Abstract
Background and purpose
World Health Organization estimated that there were 600,000 new cases of head and neck cancers and 300,000 deaths each year worldwide. Scientific modalities to predict the treatment outcomes are not available yet. We conducted this study to (1) compare CT perfusion parameters before and after chemoradiation among patients with head and neck squamous cell carcinoma and (2) to evaluate the prognostic value of each perfusion parameter in predicting the response to chemoradiation.
Materials and methods
We conducted a prospective study among all patients with head and neck squamous cell carcinoma registered for chemoradiotherapy (CRT) at Regional Cancer Research Center, Shimla, Himachal Pradesh, India during the period June 2012 through June 2013. CTp data were acquired on a 64-slice CT scanner (Light speed VCT Xte; GE Healthcare) with 14 cm z-axis coverage using Volume Helical Shuttle (VHS) feature at baseline, on completion of 40 Gy and 66 Gy of chemoradiation. We dichotomised the treatment outcome as complete response and non-response (partial responders/stable disease/progressive disease) using RECIST 1.1 criteria. We compared all perfusion parameters at baseline, 40 Gy and 66 Gy of CRT between responders and non-responders. We dichotomised the perfusion parameters as high (>median value) and low (≤median value) to analyze association between perfusion parameters and treatment outcome. We calculated the sensitivity, specificity, predictive values, and likelihood ratios for each dichotomized perfusion parameter using Wilson Score method.
Results
We followed 24 patients (23 of them men) from start of the treatment till completion of it. All had Stage III or Stage IV of the disease. Blood flow (BF) and blood volume (BV) decreased and Mean Transit Time (MTT) increased significantly (p < 0.05) at 66 Gy among responders to CRT as compared to non-responders. Patients with high BF (>106 ml/100 g/min) at baseline were five times more likely (p = 0.004) to respond to treatment as compared to those with low BF. BF was found to be 83.3% predictive of complete response. Other perfusion parameters were not significantly predictive of outcome (p > 0.05) Combination of high BF (>106 ml/100 g/min) and low (≤47 ml/100 g/min) permeability surface (PS) was 100% predictive of response to CRT irrespective of the stage of tumor.
Conclusions
High BF at baseline is the single best predictor of response to chemoradiaton. A combination of high BF and low PS was found to be 100% predictive of complete response irrespective of the stage of the tumor.
Abbreviations: BF, blood flow; BV, blood volume; CECT, contrast enhanced computed tomography; CR, complete responder; CRT, chemotherapy and radiation therapy; CTP, perfusion computed tomography; CT, computed tomography; HNSCC, head and neck squamous cell carcinoma; MVD, microvascular density; PD, progressive disease; PR, partial responder; PS, permeability surface area product; RECIST, response evaluation criteria in solid tumors; ROI, region of interest; SCC, squamous cell carcinoma; SCCA, squamous cell carcinoma of aerodigestive tract
Keywords: CT perfusion, Perfusion parameters, Chemoradiation, Head and neck squamous cell carcinoma, RECIST 1.1
1. Introduction
CTp (computed tomography perfusion) has emerged as a non-invasive functional imaging tool providing quantitative parameters regarding angiogenesis and tumor perfusion which can predict the response, monitor the effects and assess long term treatment outcome in HNSCC (head and neck squamous cell carcinoma) [1], [2], [3]. CTp can explore the molecular nature of tumor perfusion in addition to information about size and enhancement of tumor as done with conventional CT (computed tomography) [4], [5], [6], [7].
MVD (microvascular density) which is pathological marker of angiogenesis is considered as predictor of response in HNSCC. MVD requires endoscopic biopsy and is an invasive procedure accompanied by its own risks. CTp is a non-invasive measurement of intra tumoral MVD which has a prognostic value [7], [8].
Studying dynamics of perfusion parameters of tumor help in understanding the therapy induced functional changes in tumor tissue and help in distinguishing responders from non-responders [9].
CTp studies revealed that HNSCC have increased BF, BV, PS and reduced MTT as compared to normal tissue and benign lesions [10], [11]. In different studies conducted, during the course of chemo-radiation, responders showed a significant reduction in BF and BV whereas non-responders showed a non-significant elevation of BF and BV [9], [12], [13].
The purpose of our study was to evaluate the role of CTp in histologically proven HNSCC in predicting the treatment response before the commencement of therapy, determine the changes in perfusion parameters during the course of treatment and after completion of chemoradiation. Four perfusion parameters namely BF, BV, PS and MTT were assessed quantitatively and analyzed to determine which one of these perfusion parameters co-related well with treatment outcome as per RECIST 1.1 criteria.
2. Methods
2.1. Patient selection
We conducted this prospective study among patients who met the following criteria:
-
1.
Histopathologic or cytologic evidence of squamous cell carcinoma of head and neck staged according to 2002 American Joint Committee on Cancer Classification.
