Abstract
Background
Glycogen synthase deficiency (glycogen storage disease 0 — GSD 0) caused by mutations in the GYS2 gene is characterized by a lack of glycogen synthesis in the liver. It is a rare condition of disturbed glycogen homeostasis in the liver with less than 30 cases reported in the literature so far.
Case report
We report a 9 year old boy diagnosed with GSD 0 due to the newly identified, highly pathogenic homozygous mutation: NM_021957.3:p.Phe574Leu/c.1720T > C in ex. 14.
A random, asymptomatic hypoglycemia with ketonuria was found in this patient at the age of 7. His developmental parameters were within normal ranges. Oral glucose tolerance test showed normal baseline blood levels of glucose, insulin and lactate, and their increase following glucose intake. Eight-hour fasting plasma glucose test, revealed glucose blood level of 34 mg/dl with no clinical symptoms. The results of these tests suggested GSD 0. Molecular analysis of the GYS2 gene was not feasible, but this particular gene was included in the panel of hypoglycemia of whole exome sequencing (WES) which was at our disposal.
Keywords: Hypoglycemia, Glycogen synthase deficiency, Glycogen storage disease 0
1. Introduction
Glycogen synthase deficiency, also known as glycogenosis (GSD) type 0 is an inborn error of glycogen metabolism caused by mutations in the GYS2 gene, which is transmitted in an autosomal recessive trait [1]. It is a rare form of hepatic glycogen storage diseases with less than 30 cases reported in the literature so far [2], [3]. The disorder is characterized by fasting hyperketotic hypoglycemia without hyperalaninemia or hyperlactacidemia. It is a glycogenosis with lack of liver glycogen synthesis, therefore hepatomegaly is not observed in patients with glycogen synthetase deficiency.
Symptoms of fasting hypoglycemia in patients with GSD 0 usually appear for the first time in late infancy when weaning from overnight feeds. Children may have early-morning drowsiness, paleness, vomiting and fatigue. Seizures associated with low blood glucose may also occur, but they are rare [4]. Usually hypoglycemia is found in laboratory tests performed during differential diagnosis process [5].
In general, GSD 0 has no impact on physical or mental development, only some children may have a mild growth delay [5].
Alike other hepatic glycogenosis, the goal of management for GSD 0 is to prevent hypoglycemia by avoiding fasting. Clinical management is therefore based on frequent meals composed of high protein intake during the day and addition of uncooked cornstarch in the evening [6].
Next-generation sequencing techniques including whole-exome sequencing (WES) have now opened promising possibilities to identify the molecular background of rare metabolic disease [7]. Through the use of WES technology with one experiment, it is possible to identify pathological mutations in an ample number of selected genes that are potentially associated with the disorder being tested.
Here, we report a successful application of WES in a patient with a glycogen synthase deficiency.
2. Case report
A 7 year old boy was referred to the Department of Endocrinology and Metabolic Disorders for the diagnosis of hypoglycemia. A random hypoglycemia of 32 mg/dl with no clinical symptoms, and massive ketonuria were found during his hospitalization at the pediatric ward due to infection.
He was born at term, as the first child of parents of Polish origin who declared non-consanguinity, however having lived in the neighboring villages. His body mass was 3150 g, head circumference 34 cm, and Apgar score 10p.
Boy's psycho-motor and physical development were normal; 50th weight-for-age percentile and 25th height–for-age percentile, but his mid parental height was 167.5 cm.
Patient's glucose profile studied during hospitalization revealed a random hypoglycemia without post-prandial hyperglycemia. Lactates were measured during function tests. Glucose challenge showed baseline blood levels of glucose, insulin and lactate within normal values, and their increase following glucose intake. After 8 h of prolonged fasting test, hypoglycemia of 34 mg/dl with no clinical symptoms, and poor response to glucagon were found (Table 1). The glucose and lactate monitoring was performed using both laboratory readings (glucose and lactate) and meter determinations (glucose).
Table 1.
Laboratory results of patient's function tests: prolonged fasting test and glucose challenge.
| Parameter | Prolonged fasting test | Glucose challenge |
|---|---|---|
| Glucose 0′ (mg/dl) | 34 | 64 |
| Glucose 5′ (mg/dl) | 45 | – |
| Glucose 15′ (mg/dl) | 64 | – |
| Glucose 30′ (mg/dl) | 164 | 164 |
| Glucose 60′ (mg/dl) | 142 | 142 |
| Glucose 90′ (mg/dl) | 125 | 125 |
| Glucose 120′ (mg/dl) | 108 | 108 |
| Glucose 150′ (mg/dl) | 130 | 130 |
| Glucose 180′ (mg/dl) | 77 | 77 |
| Lactate 0′ (mg/dl) | 7.2 | 7.9 |
| Lactate 60′ (mg/dl) | 35.2 | 35.2 |
| Lactate 120′ (mg/dl) | 30.4 | 30.4 |
| Lactate 180′ (mg/dl) | 21.4 | 21.4 |
| Beta-OH-butyrate (mmol/l) | 4.41 | – |
Blood lactate normal values: 4.5–18.8 mg/dl.
