Highlights
-
•
Median craniocaudal extension of uterine cervical cancer on MRI is systematically slightly smaller compared to histopathology (2.1 vs. 2.5 cm). Pearson’s correlation: 0.83 (p < 0.001).
-
•
Regarding the craniocaudal extension on MRI, 91% (19/21) of the extension would have been covered by a clinical uncertainty margin of 1.2 cm.
-
•
Comparison of tumour measurements on MRI and surgical specimen is hampered by organ deformation. However, this methodological limitation might be solved by use of deformable image registration.
Abbreviations: CI, confidence interval; CRF, case record form; CTV, clinical target volume; EBRT, external beam radiation therapy; ESTRO, European society of therapeutic radiology and oncology; FIGO, international federation of gynaecology and obstetrics; ICC, intraclass correlation coefficient; JCOG, Japan clinical oncology group; LLETZ, large loop excision of the transformation zone; MRI, magnetic resonance imaging; NCIC, national cancer institute of Canada; PTV, planning target volume; RT, radiation therapy; SD, standard deviation; VMAT, volumetric modulated arc therapy
Keywords: Uterine cervical cancer, MRI, Accuracy, Craniocaudal, Extension
Abstract
Purpose
To assess the reliability of magnetic resonance imaging (MRI) for evaluation of craniocaudal tumour extension by comparing the craniocaudal tumour extension on the pre-operative MRI and post-operative hysterectomy specimen in patients with early stage uterine cervical cancer.
Materials and methods
After approval of the institutional review board was acquired, pre-operative MRI and hysterectomy specimen of 21 women with early stage cervical cancer were re-evaluated. The craniocaudal extension on MRI was measured separately by two experienced radiologists and compared with corresponding measurements from the hysterectomy specimen, which were re-evaluated by an experienced pathologist.
Results
Median craniocaudal extension of uterine cervical cancer on MRI was slightly smaller compared to histopathology (2.1 cm vs. 2.5 cm). The median underestimation was 0.4 cm (range −0.6 cm to 2.2 cm, mean 0.4 cm, standard deviation (SD) ±0.7 cm); Pearson’s correlation was 0.83 (p < 0.001). In two patients (9%) MRI underestimated tumour craniocaudal extension by more than 1.8 cm.
Conclusion
MRI represents the histopathological craniocaudal tumour extension in the majority of patients with early stage uterine cervical cancer, but with a systematic small underestimation of the real craniocaudal tumour extension.
1. Introduction
In patients with uterine cervical cancer, early recognition of involvement into and beyond the uterine internal os is pivotal for treatment decision-making in both surgical gynaecology and radiation therapy (RT). For instance, assessment of craniocaudal extension is crucial to assess the feasibility and safety of fertility-sparing surgery, in young women with early-stage uterine cervical cancer [1], [2]. Possible extension of cervical cancer into the uterine body is a well-known uncertainty in cancer staging and treatment of locally advanced uterine cervical cancer treated with (chemo) radiation [International Federation of Gynaecology and Obstetrics (FIGO) stage IIB–IVA] [3], [4]. Most RT guidelines for cervical cancer recommend inclusion of the whole uterus in the clinical target volume (CTV); this is mainly because, in the past, craniocaudal uterine invasion could not be excluded by physical examination or computed tomography [4], [5].
However, inclusion of the whole uterine corpus into the CTV, plus a large uncertainty margin for position uncertainty, usually results in large planning target volumes (PTV) for external beam RT (EBRT). Large radiation volumes are responsible for the substantial risk of late complications, such as radiation enteritis, proctitis and cystitis, as well as fistulae and sexual dysfunction, all of which are important constraining factors for dose escalation [6], [7], [8]. Therefore, it remains debatable whether the whole uterine body should be included in the CTV, both in EBRT and in brachytherapy, as recommended in international guidelines [9], [10], [11], [12]. Although in 2010, 16 representatives from the National Cancer Institute of Canada (NCIC), the Japan Clinical Oncology Group (JCOG), and the European Society of Therapeutic Radiology and Oncology (ESTRO) agreed that the complete uterus should be included in the CTV, 42% of the expert respondents felt that it was not always necessary [5]. With the ever-increasing precision of RT techniques, such as volumetric modulated arc therapy (VMAT) and magnetic resonance imaging (MRI)-guided brachytherapy, reconciliation of expert recommendations urgently requires more reliable imaging to determine tumour involvement in surrounding tissues.
Assessment of the craniocaudal tumour extension is required, in particular, for safe reduction of the radiation volume in inoperable patients, for which MRI could be used in radiation practice as it increased the visual identification of pelvic tumours and tumour extension [13], [14], [15], [16], [17]. However, very few studies have investigated the reliability of tumour extension assessed on MRI compared to histopathology [18].
