Abstract
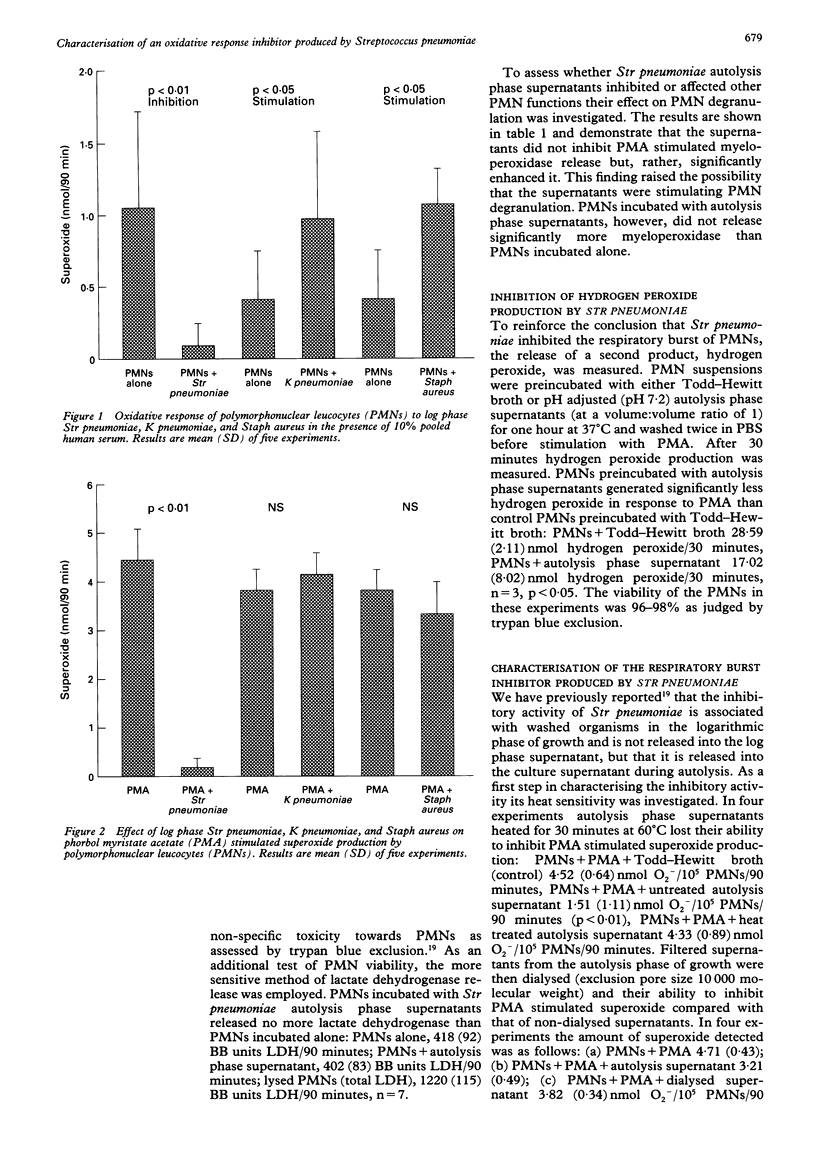
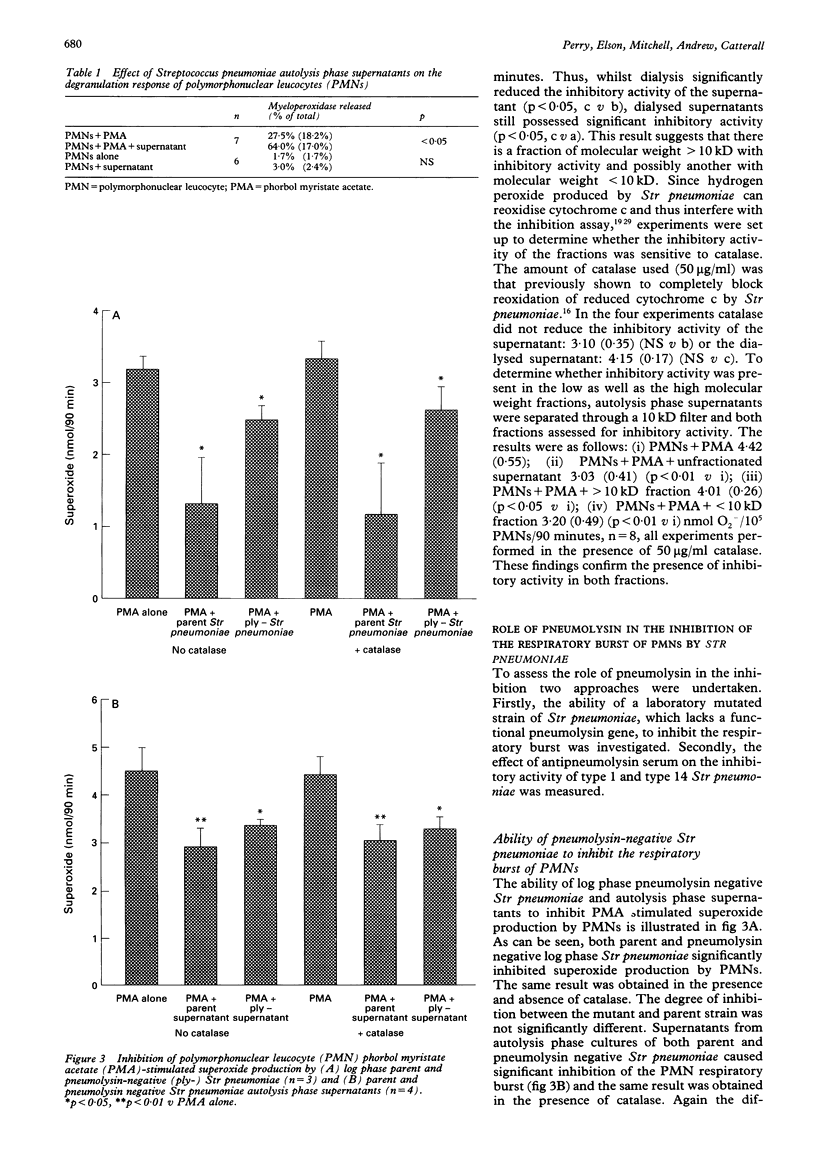
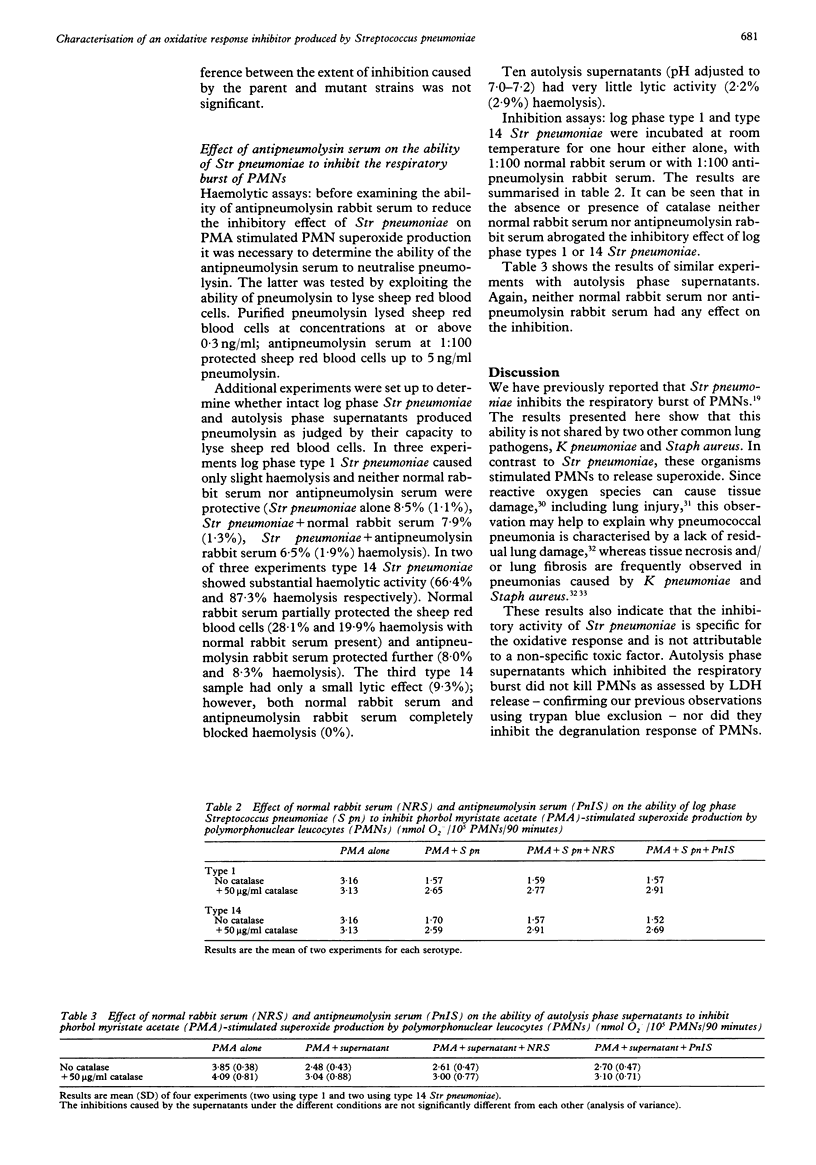
BACKGROUND--Pneumonia caused by infection with Streptococcus pneumoniae is still a major clinical problem. Reactive oxygen species contribute to the killing of these bacteria by polymorphonuclear leucocytes (PMNs). Defence mechanisms of Str pneumoniae which counter reactive oxygen species are characterised. METHODS--PMNs were stimulated with phorbol myristate acetate (PMA) in the presence and absence of Str pneumoniae and supernatants from them, and superoxide (O2-) production was measured by the reduction of ferricytochrome c. RESULTS--Streptococcus pneumoniae, but not Klebsiella pneumoniae or Staphylococcus aureus, inhibited PMA stimulated superoxide production by PMNs. Washed PMNs which had been preincubated with Str pneumoniae autolysis phase supernatants also exhibited depressed H2O2 production in response to PMA. The inhibitory activity was not attributable to non-specific cytotoxicity as assessed by release of the cytoplasmic enzyme lactate dehydrogenase, nor did the supernatants inhibit PMA stimulated degranulation of PMNs. Fractionation of the autolysis phase supernatants revealed inhibitory activity in both the fractions greater than and less than 10 kD. Like pneumolysin the inhibitory activity was heat sensitive. However, both a parent and pneumolysin negative mutant Str pneumoniae, and autolysis phase supernatants from them, inhibited PMN superoxide production. Antisera to pneumolysin failed to abrogate