Abstract
Hunter disease is an X-linked lysosomal storage disorder characterized by progressive storage of glycosaminoglycans (GAGs) and multi-organ impairment. The central nervous system (CNS) is involved in at least 50% of cases. Since 2006, the enzymatic replacement therapy (ERT) is available but with no effect on the cognitive impairment, as the present formulation does not cross the blood–brain barrier. Here we report the outcome of 17 Hunter patients treated in a single center. Most of them (11) started ERT in 2006, 3 had started it earlier in 2004, enrolled in the phase III trial, and 3 after 2006, as soon as the diagnosis was made. The liver and spleen sizes and urinary GAGs significantly decreased and normalized throughout the treatment. Heart parameters improved, in particular the left ventricular mass index/m2 decreased significantly. Amelioration of hearing was seen in many patients. Joint range of motion improved in all patients. However, no improvement on respiratory function, eye, skeletal and CNS disease was found. The developmental quotient of patients with a CNS involvement showed a fast decline. These patients were no more testable after 6 years of age and, albeit the benefits drawn from ERT, their quality of life worsened throughout the years. The whole group of patients showed a consistent residual disease burden mainly represented by persistent skeletal disease and frequent need of surgery. This study suggests that early diagnosis and treatment and other different therapies which are able to cross the blood–brain barrier, might in the future improve the MPS II outcome.
Abbreviations: 6MWT, Six minute walking test; BAER, Brainstem auditory evoked responses; CNS, Central nervous system; EF, Ejection fraction; ERT, Enzyme replacement therapy; ENT, Ear nose and throat; GAGs, Glycosaminoglycans; HAQ, Health Assessment Questionnaire; I2S, Iduronate-2-sulfatase; JROM, Joint range of motion; LVM/LVMI, Left ventricular mass/left-ventricular mass index; MDCT, Multidetector computed tomography; MPS, Mucopolysaccharidosis; MPS II, Mucopolysaccharidosis type II; MRI, Magnetic resonance imaging; QoL, Quality of Life; UAI, Upper airway infections
Keywords: MPS II, Hunter disease, Hunter syndrome, Mucopolysaccharidosis type II, ERT, Enzymatic replacement therapy, Idursulfase
1. Introduction
Mucopolysaccharidosis type II (MPS II) or Hunter syndrome (OMIM# 309900) is a lysosomal storage disorder due to mutations of the gene encoding the enzyme iduronate-2-sulfatase (I2S); it is the only X-linked mucopolysaccharidosis (MPS). Female carriers are usually asymptomatic [1]. Prevalence is around 1:140,000–156,000 male live births [1]. Glycosaminoglycans (GAGs) deposition virtually affects all tissues and organs: this produces direct damage but also triggers secondary and tertiary mechanisms leading to distortion of tissues and loss of organ function as the final effect [2]. Symptoms and signs are clinically recognizable at any age [3], [4] depending on the severity of the disease. The main features of MPS II patients are dysmorphic facies, skeletal abnormalities, hepatosplenomegaly, cardiomyopathy and heart valve disease, airway obstructions, hearing impairment and central nervous system (CNS) involvement with mental retardation in more than 50% of the cases [4]. Treatment for Hunter syndrome was mainly palliative until enzymatic replacement therapy (ERT) became available in 2006 [1], [5]. The drug was approved on the basis of a phase II/III study involving 96 patients with the attenuated form of MPS II (no cognitive involvement) [5]. The existing ERT formulation does not cross the blood–brain barrier but it has been reported to improve many somatic symptoms and signs of the disease [6], [7]. Another treatment which is currently available is the hematopoietic stem cell transplantation (HSCT); in the last 20 years this treatment has been used in few Hunter patients but its effects on CNS and cognitive development are not encouraging, contrary to what is observed in severe MPS I [8], [9]. Here we analyze the long-term clinical outcome of 17 Hunter patients on ERT, treated in a single center. Partial data of 7 of these patients were included in the AIFA study by Tomanin et al. [10].