Histopathological verification of MRI tumour extension is of course only possible in patients who can be operated. Therefore, the present ‘radiotherapy’ study assesses the reliability of MRI for evaluation of uterine tumour extension by comparing the craniocaudal tumour extension on the pre-operative MRI and post-operative hysterectomy specimen, in patients with early stage uterine cervical cancer who had surgery alone.
2. Materials and methods
2.1. Study design
This study retrospectively compared craniocaudal extension on the pre-operative MRI with (histo) pathology of the surgical specimen in patients who had a radical hysterectomy for early stage cervical cancer. Before collecting data, approval of the institutional review board was acquired; the board decided that informed consent was not obligatory.
2.2. Patients
For all women who had a radical hysterectomy for early stage cervical cancer between May 2012 and February 2013, the MRI and histopathological records were analysed. None of the patients underwent neoadjuvant chemotherapy or radiation therapy. The present study is complementary to a technical paper wherein we used the MRIs and surgical specimens of nine of the present patients to illustrate a mathematical model for deformable registration [19]. There is no double publication of measurements and outcome reporting. MRI techniques had to be comparable: i.e. patients were excluded when MRI was performed elsewhere with different MR sequences, or if imaging quality was appraised by experienced radiologists to be insufficient (discussed in detail below). Furthermore, patients were excluded if there was no residual tumour, e.g. after prior large loop excision of the transformation zone (LLETZ) on either MRI or histopathological examination of the surgical specimen. Related characteristics were registered: age, FIGO stage, time between MRI and histopathology, histopathological type, tumour location in relation to the uterine cervix, and previous surgery of the uterine cervix.
2.3. Histopathology
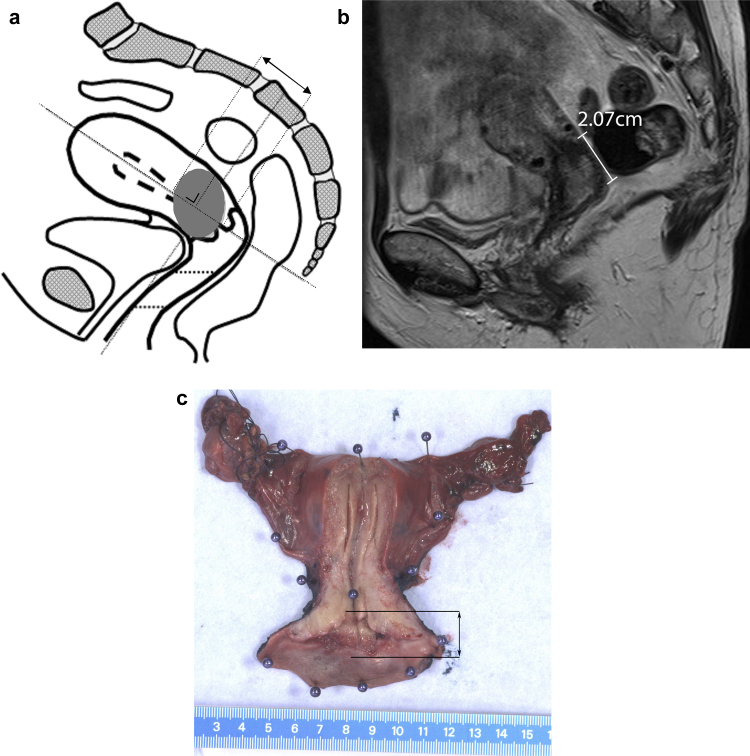
According to our hospital protocol, the pathologist made a standard ventral median incision to expose the intrauterine cavity. Then, a photograph was taken of the plane of incision with a ruler placed beside. A craniocaudal tumour extension was measured on the plane of incision in craniocaudal direction. The recorded craniocaudal diameter was primarily derived from the macroscopy report and verified by the photograph of the specimen and, if the cervix was embedded completely, from the microscopy report (Fig. 1c). Evaluation was performed by a pathologist specialized in gynaecological tumours and with 5 years related experience.
Fig. 1.
(A) Craniocaudal tumour extension of the primary tumour measured parallel to the endocervical channel in sagittal plane. (B) Example of measuring craniocaudal tumour extension in a 51-year-old woman with FIGO stage IB1 uterine cervical cancer in sagittal plane on MRI and on pathology (C). The two-headed arrow indicates the cranial and caudal extension.