Beta-OH-butyrate normal values: 0.03–0.65 mmol/l.
Outcomes of both function and laboratory tests suggested the diagnosis of glycogen synthase deficiency.
Whole exome sequencing (WES) was conducted to identify the molecular basis of the hypoglycemia in the patient. The study was approved by the Ethics Committee of the CMHI. Informed consent from the guardians of the patient undergoing large-scale sequencing was obtained.
WES was performed on a HiSeq 1500 using an Exome Enrichment Kit (Illumina) according to published protocol [8]. Generated reads were aligned to the hg19 reference human genome. Alignments were viewed with Integrative Genomics Viewer v.2.3.40. The mutations identified as pathologies were confirmed using the Sanger method following the standard protocol (BigDye® Terminator v3.1 Cycle Sequencing Kit, Applied Biosystems®).
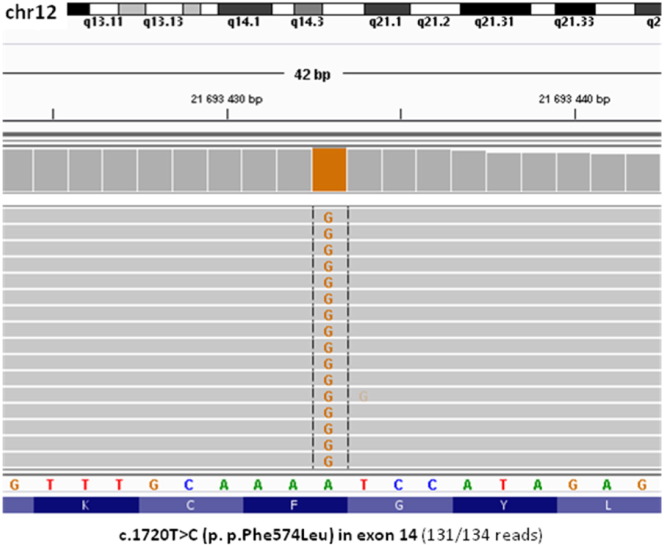
Homozygous molecular variant c.1720T > C (p.Phe574Leu) in the GYS2 gene was identified (Fig. 1). It is a novel missense substitution, which was not observed in a control group of 800 alleles and was not identified in the different Exome Sequencing Projects (e.g. ESP 6500, ExAC 65000). In silico prediction by various algorithms was assessed and pathogenic effect of mutation was revealed: CADD (result: 34), MetaSMV (Deleterious), Polyphen2 HDIV (Deleterious) and HVAR (Deleterious), Mutation Assessor (Medium), LRT (Deleterious), MetaLR (Deleterious), SIFT (Deleterious), and MutationTaster (Deleterious). In order to predict the pathogenicity of the mutation, a bioinformatic analysis was conducted instead of functional studies, which are time-consuming and long-term techniques.
Fig. 1.
Integrative Genomics Viewer view of GYS2 homozygous mutation found in the proband by whole-exome sequencing.
Nomenclature of mutation followed the guidelines of the Human Genome Variation Society using NM_021957.3 as a reference cDNA sequence for GYS2 gene.
Gen GYS2 (RefSeq NM_021957.3; NP_068776.2; CCDS8690.1).
Genotyp zgodny z HGVS v.2.0: NM_021957.3: c.[1720T > C];[1720T > C].
Protein gene description: NP_068776.2: p.[Phe574Leu];[Phe574Leu].
Parents have not been tested to confirm the significance of a homozygous variant, because the patient's father was not available for blood sample collection as he had emigrated.
Dietary management with frequent meals (every 3–4 h with late dinner and early breakfast), protein supplementation and administration of uncooked cornstarch (1–1.5 g/kg) at bedtime was introduced. Currently the patient is 9 years old and has been maintaining a fairly constant growth curve since the therapy was initiated. His baseline laboratory studies and biochemical profile following diagnosis are specified in Table 2.
Table 2.