-
2.
Registered for CRT (chemoradiotherapy) at Regional Cancer Research Center of our hospital.
-
3.
No prior history of chemotherapy/radiotherapy/CRT for any condition.
-
4.
No contra-indication to iodinated contrast medium administration.
Patients with terminal illness, inability to communicate, or unwilling to participate in the study were excluded.
A written informed consent was obtained from all participants and the proposal was approved by institutional ethics committee.
We performed non-enhanced computed tomography (NECT), CTp and contrast enhanced computed tomography (CECT) of the neck before the commencement of treatment, after 40 Gy of platinum based chemoradiotherapy and again at the end (66 Gy) of treatment.
2.2. Technique
Patients were trained for quite breathing and not to swallow during the procedure. NECT of neck was acquired to localize the tumor with following parameters – 100 kV, 50–220 mA with automated tube current modulation, 0.8 s rotation time, 5 mm thickness and total exposure time of 5 s. The scan position was adjusted to cover the entire volume.
CTp of neck was performed on a 64-slice CT scanner (Light speed VCT Xte; GE Healthcare) with Z-axis coverage of 14 cm. Fifty ml of iodinated non-ionic contrast with iodine concentration of 370 mg/ml was injected at a rate of 5 ml/s followed by saline flush of 30 ml with an automated pressure injector (Stellant, MEDRAD) through the antecubital vein on the side opposite to the tumor to reduce streak artifacts from large veins. Scanning was initiated 5 s after the start of injection and images were acquired for duration of 35.86 s. The parameters used were – 100 kV, 50–220 mA with automated tube current modulation, 0.4 s rotation time, 5 mm thickness and noise index of 15 and the processing of perfusion was done on 5/5 mm slice. Immediately after CTp, CECT of the neck was performed from thoracic inlet to the level of skull vault with the same parameters as that for NECT. Acquisition was done 40 s after the injection of 1.25 ml/kg of iodinated contrast administration.
2.3. Image processing
Images and data obtained were transferred to an image processing workstation – Advantage windows 4.2, GE Healthcare. Perfusion maps were generated by deconvolution technique using vendor's Perfusion CT 4 (GE) software. No separate motion correction program was available. The program estimates tissue perfusion as the maximum slope of the tumor time-density curve divided by the peak arterial enhancement.
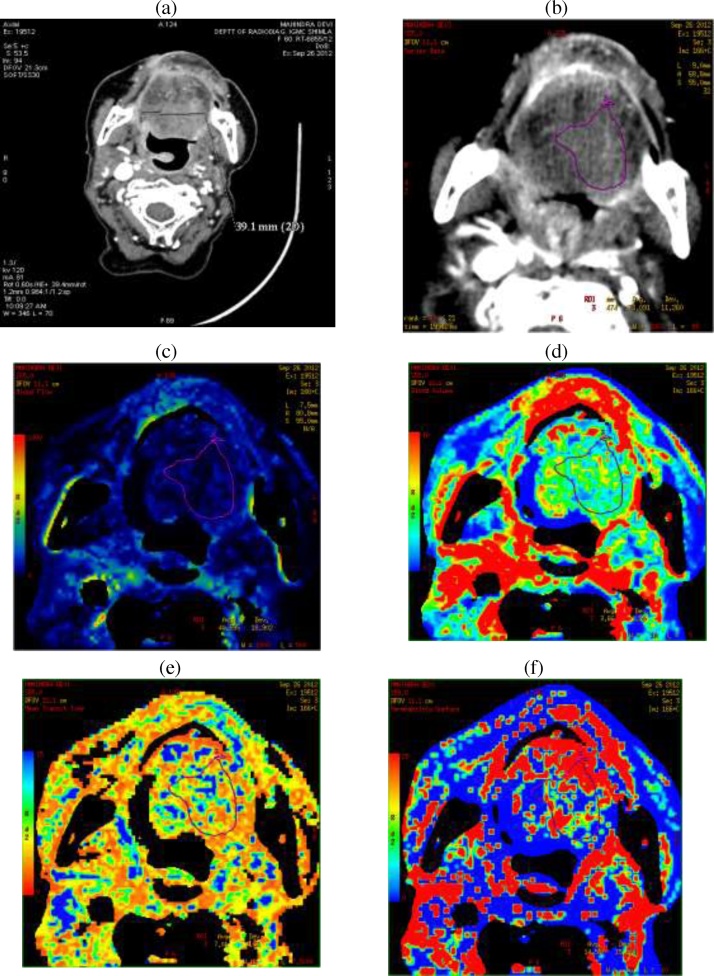
Attenuation thresholds were fixed to exclude bones. Arterial input was obtained drawing ROI 4–6 pixel size placed over ICA except in two patients in which ROI was put in ipsilateral IJV as software did not analyze parameters when ROI was placed in ICA. Last pre-enhancement and post enhancement images were chosen to generate the functional color maps. Unprocessed source images in the phase demonstrating maximal enhancement and base images were used to draw a freehand ROI at the margin of the tumor. Necrotic areas, vessels and calcifications were excluded. Values of BV, BF, and MTT & PS were measured in all sections depicting the tumor and the mean value was calculated.