2.4. MRI procedure
All MRIs acquired between May 2012 and February 2013 were part of standard preoperative staging and were performed according to a standard protocol on a 1.5 Tesla unit (Siemens Avanto, Erlangen, Germany) using a phased array coil. Sequences consisted of sagittal, axial oblique and coronal oblique fast T2-weighted turbo spin-echo (repetition time/echo time 2500/70 ms), field of view 300 × 300 mm, acquisition matrix 512 × 384 and a 4 mm slice thickness. The standard protocol also included an axial diffusion-weighted sequence. Coronal and axial T2-weighted scans were angulated orthogonal and perpendicular to the endocervical canal to reduce partial volume effects. No bowel relaxants (such as butylscopolamine) were routinely administered.
2.5. Evaluation of MRI
Before evaluation, a case record form (CRF) was developed including instructions on how to perform the measurements, based on consensus between the two radiologists and the investigator, with specific attention paid to how craniocaudal measurements were spatially made in histopathology (Fig. 1a). To avoid bias from a learning curve, the CRFs were tested, and adjusted, in 5 patients; these patients were not included in this study. The T2-weighted images were evaluated independently by two radiologists with 19 and 17 years of experience, respectively, in assessing MRI of the pelvic region. The craniocaudal extension on MRI was measured for each patient on the T2 turbo spin-echo sagittal MRI; diffusion weighted images could be used for tumour assessment. The greatest craniocaudal extension parallel to the cervical/uterine internal canal was measured on the MRI and the mean measurement between both radiologists was calculated. MRI measurements were performed on the sagittal sequence corresponding to the sagittal plane in which the specimen is evaluated on histopathology. The radiologists had knowledge of the patient’s medical history up until the time MRI was performed and were blinded to the histopathology findings (Fig. 1b). Image quality (good, acceptable, unacceptable) was registered. If the image quality was scored as ‘acceptable’ or ‘unacceptable’, the suboptimal condition was specified (i.e. due to angulation, movement artefacts, etc.). In addition, the position of the tumour in relation to the cervix (central, dorsal, ventral, left, right) was determined.
2.6. Correlation between radiologists
The intraclass correlation coefficient (ICC) of craniocaudal tumour extension measurements between the observers was calculated. The ICC is interpreted in the following way: 0 = poor agreement; 0–0.20 = minor agreement; 0.21–0.40 fair agreement; 0.41–0.60 moderate agreement; 0.61–0.80 major agreement; and 0.81–1.00 almost perfect agreement [20].
2.7. Comparison between MRI and (histo) pathology
The craniocaudal MRI measurement of tumour extension was compared to the histopathology measurement of craniocaudal tumour extension. The difference in measured craniocaudal extension between histopathology and MRI was presented in a Bland–Altman plot to show the agreement between the two modalities. If this difference was ≥1.0 cm a radiation oncologist and the pathologist discussed possible causes separately. Descriptive statistics were performed and Pearson’s correlation was calculated of the measured craniocaudal extension in MRI and histopathology. To detect possible correlations between the difference in measured craniocaudal extension and baseline characteristics (age, time between MRI and histopathology, histopathological type, tumour location in relation to the uterine cervix, and previous surgery of the uterine cervix), univariate analysis of variance was calculated. Data were analysed using IBM SPSS Statistics version 21.0.0.1 for Windows.
3. Results
3.1. Patients
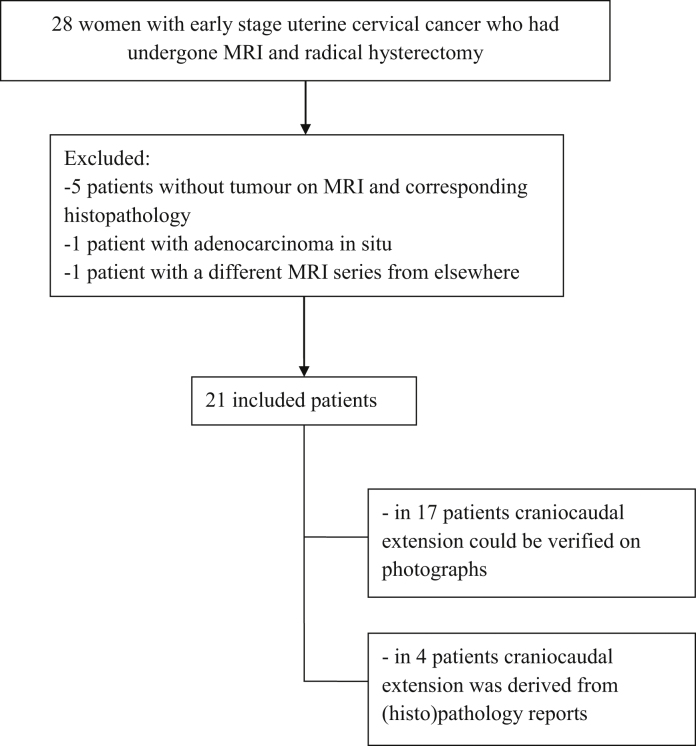
Between May 2012 and February 2013, 28 patients with early stage uterine cervical cancer underwent radical hysterectomy. Of these, six were excluded because no residual tumour was identified on MRI and corresponding histopathology after irradical LLETZ or conisation. One patient with MRI from another hospital was excluded because of substantial differences compared with our MRI sequences. Finally, 21 patient records were available for further analysis (Fig. 2).
