google Scholar]
- 2.Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, Campbell DA., Jr Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110:505–515. doi: 10.1097/ALN.0b013e3181979440. [DOI] [PubMed] [Google Scholar]
- 3.Gamulin Z, Forster A, Morel D, Simonet F, Aymon E, Favre H. Effects of infrarenal aortic cross-clamping on renal hemodynamics in humans. Anesthesiology. 1984;61:394–399. doi: 10.1097/00000542-198410000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Colson P, Ribstein J, Séguin JR, Marty-Ane C, Roquefeuil B. Mechanisms of renal hemodynamic impairment during infrarenal aortic cross-clamping. Anesth Analg. 1992;75:18–23. doi: 10.1213/00000539-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 6.Parmer SS, Fairman RM, Karmacharya J, Carpenter JP, Velazquez OC, Woo EY. A comparison of renal function between open and endovascular aneurysm repair in patients with baseline chronic renal insufficiency. J Vasc Surg. 2006;44:706–711. doi: 10.1016/j.jvs.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 7.Gawenda M, Brunkwall J. Renal response to open and endovascular repair of abdominal aortic aneurysm: a prospective study. Ann Vasc Surg. 2008;22:1–4. doi: 10.1016/j.avsg.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Brown LC, Brown EA, Greenhalgh RM, Powell JT, Thompson SG. UK EVAR, trial participants. Renal function and abdominal aortic aneurysm (AAA): the impact of different management strategies on long-term renal function in the UK Endovascular Aneurysm Repair (EVAR) Trials. Ann Surg. 2010;251:966–975. doi: 10.1097/SLA.0b013e3181d9767c. [DOI] [PubMed] [Google Scholar]
- 9.Chronopoulos A, Rosner MH, Cruz DN, Ronco C. Acute kidney injury in the elderly: a review. Contrib Nephrol. 2010;165:315–321. doi: 10.1159/000313772. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liss P, Nygren A, Erikson U, Ulfendahl HR. Injection of low and iso-osmolar contrast medium decreases oxygen tension in the renal medulla. Kidney Int. 1998;53:698–702. doi: 10.1046/j.1523-1755.1998.00811.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen HS, Morcos SK, Barrett BJ. Contrast-induced nephropathy: the wheel has turned 360°. Acta Radiol. 2008;49:646–657. doi: 10.1080/02841850801995413. [DOI] [PubMed] [Google Scholar]
- 13.Geenen RW, Kingma HJ, van der Molen AJ. Contrast-induced nephropathy: pharmacology, pathophysiology and prevention. Insights Imaging. 2013;4:811–820. doi: 10.1007/s13244-013-0291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, Star RA. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grootendorst DC, Michels WM, Richardson JD, Jager KJ, Boeschoten EW, Dekker FW. Krediet RT; NECOSAD Study Group. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant. 2011;26:1932–1937. doi: 10.1093/ndt/gfq667. [DOI] [PubMed] [Google Scholar]
- 16.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 17.Matsui K, Kamijo-Ikemori A, Sugaya T, Yasuda T, Kimura K. Usefulness of urinary biomarkers in early detection of acute kidney injury after cardiac surgery in adults. Circ J. 2012;76:213–220. doi: 10.1253/circj.CJ-11-0342. [DOI] [PubMed] [Google Scholar]
- 18.Kamijo-Ikemori A, Sugaya T, Kimura K. Urinary fatty acid binding protein in renal disease. Clin Chim Acta. 2006;374:1–7. doi: 10.1016/j.cca.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Sugaya T, Node K, Ueda Y, Koide H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am J Kidney Dis. 2006;47:439–444. doi: 10.1053/j.ajkd.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Ueta K, Watanabe M, Iguchi N, Uchiyama A, Shirakawa Y, Kuratani T, Sawa Y, Fujino Y. Early prediction of acute kidney injury biomarkers after endovascular stent graft repair of aortic aneurysm: a prospective observational study. J Intensive Care. 2014;2:45. doi: 10.1186/s40560-014-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori Y, Sato N, Kobayashi Y, Ochiai R. Low levels of urinary liver-type fatty acid-binding protein may indicate a lack of kidney protection during aortic arch surgery requiring hypothermic circulatory arrest. J Clin Anesth. 2014;26:118–124. doi: 10.1016/j.jclinane.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Parr SK, Clark AJ, Bian A, Shintani AK, Wickersham NE, Ware LB, Ikizler TA, Siew ED. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2015;87:640–648. doi: 10.1038/ki.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto T, Noiri E, Ono Y. Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid-binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Sugaya T, Koide H. Urinary liver-type fatty acid-binding protein in septic shock: effect of polymyxin B-immobilized fiber hemoperfusion. Shock. 2009;31:454–459. doi: 10.1097/SHK.0b013e3181891131. [DOI] [PubMed] [Google Scholar]
- 28.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Yi Li J, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, Ebihara K, Omata M, Sotoh N, Sugawara A, Barasch J, Nakao K. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–294. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 30.Kitao T, Kimata T, Yamanouchi S, Kato S, Tsuji S, Kaneko K. Urinary biomarkers for screening for renal scarring in children with febrile urinary tract infection: pilot study. J Urol. 2015;15:766–771. doi: 10.1016/j.juro.2015.04.091. [DOI] [PubMed] [Google Scholar]
- 31.Urbschat A, Obermüller N, Paulus P, Reissig M, Hadji P, Hofmann R, Geiger H, Gauer S. Upper and lower urinary tract infections can be detected early but not be discriminated by urinary NGAL in adults. Int Urol Nephrol. 2014;46:2243–2249. doi: 10.1007/s11255-014-0831-x. [DOI] [PubMed] [Google Scholar]
- 32.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 33.Itoh Y, Yano T, Sendo T, Sueyasu M, Hirano K, Kanaide H, Oishi R. Involvement of de novo ceramide synthesis in radiocontrast-induced renal tubular cell injury. Kidney Int. 2006;69:288–297. doi: 10.1038/sj.ki.5000057. [DOI] [PubMed] [Google Scholar]
- 34.Peyton PJ, Myles PS, Silbert BS, Rigg JA, Jamrozik K, Parsons R. Perioperative epidural analgesia and outcome after major abdominal surgery in high-risk patients. Anesth Analg. 2003;96:548–554. doi: 10.1097/00000539-200302000-00046. [DOI] [PubMed] [Google Scholar]