2.4. Statistical analysis
We dichotomised the treatment outcome as complete response and non-response (partial responders/stable disease/progressive disease) using RECIST 1.1 criteria. For all measurements taken at different points in time, we tested for normality of distribution by Shapiro Wilk test and for inequality of variances by Bartlett's test. A p-value of 0.05 or above was taken as indicative of normality of data distribution and homogeneity of variances respectively. The normally distributed independent sets of data with homogenous variances were compared by t-test or one way ANOVA and data with non-normal distribution were compared by non-parametric tests (Kruskal Wallis test or Friedman test). We compared means of each perfusion parameter at baseline for RECIST outcome category. The changes in perfusion parameters overtime were compared by paired t-test or Kruskal Wallis test for each category of RECIST response. We dichotomotized the perfusion parameter values by taking median as the cut off – high (>median value) and low (≤median value). We conducted a bivariate analysis with dichotomized perfusion parameter values as exposure variable and dichotomized response as outcome variable. We compared the response rates (RR) in each exposure group and constructed 95% confidence intervals around the point estimates. We calculated point estimates and 95% confidence intervals for sensitivity, specificity, predictive positive values, negative predictive values, likelihood ratios positive and likelihood ratios negative by Wilson Score method. Except for statistical tests for normality of data and homogeneity of variances, p-values ≤ 0.05 were treated as statistically significant. We analyzed data using EpiInfo version 7.1.4 for windows (http://wwwn.cdc.gov/epiinfo/7/).
3. Results
3.1. Characteristics of the study participants
Twenty four patients (23 of them men) with HNSSC and a mean age of 57.9 ± 4.5 years were included in our study. Nineteen (79.1%) patients were in stage III of the disease and remaining in stage IV disease according to 2002 American Joint Committee on Cancer Classification. Majority (10; 41.7%) patients had well differentiated SCC, followed by seven (29.1%) with moderately differentiated carcinoma histo-pathologically (Table 1). Measurements of target lesions, lymph node size and all perfusion parameters did not differ statistically by the type of squamous cell carcinoma (p > 0.05).
Table 1.
Baseline characteristics of the lesion type, size, and perfusion parameters (all values expressed in mean ± sd).a
| Measurement | Type of squamous cell carcinoma |
||||
|---|---|---|---|---|---|
| Well differentiated (n = 10) | Moderately differentiated (n = 7) | Poorly differentiated (n = 2) | Un-classified (n = 5) | All lesions (n = 24) | |
| Target lesion size (cm.) | 4.1 ± 1.1 | 4.0 ± 1.3 | 3.9 ± 0.9 | 5.7 ± 2.8 | 4.4 ± 1.7 |
| Lymph node size (cm.) | 1.6 ± 1.1 | 1.4 ± 0.5 | 1.3 ± 0.6 | 1.8 ± 0.6 | 1.5 ± 0.8 |
| Perfusion parameters | |||||
| BFb (ml/100 g/min) | 124.5 ± 33.5 | 103.7 ± 34.7 | 102.7 ± 9.4 | 81.8 ± 17.8 | 107.7 ± 33.0 |
| BVc (ml/100 g) | 12.3 ± 4.5 | 11.3 ± 3.1 | 8.3 ± 7.0 | 8.5 ± 2.2 | 10.9 ± 4.0 |
| MTTd (s) | 6.9 ± 0.9 | 7.8 ± 1.9 | 5.0 ± 4.2 | 7.8 ± 1.5 | 7.2 ± 1.8 |
| PSe (ml/100 g/min) | 45.7 ± 14.3 | 57.4 ± 17.9 | 62.5 ± 24.8 | 49.9 ± 12.7 | 51.4 ± 15.9 |
Standard deviation.
Blood flow.
Blood volume.
Mean transit time
Permeability surface.
At the end of chemoradiation, complete remission was achieved by 12 (50%), partial response by nine patients and three patients had static disease. None of the patients had progressive disease (Table 2).
Table 2.