Fig. 2.
Flow diagram of patient inclusion.
3.2. Related baseline characteristics
Table 1 summarizes the baseline characteristics. The median age was 50 (range 30–68, standard deviation (SD) 9.5) years; 17 (81%) of the patients had FIGO stage IB1 and four (19%) patients had another tumour stage. Three patients (14%) had a conisation before MRI and three patients (14%) had a LLETZ before MRI. One patient (5%) had abdominal radical trachelectomy instead of a radical hysterectomy. The location of the tumour in relation to the uterine cervix was assessed on MRI: in 11 patients (52%) tumours were centrally located, and in two patients (10%) no (residual) tumour could be recognised on MRI. In the remaining 8 patients (38%) tumours were located more to one side of the cervix. The median time between MRI and surgery was 28 (range 14–44, SD 8) days. In 16 patients (76%) a squamous cell carcinoma was found and the remaining five patients (24%) had adenocarcinoma. For the 21 included patients, the two radiologists assessed the quality of MRI as ‘good’ in 12 and 14 patients, respectively, and acceptable in 9 and 7 patients, respectively; the slightly lower quality was attributed to the presence of movement artefacts or suboptimal sequence angulation.
Table 1.
Related baseline characteristics.
| Related baseline characteristics | |
|---|---|
| Parameter | No. of patients (n = 21) |
| Age in years: median (range) | 50 (30–68) |
| FIGO stage: | |
| IA | 1 (5) |
| IB1 | 17 (81) |
| IB2 | 2 (9) |
| IIA | 1 (5) |
| Pre-treatment: | |
| Biopsy only | 10 (48) |
| LLETZ | 5 (24) |
| Conisation | 6 (28) |
| Tumour location in relation to the uterine cervix on MRI: | |
| Central | 10 (48) |
| Ventral | 4 (20) |
| Dorsal | 2 (9) |
| Left | 2 (9) |
| Right | 1 (5) |
| No visible tumour | 2 (9) |
| Time between MRI and hysterectomy: median days (range) | 28 (14–44) |
| Histopathological type: | |
| Squamous cell carcinoma | 16 (76) |
| Adenocarcinoma | 5 (24) |
Note: Unless otherwise indicated, numbers in parentheses are percentages.
FIGO—International Federation of Gynaecology and Obstetrics, LLETZ—Large Loop Excision of the Transformation Zone, MRI—Magnetic Resonance Imaging.
3.3. Histopathology
For all 21 patients, the craniocaudal extension could be extracted from the pathology reports. In 14 of these patients the craniocaudal extension could be reassessed based on photographs of the specimens. In six patients the photographs were taken after formalin fixation, and in eight patients the photographs showed fresh specimens. In two patients, the craniocaudal extensions needed to be reassessed by microscopic revision of the slices due to uncertainties in the pathology report. The median craniocaudal extension of the tumour was 2.5 (range 0.5–4.4, SD 1.2) cm.
3.4. MRI
For both radiologists, on MRI the median craniocaudal extension of the tumour was 2.1 cm (range 0–3.5 cm and 0–4.9 cm respectively; SD 1.2 cm and 1.4 cm, respectively). The combined data from the two radiologists resulted in the following averages per measurement: range 0–4.2 (median 2.1, SD 1.3) cm.
3.5. Correlation between radiologists
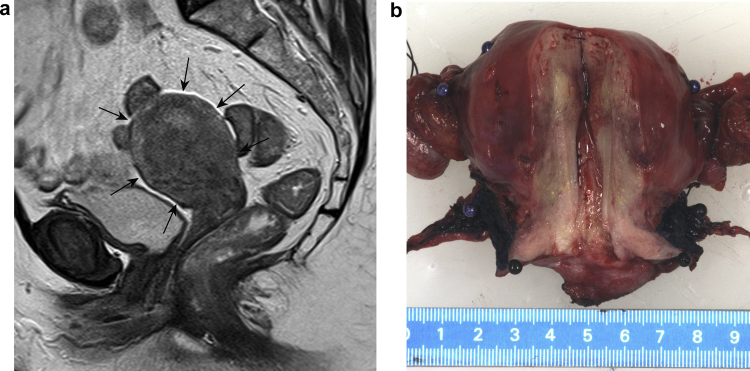
The mean difference in craniocaudal tumour extension between the radiologists was 0.1 (range −1.4 to 1.5, SD 0.6) cm. The ICC between the two radiologists was 0.94 with a 95% confidence interval (CI) of 0.85–0.98. In four cases there was a disagreement of ≥1.0 cm. In two of these latter patients, because MRI was performed after conisation or LLETZ, the tumour extension and surgical scar could not be well distinguished, leading to differences in measurement of the craniocaudal tumour extension between the radiologists. In one patient, the first radiologist found a tumour in the anterior side of the cervix only, but the second radiologist found that the endocervical canal also contained a tumour. Both radiologists found the MRI quality to be adequate but, in hindsight, the interobserver difference of 1.2 cm could be attributed to a suboptimal sequence angulation, a situation which also occurs in common practice. In the latter case, tumour extension into the uterus was seen on the MRI, but the boundaries could hardly be discriminated on either MRI or macroscopy; microscopically the tumour was a diffusely growing vaso-invasive squamous cell carcinoma infiltrating the whole uterus (Fig. 3). In two patients no (residual) tumour could be recognized on MRI by either of the radiologists.