the inhibitory effect of intact Str pneumoniae or autolysis phase supernatants from types 1 or 14 Str pneumoniae. CONCLUSIONS--The inhibitory effect of Str pneumoniae on the respiratory burst of PMNs is not shared by two other common lung pathogens. The existence of a novel inhibitor of the PMN respiratory burst, distinct from pneumolysin, has been demonstrated. The inhibitor is specific for the respiratory burst and is active both in the logarithmic phase of growth and during autolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry A. M., Yother J., Briles D. E., Hansman D., Paton J. C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braconier J. H., Odeberg H. Granulocyte phagocytosis and killing virulent and avirulent serotypes of Streptococcus pneumoniae. J Lab Clin Med. 1982 Aug;100(2):279–287. [PubMed] [Google Scholar]
- Casal J., Fenoll A., Vicioso M. D., Muñoz R. Increase in resistance to penicillin in pneumococci in Spain. Lancet. 1989 Apr 1;1(8640):735–735. doi: 10.1016/s0140-6736(89)92260-5. [DOI] [PubMed] [Google Scholar]
- Clark R. A. Oxidative inactivation of pneumolysin by the myeloperoxidase system and stimulated human neutrophils. J Immunol. 1986 Jun 15;136(12):4617–4622. [PubMed] [Google Scholar]
- Dooley D. C., Simpson J. F., Meryman H. T. Isolation of large numbers of fully viable human neutrophils: a preparative technique using percoll density gradient centrifugation. Exp Hematol. 1982 Aug;10(7):591–599. [PubMed] [Google Scholar]