Comparison of perfusion parameters at baseline, 40 GYa CRTb and 66 Gy CRT stratified by response to CRT (n = 24).
| Perfusion parameter | Responders (n = 12) |
Partial responders (n = 9) |
Static disease (n = 3) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 40 Gy CRT | 66 Gy CRT | p-Value | Baseline | 40 Gy CRT | 66 Gy CRT | p-Value | Baseline | 40 Gy CRT | 66 Gy CRT | p-Value | |
| BFc (ml/100 g/min) | 125.3 ± 25.7 | 122.7 ± 32.4 | 82.5 ± 36.3 | 0.002 | 86.7 ± 20.8 | 118.9 ± 39.1 | 85.4 ± 21.0 | 0.050 | 100.3 ± 56.5 | 92.1 ± 9.3 | 72.0 ± 28.5 | 0.264 |
| BVd (ml/100 g) | 12.2 ± 3.5 | 13.8 ± 6.6 | 10.1 ± 5.9 | 0.005 | 8.4 ± 2.6 | 12.5 ± 7.0 | 7.5 ± 2.7 | 0.147 | 13.4 ± 6.7 | 7.7 ± 0.7 | 7.3 ± 3.1 | 0.097 |
| MTTe (seconds) | 7.0 ± 1.2 | 7.1 ± 2.4 | 8.4 ± 2.2 | 0.024 | 7.1 ± 2.4 | 7.3 ± 2.2 | 6.2 ± 1.6 | 0.124 | 8.4 ± 1.5 | 5.7 ± 1.6 | 7.7 ± 0.9 | 0.178 |
| PSf (ml/100 g/min) | 48.7 ± 10.6 | 48.7 ± 14.9 | 41.2 ± 23.6 | 0.233 | 58.4 ± 16.3 | 38.3 ± 20.2 | 36.7 ± 13.4 | 0.032 | 41.0 ± 28.6 | 54.7 ± 38.1 | 18.5 ± 8.3 | 0.097 |
Gray unit.
Chemo-radiotherapy.
Blood flow.
Blood volume.
Mean transit time.
Permeability Surface.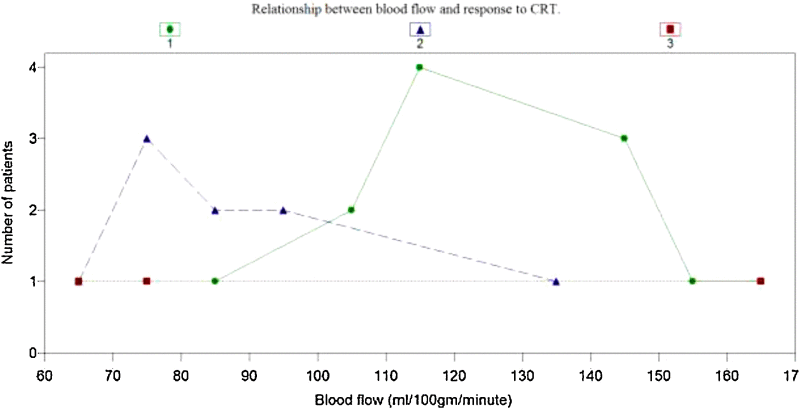
3.2. Perfusion parameters
At baseline BF was the only perfusion parameters that was significantly higher (p = 0.006) among responders as compared to non-responders (Table 3).
Table 3.
Comparison of perfusion parameters between complete responders and non-responders to CRT at baseline, 40 Gya CRTb and 66 Gy CRT (n = 24).
| Perfusion parameter | Baseline |
40 Gy CRT |
66 Gy CRT |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Responders (n = 12) | Non-responders (n = 12) | p-Value | Responders (n = 12) | Non-responders (n = 12) | p-Value | Responders (n = 12) | Non-responders (n = 12) | p-Value | |
| BFc (ml/100 g/min) | 125.3 ± 25.7 | 90.1 ± 30.5 | 0.006 | 122.7 ± 32.4 | 112.2 ± 35.7 | 0.461 | 82.5 ± 36.3 | 82.1 ± 22.5 | 0.974 |
| BVd (ml/100 g) | 12.2 ± 3.5 | 9.6 ± 4.3 | 0.128 | 13.8 ± 6.6 | 11.3 ± 6.4 | 0.351 | 10.1 ± 5.9 | 7.4 ± 2.6 | 0.356 |
| MTTe (s) | 7.0 ± 1.2 | 7.4 ± 2.2 | 0.523 | 7.1 ± 2.4 | 6.9 ± 2.1 | 0.860 | 8.4 ± 2.2 | 6.6 ± 1.6 | 0.034 |
| PSf (ml/100 g/min) | 48.7 ± 10.6 | 54.1 ± 20.1 | 0.386 | 48.7 ± 14.9 | 42.4 ± 24.8 | 0.456 | 41.2 ± 23.6 | 32.2 ± 14.6 | 0.272 |
Gray unit.
Chemo-radiotherapy.
Blood flow.
Blood volume.
Mean transit time.
Permeability surface.
During the course of chemoradiation, BF and BV both reduced significantly at 66 Gy in complete responders (p < 0.05) as compared to partial responders or patients with static disease. MTT in complete responders showed a slight temporary reduction at 40 Gy, which was followed by a rebound as measured at 66 Gy (p = 0.024) but changes in partial-responders and non-responders were not significant. PS values in the complete responders were almost stable after 40 Gy and insignificantly decreased at 66 Gy (p = 0.233) whereas in partial responders PS showed reduction at 40 Gy, which continued after 66 Gy (p = 0.032). In non-responders there was statistically insignificant elevation of PS at 40 Gy and reduction at 66 Gy (p = 0.097; Table 2).