Fig. 3.
(A) Due to diffuse vaso-invasion, the craniocaudal extension of uterine cervical cancer (arrows) could not be properly measured in sagittal plane on MRI (B) or on macroscopic images.
3.6. Comparison of MRI and histopathology
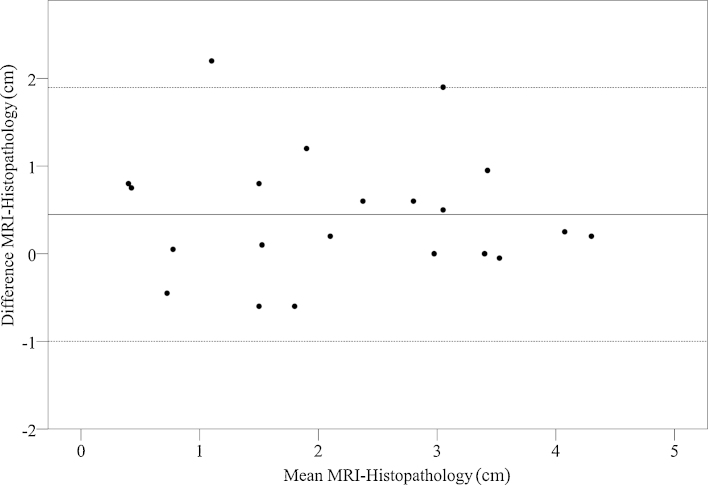
Fig. 4 shows the differences between histopathology vs. MRI in a Bland–Altman plot; the differences were smaller in the larger tumours. MRI gives a median underestimation of histopathological extension of 0.2 (mean 0.4, range −0.6 to 2.2, mean SD 0.7) cm; Pearson’s correlation 0.83 (p < 0.001).
Fig. 4.
Bland–Altman plot for craniocaudal extension measured on MRI and histopathology. The dotted lines show a 95% confidence interval (−1.0 to1.9 cm). It can be seen that MRI has a systematic error of −0.4 cm or, in other words, MRI slightly underestimates histopathology by (on average) 0.4 cm. The difference between MRI and histopathology seems to get smaller with larger tumours. There were two outlying cases (see Section 3).
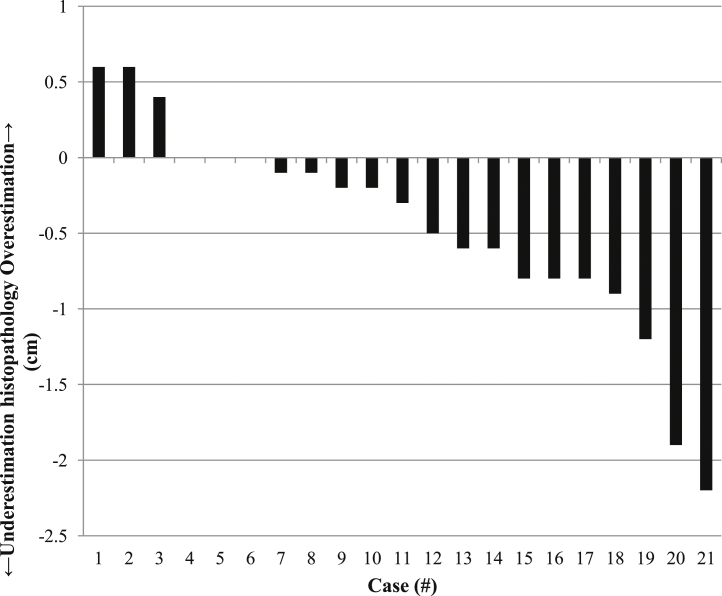
In three patients the craniocaudal tumour extension was underestimated on MRI by ≥1 cm (Fig. 4, Fig. 5). In one patient the tumour signal intensity on T2-weighted and diffusion-weighted images resembled endocervical tissue; this tumour extension was not recognized by either of the radiologists and was recorded as 0.0 cm. However, on (histo) pathological examination there was an evident macroscopic tumour with a craniocaudal extension of 2.2 cm. Further examination by a radiation oncologist, and the pathologist involved in the other two cases, showed that the spatial shape of the tumour differed substantially between MRI and histopathology. This was probably caused by post-operative change in tumour shape of the surgical specimen (Fig. 6). This may also play a role in the smaller differences in craniocaudal extensions between MRI and histopathology.