Baseline laboratory studies and his biochemical profile following diagnosis.
| Parameter | Baseline | Following diagnosis |
|---|---|---|
| AST (U/l) | 27 | 23 |
| ALT (U/l) | 15 | 14 |
| Alanine (μmol/l) | 102 | – |
| Cholesterol (mg/dl) | 161 | 185 |
| Triglycerides (mg/dl) | 68 | 94 |
| Total protein (g/l) | 65 | 69 |
| Albumin (g/l) | 45 | 49.1 |
| Lactate (mg/dl) | 11.54 | 9 |
3. Discussion
Infantile hypoglycemia due to the deficiency of liver glycogen synthase was demonstrated for the 1st time in a well-studied family by Lewis et al. in 1963 [9]. Since then, only 20 cases have been reported [3], [10], [11], [12], [13], [14], [15]. Therefore, hepatic glycogen synthase deficiency is considered a very rare disorder. However, reported cases (Table 3) demonstrate, that its course may be asymptomatic, and thus the disease may be more common than previously thought. Unlike other hepatic GSD patients, individuals with glycogen synthase deficiency do not present growth, developmental, and physical, such as enlarged liver, disorders. In all reported patients, including our case, the developmental parameters were normal.
Table 3.
Characteristics of patients with GSD 0 in the literature review.
| Patient | Symptoms and metabolic profile |
|||
|---|---|---|---|---|
| Symptoms | Ketonuria | Baseline lactate and glucose | References | |
| An 8 month-old infant | Transient neurological symptoms improved after the feed | – | N | Lewis et al. [9] |
| A 9-year-old girl | Hypoglycemic seizures at the age of 7 years | P | N | Aynsley-Green et al. [10] |
| A boy of Italian ancestry at the age 21 months | Signs of hepatic deficiency with mild clinical symptoms contrasted with a remarkable fatty liver degeneration + atypic reaction to fructose overload | P | ↑ (L)/N(G) | de Kremer et al. [14] |
| 3 children from 2 German families | - 2 cases: morning fatigue rapidly disappearing after heating - 1 case: asymptomatic |
P | N | Gitzelmann et al. [11] |
| A 15 month-old child | Generalized tonic–clonic seizures after night fasting | P | N | Rutledge et al. [12] |
| A 7 year-old Canadian girl | Asymptomatic | P | N | Laberge et al. [13] |
| 2 children | Glucosuria and hyperglycemia | P | ↑ | Bachrach et al. [15] |
| Our patient | Asymptomatic | P | N | Our patient |
P — present.
N — normal.
Reported patient is the first known case of glycogen synthase deficiency in Poland. The described mutation is also novel, and it is likely to be mild, which may lead to misdiagnosis as short stature or ketotic hypoglycemia. Therefore, we suspect that there are more cases present in our country. This only supports the fact, that GSD 0 is more common than it has been appreciated. Nonetheless, if the patient presents with asymptomatic hyperketotic hypoglycemia, as it was in our case, no clinical signs allow for the diagnosis to be made earlier. In our case, clinical diagnosis was established when he was 7 years old, and confirmed 2 years later. Only random glucose intolerance found either on control pediatric checkup, or during endocrinological diagnosis of short stature may suggest the diagnosis of GSD 0.
Most children with glycogen synthase deficiency are indeed identified incidentally when hypoglycemia due to the increased energy expenditure, such as prolonged fasting, illness, pregnancy, increased activity, or interrupted normal enteral intake is discovered [3], [16].
Since testing for type 0 glycogen storage disease has become available just recently, it is not feasible in many countries including Poland. Therefore, we decided to conduct WES analysis which included GYS2 gene within the panel of hypoglycemia and was at our disposal. However, we are aware of a poor access to such sophisticated molecular techniques in most hospitals. Thus, home blood glucose and urine ketone monitoring, and function tests including prolonged fasting test and glucose challenge may be recommended to screen for this disorder.
Dietary management with frequent meals given every 3–4 h during the day and addition of uncooked cornstarch acting as a “slow release” form of glucose for the body at the evening with protein supplementation is recommended in GSD 0.
4. Conclusion
Glycogenosis type 0 should be suspected in children, who usually present with fasting hypoglycemia and urinary ketons.
However, the disorder may also be asymptomatic, and since it is considered a very rare form of hepatic GSDs, its prevalence may be underestimated then. Moreover, any genetically determined disease requires a confirmed molecular diagnosis.
Compliance with ethics guidelines
The following authors: Edyta Szymańska, Urszula Wątrobinska, Dariusz Rokicki, Elżbieta Ciara, Paulina Halat, Rafał Płoski, and Anna Tylki-Szymańka declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by the any of the authors — it is not a study/trial but a case report.
Details of the contributions of individual authors
Edyta Szymańska — planning, conduct, and reporting.