Prognostically, patients with high BF were five times more likely to respond to chemoradiation as compared to patients with low BF (Table 4) and the difference was statistically significant (p = 0.004). High BF in our study was found to have 83.3% prognostic accuracy, sensitivity, specificity and positive predictive value each (Table 5).
Table 4.
Association between perfusion parameter value at baseline and response to chemo radiation (n = 24).
| Perfusion parameter | Complete response |
Risk Ratio | 95% CIa | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| High value (>median) |
Low value (≤median) |
||||||||
| No. | Total | % | No. | Total | % | ||||
| Blood Flow (median = 106 ml/100 g/min) | 10 | 12 | 83.3 | 2 | 12 | 16.7 | 5.00 | 1.38–18.2 | 0.004 |
| Blood volume (median = 10.1 ml/100 g) | 7 | 11 | 63.6 | 5 | 13 | 38.5 | 1.65 | 0.73–3.76 | 0.413 |
| Mean transit time (median = 6.9 s) | 4 | 11 | 36.4 | 8 | 13 | 61.5 | 0.59 | 0.24–1.44 | 0.413 |
| Permeability surface (median = 47.0 ml/100 g/min) | 5 | 12 | 41.7 | 7 | 12 | 58.3 | 0.71 | 0.31–1.62 | 0.683 |
Confidence interval.
Table 5.
Sensitivity, specificity, PPVa, NPVb, LHRc (positive) and LHR (negative) of dichotomised (high/low) values of perfusion parameters at baseline and there combinations.
| Perfusion parameters | Prognostic accuracy (%) | Sensitivity |
Specificity |
PPV |
NPV |
LHR (positive) |
LHR (negative) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CId | % | 95% CI | % | 95% CI | % | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| Highe BFf (n = 12) | 83.3 | 83.3 | 55.2–95.3 | 83.3 | 55.2–95.3 | 83.3 | 55.2–95.3 | 83.3 | 55.2–95.3 | 5.0 | 1.8–13.9 | 0.20 | 0.07–0.55 |
| High BVg (n = 11) | 62.5 | 58.3 | 32.0–80.7 | 66.7 | 39.1–86.2 | 63.6 | 35.4–84.8 | 61.5 | 35.5–82.3 | 1.8 | 0.9–3.5 | 0.63 | 0.37–1.05 |
| Lowh MTTi (n = 13) | 62.5 | 66.7 | 39.1–86.2 | 58.3 | 32.0–80.7 | 61.5 | 35.5–82.3 | 63.6 | 35.4–84.8 | 1.6 | 1.0–2.7 | 0.57 | 0.29–1.14 |
| Low PSj (n = 12) | 58.3 | 58.3 | 32.0–80.7 | 58.3 | 32.0–80.7 | 58.3 | 32.0–80.7 | 58.3 | 32.0–80.7 | 1.4 | 0.8–2.5 | 0.71 | 0.40–1.29 |
| High BF & high BV (n = 9) | 70.8 | 58.3 | 32.0–80.7 | 83.3 | 55.2–95.3 | 77.8 | 45.3–93.7 | 66.7 | 41.7–84.8 | 3.5 | 1.1–11.4 | 0.50 | 0.32–0.77 |
| High BF and low MTT (n = 8) | 75.0 | 58.3 | 32.0–80.7 | 91.7 | 64.6–98.5 | 87.5 | 52.9–97.8 | 68.8 | 44.4–85.8 | 7.0 | 0.8–60.7 | 0.45 | 0.30–0.68 |
| High BF and low PS (n = 6) | 75.0 | 50.0 | 25.4–74.6 | 100.0 | 75.8–100.0 | 100.0 | 61.0–100.0 | 66.7 | 43.8–83.7 | Undefined | Undefined | 0.50 | 0.36–0.69 |
Positive predictive value.
Negative predictive value.
Likelihood ratio.
Confidence interval.
High > median value.
Blood flow (ml/100 g/min).
Blood volume (ml/100 g).
Low (≤median value).
Mean transit time (s).
Permeability surface (ml/100 g/min).
On graphical analysis (Fig. 1) BF of ≥85 ml/100 g/min was predictor of complete response to treatment in 11 (73.3%) out of 15 patients. Out of 12 completer responders, 11 (92.0%) had BF above 85 ml/100 g/min.
Fig. 1.
Post contrast CT (a), source image (b), BF (c), BV (d) MTT (e) and PS (f) maps.
Positive predictive value (PPV) of high BV was next to high BF, followed by low MTT and low PS (Table 5).