Fig. 5.
Estimation of craniocaudal tumour extension by MRI compared to (histo) pathology as reference standard. For instance, in patient 21, MRI underestimates histopathology by 2.2 cm.
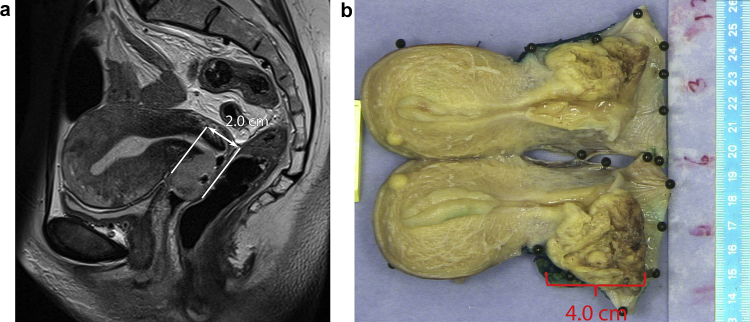
Fig. 6.
Images from a 45-year old woman with FIGO stage IB uterine cervical cancer. On MRI the craniocaudal tumour extension measured in sagittal plane parallel to the endocervical channel is 1.9 cm (A). Despite measurements in exactly the same sagittal plane both on MRI and pathology, the spatial shape of the tumour is substantially altered at pathology and a craniocaudal tumour extension of 4.0 cm is measured (B). Note that on both images the tumour is very well distinguishable from normal tissue.
Univariate analysis of variance of the differences between MRI and histopathology showed no significant influence of patients’ baseline clinical characteristics (age, time between MRI and histopathology, histopathological type, tumour location in relation to the uterine cervix and previous surgery of the uterine cervix).
4. Discussion
The reliability of MRI for craniocaudal extension of cervical cancer was assessed by comparing the pre-operative MRI with the surgical specimen in patients who had undergone hysterectomy for early stage cervical cancer. The study shows that MRI slightly underestimated the mean craniocaudal extension of uterine cervical cancer extension compared with histopathology. Craniocaudal tumour extension is particularly important for determining the feasibility of organ-sparing surgery of early cervical cancer, and for potential sparing of the uterine corpus in RT.
Earlier retrospective analyses showed the presence/absence of uterine internal os involvement on MRI with a promising specificity (93–98%) and sensitivity (86–100%) [21], [22], [23]. Furthermore, a maximal underestimation of 15 mm was reported when measuring maximal tumour diameter of uterine cervical cancer on MRI compared to histopathology [24]. However, these latter studies were designed to determine the largest tumour dimension irrespective of the orientation and not specifically to measure craniocaudal tumour extension. Secondly, although histopathology is usually considered the gold standard, it is questionable whether (and to what extent) changes in the shape of the surgical specimen after hysterectomy and during processing may have affected maximum tumour diameter. However, we aimed to limit these uncertainties by systematically measuring craniocaudal tumour extension parallel to the uterine internal canal in the same spatial plane on both MRI and histopathology.
The current RT treatment planning guidelines for uterine cervical cancer recommend irradiation of the whole uterus but with the advent of high-precision RT techniques, such as VMAT and image-guided RT, it is debatable whether uninvolved parts of the uterine corpus can be safely excluded to reduce toxicity to the bladder and intestines [3], [5], [25], [26]. In addition, radiobiological rules support the treatment of microscopic disease using lower doses than for macroscopic disease; ultimately, however, the efficacy of that idea can only be proven based on clinical trials [27]. Sanuki et al. found that if cervical tumours were ≤2 cm, then no cases of uterine invasion exceeded ≥1/3 of the uterine body; the authors suggested that, if these patients had received RT, it would not have been necessary to irradiate the uterine fundus with brachytherapy [25]. Based on a similar rationale, more normal intestine and bladder would be spared by excluding the uterine fundus during external beam elective pelvic RT, and by including the fundus ‘electively’ during brachytherapy.