- Edwards S. W., Nurcombe H. L., Hart C. A. Oxidative inactivation of myeloperoxidase released from human neutrophils. Biochem J. 1987 Aug 1;245(3):925–928. doi: 10.1042/bj2450925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito A. L., Clark C. A., Poirier W. J. An assessment of the factors contributing to the killing of type 3 Streptococcus pneumoniae by human polymorphonuclear leukocytes in vitro. APMIS. 1990 Feb;98(2):111–121. [PubMed] [Google Scholar]
- Finch R. Is pneumococcal infection a preventable disease? J Infect. 1988 Sep;17(2):95–98. doi: 10.1016/s0163-4453(88)91459-4. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Kirk J. L., Schiffman G., Schweinle J. E., Guckian J. C. Analysis of humoral and phagocytic defenses against Streptococcus pneumoniae serotypes 1 and 3. J Lab Clin Med. 1988 Oct;112(4):487–497. [PubMed] [Google Scholar]
- Gillespie S. H. Aspects of pneumococcal infection including bacterial virulence, host response and vaccination. J Med Microbiol. 1989 Apr;28(4):237–248. doi: 10.1099/00222615-28-4-237. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman K. P., Koornhof H. J. Worldwide increase in pneumococcal antibiotic resistance. Lancet. 1989 Aug 19;2(8660):444–444. doi: 10.1016/s0140-6736(89)90617-x. [DOI] [PubMed] [Google Scholar]
- Mitchell T. J., Walker J. A., Saunders F. K., Andrew P. W., Boulnois G. J. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochim Biophys Acta. 1989 Jan 23;1007(1):67–72. doi: 10.1016/0167-4781(89)90131-0. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Lock R. A., Lee C. J., Li J. P., Berry A. M., Mitchell T. J., Andrew P. W., Hansman D., Boulnois G. J. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect Immun. 1991 Jul;59(7):2297–2304. doi: 10.1128/iai.59.7.2297-2304.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penicillin-resistant strains of Neisseria meningitidis in Spain. Lancet. 1988 Jun 25;1(8600):1452–1453. [PubMed] [Google Scholar]
- Perry F. E., Elson C. J., Greenham L. W., Catterall J. R. Interference with the oxidative response of neutrophils by Streptococcus pneumoniae. Thorax. 1993 Apr;48(4):364–369. doi: 10.1136/thx.48.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E. J., Allen K. D. Penicillin resistance in pneumococci. J Antimicrob Chemother. 1991 Feb;27(2):251–252. doi: 10.1093/jac/27.2.251. [DOI] [PubMed] [Google Scholar]
- Shapiro E. D., Berg A. T., Austrian R., Schroeder D., Parcells V., Margolis A., Adair R. K., Clemens J. D. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991 Nov 21;325(21):1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983 Jul 15;132(2):345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Thore M., Löfgren S., Tärnvik A., Monsen T., Selstam E., Burman L. G. Anaerobic phagocytosis, killing, and degradation of Streptococcus pneumoniae by human peripheral blood leukocytes. Infect Immun. 1985 Jan;47(1):277–281. doi: 10.1128/iai.47.1.277-281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle P. L., Petersen N. O. Oxidation of reduced cytochrome c by hydrogen peroxide. Implications for superoxide assays. FEBS Lett. 1987 Jan 5;210(2):195–198. doi: 10.1016/0014-5793(87)81336-4. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]