After stratifying the patients in four different patterns of combination of perfusion parameters [15] i.e. High BF with high PS, High BF with low PS, low BF with high PS and low BF with low PS, there was 100% response to treatment in the perfusion parameter combination of high BF and low PS while 66.6% complete responders were seen with high BF and high PS combination irrespective of the stage of HNSCC (Table 6).
Table 6.
Predictive value of different combination of baseline blood flow (BF) and permeability surface (PS).
| Blood flow and permeability | Participants (n = 24) |
Stage of the tumor (n = 24) |
Complete response |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | Stage III |
Stage IV |
No. | % | |||
| No. | % | No. | % | |||||
| Higha BF and Lowb PS | 6 | 25.0 | 4 | 66.7 | 2 | 33.3 | 6 | 100.0 |
| High BF and High PS | 6 | 25.0 | 6 | 100.0 | 0 | 0.0 | 4 | 66.7 |
| Low BF and High PS | 6 | 25.0 | 4 | 66.7 | 2 | 33.3 | 1 | 16.7 |
| Low BF and Low PS | 6 | 25.0 | 4 | 66.7 | 2 | 33.3 | 1 | 16.7 |
>Median value.
≤Median value
3.3. Bivariate analysis
On bivariate analysis, patients with high blood flow were found to be five times more likely to respond as compared to the patients with low BF at baseline. Other perfusion parameters were not significantly associated with the response probably due to low power of the study (Table 4).
4. Discussion
Angiogenesis is an important aspect of tumorigenesis. Primary stimulus for new vessel formation is presumed to be hypoxia induced by expansion of growing cellular tumor mass. Hypoxia induces the expression of HIF-1, VEGF, PDGF and carbonic anhydrase IX [14]. Goh at el [15] have reported that tumor vessels are different and show arteriovenous shunting, intermittent or reverse flow. In the center of the tumor there is decline of vascular density with higher number of elongated compressed vessels having high intervessel and interbranch distances. There is acute vascular collapse due to raised interstitial pressure. The endothelial lining of tumor vessel wall has loose endothelial connections, bigger openings and lack in smooth muscle and pericyte covering. Defective endothelium causes vascular hyperpermeability which results in raised intratumoral interstitial pressure.
The changes happening at vascular level cannot be assessed by RECIST 1.1 which is still considered as gold standard for assessing the tumor response to chemo-radiotherapy [16], [17]. CTp assesses not only the tumor morphology but is a functional imaging biomarker reflecting angiogenesis and can predict tumor response to treatment and vascular changes after treatment even before there is reduction in the tumor size [12].
The Z-axis coverage in CT perfusion of head and neck varied from 1 cm to 2.5 cm in different studies [8], [18]. Limited coverage resulted in centering of the perfusion study at the level of the largest tumor diameter only. However due to heterogeneity of the tumor and its perfusion parameters whole tumor volume should be encompassed in the perfusion protocols.
Dynamic CT perfusion can be performed using cine mode, a combination of cine and axial modes or a toggling table technique [19]. Axial CT perfusion allows discontinuous scanning and the area of coverage is 4 cm only as there is no table toggling motion. Though cine CT perfusion allows axial continuous scanning but area of coverage of 4 cm only. In the present study, CTp was done with Z-axis coverage of 14 cm using the Volume Helical Shuttle [VHS] that enabled visualization of entire tumuor. With wider z-axis coverage tumor heterogeneity is better assessed because whole tumor perfusion parameters can be quantified at all levels and necrotic areas can be excluded while calculating the perfusion parameters. According to ACR–ASNR–SPR practice guideline for the performance of computed tomography (CT) perfusion in neuroradiologic imaging [20], “in toggling table technique, the temporal resolution at each location should be no less than 1 image per second.” In the VHS with use of Dynamic Pitch Cone Beam Reconstruction (DPCB), VT patient table and advanced real-time control, extended z-coverage and Tuned Auto mA the resulting image at the central location of scan is scanned or has an average temporal sampling of 1.5 s.
Our results show that responders had significant higher baseline BF values than the non-responders. Similar results have been found not only in HNSCC but also in rectal and lung malignancies. Tumors adapt to hypoxia by promoting more neo-angiogenesis leading to higher BF and PS. Low tumor perfusion indicates less blood flow to the tumor which in turn impairs the delivery of the chemotherapeutic agents, hypoxia also decreases radiosensitivity [21]. Another study [22] concluded that pretreatment tumor BF was significantly higher in responders. Our findings were in partial agreement with another study [23] which showed the long-term predictive value of baseline BF and PS measurements from CTp imaging for local control in 84 patients undergoing chemoradiation.
Perfusion parameters were compared according to the volume of the tumor. Patients having higher BF at baseline showed significant reduction in volume while patient having low BF were non-responder for volume reduction [p = 0.003]. There was no statistically significant correlation in BV, MTT and PS values among responders and non-responders. Similar methodology has been done by Zima et al. [9] but they employed invasive endoscopic procedure for this calculation.