In the present study, in 19 patients (91%) craniocaudal extension would have been covered by a clinical uncertainty margin of 1.2 cm (including a mean underestimation of MRI of 0.4 cm) and all patients with a margin of 2.2 cm, despite spatial uncertainties. In one patient, tumour extension could not be recognized; in this case the tumour was highly symmetrical and the MRI signal resembled endocervical cavity tissue. To our knowledge no similar cases have been reported and this might be very rare in a cervical tumour with a diameter of 2.2 cm.
A limitation of the present study is the small number of patients and its retrospective design. A more fundamental question is whether the results from surgical patients with early stage cervical cancer can be safely extrapolated to RT patients with inoperable advanced stage tumours. However, as it is impossible to make a direct comparison of MRI and histopathology in large tumours, at the moment this is the best we can do. Also, this study emphasizes that, on pathology, the shape of the uterus in vivo can differ from that ex vivo; this limitation is inherent to all studies on this topic. This problem could partially be solved by deformable image registration and digital correction of deformations between MRI and histopathology [28], [29]. Moreover, these uncertainties can be further reduced by embedding of the whole hysterectomy specimen and 3D reconstruction of multiple cross-sections [29]. In the present study, in those cases where craniocaudal tumour extension differed ≥1.0 cm between MRI and histopathology, we show clear examples of perfectly distinguishable tumours on MRI and histopathology which differ in measured size due to in vivo/ex vivo deformations (Fig. 6). Therefore, ex vivo post-surgical tissue deformation plays an important role in all these measurements.
5. Conclusions
In the present study, MRI represents the histopathological craniocaudal tumour extension in the majority of patients with cervical cancer, but with a systematic small underestimation. The next step is to perform prospective validation of MRI while ruling out more spatial uncertainties.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Peter de Boer, Email: p.deboer@amc.uva.nl.
Maaike C.G. Bleeker, Email: m.c.bleeker@amc.uva.nl.
Anje M. Spijkerboer, Email: a.mSpijkerboer@amc.uva.nl.
Agustinus J.A.J. van de Schoot, Email: a.j.schootvande@amc.uva.nl.
Shandra Bipat, Email: s.bipat@amc.uva.nl.
Marrije R. Buist, Email: m.r.buist@amc.uva.nl.
Coen R.N. Rasch, Email: c.r.rasch@amc.uva.nl.
Jaap Stoker, Email: j.stoker@amc.uva.nl.
Lukas J.A. Stalpers, Email: l.stalpers@amc.uva.nl.
References
- 1.Plante M., Gregoire J., Renaud M.C., Roy M. The vaginal radical trachelectomy: an update of a series of 125 cases and 106 pregnancies. Gynecol. Oncol. 2011;121:290–297. doi: 10.1016/j.ygyno.2010.12.345. [DOI] [PubMed] [Google Scholar]
- 2.Rob L., Skapa P., Robova H. Fertility-sparing surgery in patients with cervical cancer. Lancet Oncol. 2011;12:192–200. doi: 10.1016/S1470-2045(10)70084-X. [DOI] [PubMed] [Google Scholar]
- 3.Tanderup K., Georg D., Pötter R., Kirisits C., Grau C., Lindegaard J.C. Adaptive management of cervical cancer radiotherapy. Semin. Radiat. Oncol. 2010;20:121–129. doi: 10.1016/j.semradonc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Rutledge F.N., Wharton J.T. Surgical procedures associated with radiation therapy for cervical cancer. In: Fletcher G.H., editor. Textbook of Radiotherapy. 2nd ed. Lea & Febiger; Philadelphia, Pa: 1973. pp. 705–719. [Google Scholar]
- 5.Lim K., Small W., Portelance L. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:348–355. doi: 10.1016/j.ijrobp.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 6.Bipat S., van den Berg a R., van der Velden J., Stoker J., Spijkerboer A.M. The role of magnetic resonance imaging in determining the proximal extension of early stage cervical cancer to the internal os. Eur. J. Radiol. 2011;78:60–64. doi: 10.1016/j.ejrad.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Groenendaal G., Moman M.R., Korporaal J.G. Validation of functional imaging with pathology for tumor delineation in the prostate. Radiother. Oncol. 2010;94:145–150. doi: 10.1016/j.radonc.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 8.Georg P., Boni A., Ghabuous A. Time course of late rectal- and urinary bladder side effects after MRI-guided adaptive brachytherapy for cervical cancer. Strahlenther. Onkol. 2013;189:535–540. doi: 10.1007/s00066-013-0365-7. [DOI] [PubMed] [Google Scholar]