Urszula Wątrobinska — conduct.
Dariusz Rokicki — conduct, and reporting.
Elżbieta Ciara — conduct, and reporting.
Paulina Halat — conduct.
Rafał Płoski — conduct.
Anna Tylki-Szymańka — planning, conduct, and reporting.
Acknowledgments
Support: This work has been supported by the Children's Memorial Health Institute project no CMHI 216/12.
References
- 1.http://www.orpha.net/serach: glycogen storage disease0.
- 2.http://omim.org/search=glycogen storage disease0.
- 3.Weinstein D.A., Correia C.E., Saunders A.C., Wolfsdorf J.I. Hepatic glycogen synthase deficiency: an infrequently recognized cause of ketotic hypoglycemia. Mol. Genet. Metab. 2006;87(4):284–288. doi: 10.1016/j.ymgme.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes J., GPA S. 3rd rev Edn. Springer; Berlin: 2000. The Glycogen Storage Disease. Inborn Metabolic Diseases. 86-101. [Google Scholar]
- 5.Bachrach B.E., Weinstein D.A., Orho-Melander M. Glycogen synthase deficiency (glycogen storage disease type 0) presenting with hyperglycemia and glucosuria: report of three new mutations. J. Pediatr. 2002;140(6):781–783. doi: 10.1067/mpd.2002.124317. [DOI] [PubMed] [Google Scholar]
- 6.Raghuveer T.S., Gardg U. Inborn errors of metabolism in infancy and early childhood: an update. Am. Fam. Physician. 2006;11:1981–1990. [PubMed] [Google Scholar]
- 7.Choi R., Woo H.I., Choe B.H. Application of whole exome sequencing to a rare inherited metabolic disease with neurological and gastrointestinal manifestations: a congenital disorder of glycosylation mimicking glycogen storage disease. Clin. Chim. Acta. 2015;15(444):50–53. doi: 10.1016/j.cca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Ploski R., Pollak A., Müller S. Does p.Q247X in TRIM63 cause human hypertrophic cardiomyopathy? Circ. Res. 2014;17(114(2)):2–5. doi: 10.1161/CIRCRESAHA.114.302662. [DOI] [PubMed] [Google Scholar]
- 9.Lewis G.M., Spencer-Peet J., Stewart K.M. Infantile hypoglycaemia due to inherited deficiency of glycogen synthetase in liver. Arch. Dis. Child. 1963;38:40–48. doi: 10.1136/adc.38.197.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aynsley-Green A., Williamson D.H., Gitzelmann R. Hepatic glycogen synthetase deficiency: definition of syndrome from metabolic and enzyme studies on a 9-year-old girl. Arch. Dis. Child. 1977;52:573–579. doi: 10.1136/adc.52.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitzelmann R., Spycher M.A., Feil G., Muller J. Liver glycogen synthase deficiency: a rarely diagnosed entity. Eur. J. Pediatr. 1996;155:561–567. doi: 10.1007/BF01957905. [DOI] [PubMed] [Google Scholar]
- 12.Rutledge S.L., Atchison J., Bosshard N.U., Steinmann B. Case report: liver glycogen synthase deficiency—a cause of ketotic hypoglycemia. Pediatrics. 2001;108:495–497. doi: 10.1542/peds.108.2.495. [DOI] [PubMed] [Google Scholar]
- 13.Laberge A.M., Mitchell G.A., van de Werve G., Lambert M. Long-term follow-up of a new case of liver glycogen synthase deficiency. Am. J. Med. Genet. 2003;2003:19–22. doi: 10.1002/ajmg.a.20110. [DOI] [PubMed] [Google Scholar]
- 14.de Kremer R.D., de Capra A.P., de Boldini C.D. Hepatic glycogen synthetase deficiency or glycogen storage disease-zero. Mild phenotype with partial enzymatic defect. Medicina (B Aires) 1990;50:299–309. [PubMed] [Google Scholar]
- 15.Bachrach B.E., Weinstein D.A., Orho-Melander M. Glycogen synthase deficiency (glycogen storage disease type 0) presenting with hyperglycemia and glucosuria: report of three new mutations. J. Pediatr. 2002;140:781–783. doi: 10.1067/mpd.2002.124317. [DOI] [PubMed] [Google Scholar]
- 16.Byrne B.M., Gillmer M.D., Turner R.C., Aynsley-Green A. Glucose homeostasis in adulthood and in pregnancy in a patient with hepatic glycogen synthetase deficiency. Br. J. Obstet. Gynaecol. 1995;102:931–933. doi: 10.1111/j.1471-0528.1995.tb10886.x. [DOI] [PubMed] [Google Scholar]