Goh et al. [15] divided perfusion parameters of a tumor into four different patterns of vascularization – high BF and high PS as matched vascularization, high BF and low PS as high interstitial pressure, low BF and high PS as mismatched vascularization and low BF and low PS as necrosis. In our study we combined BF and PS in these four categories and to predict the response to treatment. After stratifying the patients in four different patterns of combination of perfusion parameters i.e. high BF with high PS, high BF with low PS, low BF with high PS and low BF with low PS, there was 100% response to treatment in the perfusion parameter combination of high BF and low PS while 66.6% complete responders were seen with high BF and high PS combination irrespective of the stage of HNSCC. We observed that relying only on morphological criteria for staging may be inadequate and perfusion status of tumor plays more important role in determining the response of the tumor to the treatment. As this doctrine has already been included in the PERCIST criteria for solid tumor evaluation, we propose that CTP parameters should also be incorporated along with RECIST criteria or alone for tumor evaluation. Due to wide availability of MDCT as compared to PET-CT, CTp appears to be good alternative to PET-CT especially in the developing countries.
We attempted to classify HNSCC into histopathological types and TNM staging and evaluate their perfusion parameters to compare them with RECIST 1.1 criteria. However, changes in them were not statistically significant. CTp values, therefore, are not dependent on the histopathological subtypes.
In responders, BF showed decreasing trend at 40 Gy and 66 Gy of treatment and this change was statistically significant (p = 0.002). BV increased at 40 Gy but decreased at 66 Gy of CRT which was statistically significant (p = 0.005). MTT also showed statistically significant (p = 0.024) increase at 40 Gy and 66 Gy. This is attributed to cytotoxic effect of radiotherapy to the vascular endothelial cells. The fluctuation of perfusion parameters during the course of therapy is due to hypoxia and intratumoral inflammation as hypoxia may lead to decrease in perfusion and inflammation further lead to increase in BF and PS. Changes in PS values were not statistically significant (p = 0.233).
In partial responders, BF increased at 40 Gy and thereafter decreased significantly (p = 0.050) at 66 Gy. The changes in values of BV, MTT and PS were statistically insignificant.
In patients with stable disease (non-responders) changes in all the perfusion parameters at baseline, 40 Gy and 66 Gy were statistically insignificant.
The serial study done at baseline, 40 Gy and 66 Gy revealed that the change in perfusion parameters at 40 Gy is not reliable to predict response as compared to changes at completion of therapy. Therefore CTp done at 40 Gy appears to be clinically insignificant and can be done away with.
5. Conclusions
High BF at baseline is the single best predictor of response to chemoradiaton. A combination of high BF and low PS was found to be 100% predictive of complete response irrespective of the stage of the tumor. CTp parameter measurements at 40 GY of chemoradation are not indicative for response to treatment.
Conflict of interest
None declared.
Contributor Information
Lokesh Rana, Email: poojalokesh2007@gmail.com.
Sanjiv Sharma, Email: sanjeev_sai_2@yahoo.co.in.
Shikha Sood, Email: manishsharma57@yahoo.com.
Balraj Singh, Email: drbalraj@gmail.com.
Manoj K. Gupta, Email: mkgupta62@yahoo.co.in.
R.S. Minhas, Email: raviminhas@yahoo.com.
Anupam Jhobta, Email: bhavuvasu@rediffmail.com.
Vikas Bhatia, Email: drvikasbhatia@gmail.com.
Bargavee Venkat, Email: bargavee@gmail.com.