- 9.Haie-Meder C., Pötter R., Van Limbergen E. Recommendations from gynaecological (GYN) GEC-ESTRO working group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother. Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Taylor A., Rockall A.G., Reznek R.H., Powell M.E.B. Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:1604–1612. doi: 10.1016/j.ijrobp.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 11.Gay a H., Barthold H.J., O'Meara E. Pelvic normal tissue contouring guidelines for radiation therapy: a radiation therapy oncology group consensus panel atlas. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Small W., Mell L.K., Anderson P., Creutzberg C. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:428–434. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirumani S.H., Shanbhogue A.K., Prasad S.R. Current concepts in the diagnosis and management of endometrial and cervical carcinomas. Radiol. Clin. North Am. 2013;51:1087–1110. doi: 10.1016/j.rcl.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Follen M., Levenback C.F., Iyer R.B. Imaging in cervical cancer. Cancer. 2003;98:2028–2038. doi: 10.1002/cncr.11679. [DOI] [PubMed] [Google Scholar]
- 15.Naganawa S., Sato C., Kumada H., Ishigaki T., Miura S., Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur. Radiol. 2005;15:71–78. doi: 10.1007/s00330-004-2529-4. [DOI] [PubMed] [Google Scholar]
- 16.Hoogendam J.P., Klerkx W.M., de Kort a G.P. The influence of the b-value combination on apparent diffusion coefficient based differentiation between malignant and benign tissue in cervical cancer. J. Magn. Reson. Imaging. 2010;32:376–382. doi: 10.1002/jmri.22236. [DOI] [PubMed] [Google Scholar]
- 17.Sala E., Wakely S., Senior E., Lomas D. MRI of malignant neoplasms of the uterine corpus and cervix. AJR Am. J. Roentgenol. 2007;188:1577–1587. doi: 10.2214/AJR.06.1196. [DOI] [PubMed] [Google Scholar]
- 18.De Boer P., Adam J.A., Buist M.R. Role of MRI in detecting involvement of the uterine internal os in uterine cervical cancer: systematic review of diagnostic test accuracy. Eur. J. Radiol. 2013;82:e422–e428. doi: 10.1016/j.ejrad.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 19.van de Schoot A.J., de Boer P., Buist M.R. Quantification of delineation errors of the gross tumor volume on magnetic resonance imaging in uterine cervical cancer using pathology data and deformation correction. Acta Oncol. 2014 doi: 10.3109/0284186X.2014.983655. Early Online :1–8. [DOI] [PubMed] [Google Scholar]
- 20.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 21.Manfredi R., Gui B., Giovanzana A. Localized cervical cancer (stage <IIB): accuracy of MR imaging in planning less extensive surgery. Radiol. Med. 2009;114:960–975. doi: 10.1007/s11547-009-0397-3. [DOI] [PubMed] [Google Scholar]
- 22.Sahdev A., Sohaib S.A., Wenaden A.E.T., Shepherd J.H., Reznek R.H. The performance of magnetic resonance imaging in early cervical carcinoma: a long-term experience. Int. J. Gynecol. Cancer. 2007;17:629–636. doi: 10.1111/j.1525-1438.2007.00829.x. [DOI] [PubMed] [Google Scholar]
- 23.Peppercorn P.D., Jeyarajah A.R., Woolas R. Role of MR imaging in the selection of patients with early cervical carcinoma for fertility-preserving surgery: initial experience. Radiology. 1999;212:395–399. doi: 10.1148/radiology.212.2.r99au01395. [DOI] [PubMed] [Google Scholar]
- 24.Lakhman Y., Park K.J., Sarasohn D.M., Goldman D.A., Sohn M.J., Moskowitz C.S. Stage IB1 cervical cancer: role of preoperative MR imaging in selection of patients for fertility-sparing radical trachelectomy. Radiology. 2013;269:149–158. doi: 10.1148/radiol.13121746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanuki N., Urabe S., Matsumoto H. Evaluation of microscopic tumor extension in early-stage cervical cancer: quantifying subclinical uncertainties by pathological and magnetic resonance imaging findings. J. Radiat. Res. 2013;54:719–726. doi: 10.1093/jrr/rrt004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eifel P.J., Winter K., Morris M. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J. Clin. Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 27.Joiner M.C. Quantifying cell kill and cell survival. In: Joiner M.C., van der Kogel A., editors. Basic Clinical Radiobiology. 4th ed. Hodder Arnold; London, United Kingdom: 2009. pp. 41–54. [Google Scholar]
- 28.Mazaheri Y., Bokacheva L., Kroon D.J. Semi-automatic deformable registration of prostate MR images to pathological slices. J. Magn. Reson. Imaging. 2010;32(5):1149–1157. doi: 10.1002/jmri.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson E., Gaed M., Gómez J.A. 3D prostate histology image reconstruction: quantifying the impact of tissue deformation and histology section location. J. Pathol. Inform. 2013;4:31. doi: 10.4103/2153-3539.120874. [DOI] [PMC free article] [PubMed] [Google Scholar]