References
- 1.Miles K.A. Perfusion CT for the assessment of tumour vascularity which protocol? Br J Radiol. 2003;76:S36–S42. doi: 10.1259/bjr/18486642. [DOI] [PubMed] [Google Scholar]
- 2.Miles K.A., Hayball M., Dixon A.K. Colour perfusion imaging: a new application of computed tomography. Lancet. 1991;337:643–645. doi: 10.1016/0140-6736(91)92455-b. [DOI] [PubMed] [Google Scholar]
- 3.Miles K.A. Perfusion CT: a worthwhile enhancement? Br J Radiol. 2007;6:220–231. doi: 10.1259/bjr/13564625. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan A., Mohan S., Mukherji S.K. Biologic imaging of head and neck cancer: the present and future. Am J Neuroradiol. 2012;33:586–594. doi: 10.3174/ajnr.A2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faggioni L., Neri E., Bartolozzi C. CT perfusion of head and neck tumours: how we do it. Am J Rev. 2010;194:A62–A69. doi: 10.2214/ajr.09.3187. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt P., Kotas M., Tobermann A., Haase A., Flentje M. Quantitative tissue perfusion measurements in head and neck carcinoma patients before and during radiation therapy with a non-invasive MR imaging spin-labeling technique. Radiother Oncol. 2003;67:27–34. doi: 10.1016/s0167-8140(03)00024-0. [DOI] [PubMed] [Google Scholar]
- 7.Hermans R., Meijerink M., Van den Bogaert W., Rijnders A., Weltens C., Lambin P. Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:1351–1356. doi: 10.1016/s0360-3016(03)00764-8. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi D., Chepeha D.B., Miller T., Carlos R.C., Bradford C.R., Karamchandani R. Correlation between initial and early follow-up CT perfusion parameters with endoscopic tumour response in patients with advanced squamous cell carcinomas of oropharynx treated with organ-preservation therapy. Am J Neuroradiol. 2006;27:101–106. [PMC free article] [PubMed] [Google Scholar]
- 9.Zima A., Carlos R., Gandhi D., Case I., Teknos T., Mukherji S.K. Can pretreatment CT perfusion predict response of advanced squamous cell carcinoma of the upper aerodigestive tract treated with induction chemotherapy? Am J Neuroradiol. 2007;28:328–334. [PMC free article] [PubMed] [Google Scholar]
- 10.Miles K.A., Hayball M.P., Dixon A.K. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405–411. doi: 10.1148/radiology.188.2.8327686. [DOI] [PubMed] [Google Scholar]
- 11.Shah G.V., Wesolowski J.R., Ansari S.A., Mukherji S.K. New directions in head and neck imaging. J Surg Oncol. 2008;97:644–648. doi: 10.1002/jso.21022. [DOI] [PubMed] [Google Scholar]
- 12.Ash L., Teknos T.N., Gandhi D., Patel S., Mukherji S.K. Head and neck squamous cell carcinoma: CT perfusion can help noninvasively predict intratumoral microvessel density. Radiology. 2009;251:2–6. doi: 10.1148/radiol.2512080743. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi D., Hoeffner E.G., Carlos R.C., Case I., Mukherji S.K. Computed tomography perfusion of squamous cell carcinoma of the upper aerodigestive tract. Initial results. J Comput Assist Tomogr. 2003;27:687–693. doi: 10.1097/00004728-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Figueiras R.G., Padhani A.R., Goh V.J., Vilanova J.C., González S.B., Martín C.V. Novel oncological drugs: what they do and how they affect images. Radiographics. 2011;31:2059–2091. doi: 10.1148/rg.317115108. [DOI] [PubMed] [Google Scholar]
- 15.Goh V., Glynne-Jones R. Perfusion CT imaging of colorectal cancer. Br J Radiol. 2014;87:20130811. doi: 10.1259/bjr.20130811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino M., Jagannathan J.P., Ramaiya N.H., Van den Abbeele A.D. Revised RECIST Guideline version 1.1: what oncologist wants to know and what radiologists need to know. Am J Roentgenol. 2010;195:281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 17.Padhani A.R. The RECIST criteria: implications for diagnostic radiologists. Br J Radiol. 2001;74:983–986. doi: 10.1259/bjr.74.887.740983. [DOI] [PubMed] [Google Scholar]
- 18.Bisdas S., Rumboldt Z., Surlan-Popovic K., Baghi M., Koh T.S., Vogl T.J. Perfusion CT in squamous cell carcinoma of the upper aerodigestive tract: long-term predictive value of baseline perfusion CT measurements. Am J Neuroradiol. 2010;31:576–581. doi: 10.3174/ajnr.A1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts H.C., Roberts T.P., Smith W.S., Lee T.J., Fischbein N.J., Dillon W.P. Multisection dynamic CT perfusion for acute cerebral ischemia: the “toggling table” technique. Am J Neuroradiol. 2001;22:1077–1080. [PMC free article] [PubMed] [Google Scholar]
- 20.2012. ACR–ASNR–SPR practice guideline for the performance of computed tomography (CT) perfusion in neuroradiologic imaging. [resolution 12] [Google Scholar]
- 21.Petralia G., Preda L., Raimondi S., D’Andrea G., Summers P., Giugliano G. Intra- and interobserver agreement and impact of arterial input selection in perfusion ct measurements performed in squamous cell carcinoma of the upper aerodigestive tract. Am J Neuroradiol. 2009;30:1107–1115. doi: 10.3174/ajnr.A1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong M.T., Saito N., Ozonoff A., Wang J., Lee R., Qureshi M.M. Prediction of locoregional control in head and neck squamous cell carcinoma with serial CT perfusion during radiotherapy. Am J Neuroradiol. 2011;32:1195–1201. doi: 10.3174/ajnr.A2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bisdas S., Spicer K., Rumboldt Z. Whole-tumor perfusion CT parameters and glucose metabolism measurements in head and neck squamous cell carcinomas: a pilot study using combined positron-emission tomography/CT imaging. Am J Neuroradiol. 2008;29:1376–1381. doi: 10.3174/ajnr.A1111. [DOI] [PMC free article] [PubMed] [Google Scholar]