2. Materials and methods
2.1. Setting
This is a single-center observational prospective study. Patients were treated and followed up according to a standardized clinical protocol for mucopolysaccharidosis type II [6]. No informed consent was requested as all tests were part of the regular follow-up procedures of the center.
2.2. Patients
17 male patients (Table 1) affected by MPS II were treated with idursulfase (Elaprase® Shire Human Genetic Therapies, Cambridge, MA) at a dose of 0.5 mg/kg weekly intravenously. For all patients, diagnosis of MPS II was confirmed by low I2S activity in skin fibroblasts or leukocytes and pathologic mutations in I2S gene. One patient (no. 14), in agreement with his parents, discontinued ERT after 3 years following a tracheotomy for respiratory insufficiency. He died one year after the discontinuation of the treatment at 11 years of age. The other 16 patients were treated for a minimum period of 5.1 years to a maximum period of 10.3 years (mean 7.8, median 7.8).
Table 1.
Characteristics of the 17 Hunter patients included in the study. # = brothers; * U/mg/h fibroblasts (nc 17); ⁎⁎ nmol/h/mgpt fibroblasts (nv 2.4–18.4); *** U/mg proteins (nv 9–31); **** nmol/mg/4 h fibroblasts (nv 36 ± 9). Note that patient 14 stopped ERT after 3 years and died at home one year later.
| Patient-phenotype | I2S activity in leukocytes nmol/h/mgpt (nv 2.3–7.9) |
Mutation in the I2S gene | Age at onset of first signs and/or symptoms (m) | Age at diagnosis (y) | Age at starting ERT (y) | Age at end of study (y) | Years on ERT at the last evaluation (January–July 2014) |
|---|---|---|---|---|---|---|---|
| 1-B | 0.3* | G374G | 0.5 | 3 | 10.2 | 18.1 | 7.8 |
| 2-B | 0.19* | c878G > A | 12 | 4.2 | 8 | 17.7 | 9.7 |
| 3-A | 0.019** | R468W | 24 | 2.2 | 7.7 | 15.5 | 7.9 |
| 4-A | 0.5 | Q521K | 10 | 3.8 | 8.7 | 16.5 | 7.8 |
| 5-A | 0.1*** | Q80X | 36 | 4 | 15.7 | 22.2 | 6.4 |
| 6-A | 0.23 | c.189delT | 28 | 2.7 | 5.2 | 13.1 | 7.8 |
| 7-B | 0.08 | A82V | 36 | 3.8 | 16.3 | 26.2 | 9.9 |
| 8-A | 0.4*** | Y103X | 2 | 2.5 | 11.6 | 18.6 | 7.0 |
| 9-A | 0.0 | G140V | 24 | 3.7 | 12.7 | 20.2 | 7.4 |
| 10-A | 0.008**** | del exon 8 | 4 | 4 | 12.8 | 20.6 | 7.7 |
| 11-A# | 0.02 | p.P86L | 24 | 2 | 2.3 | 7.3 | 5.1 |
| 12-A # | 0.02 | p.P86L | 4 | 3.3 | 3.7 | 8.7 | 5.1 |
| 13-B | 0.1 | R443X | 0 | 4.5 | 7.0 | 14.8 | 7.8 |
| 14-A | 0.27 | Y264X | 4 | 4.1 | 7.2 | 11.2 | 3.0 |
| 15-A/B | 0.0 | R443X | 24 | 4.2 | 25.5 | 34.0 | 7.5 |
| 16-A | 0.12 | ΔH159 | 4 | 2.8 | 12.0 | 19.8 | 7.8 |
| 17-B | 0.16 | R443X | 2 | 4.6 | 6.2 | 16.5 | 10.3 |
| Median | 11 | 4 | 8 | 7.8 | |||
| Mean | 14.9 | 3 | 9 | 7.4 |
Most of them (11) started ERT in 2006, 3 (no. 2, 7, 17) had started earlier (2004) as benefitted from enrollment in the phase III trial (two different European sites). The remaining 3 patients started at a later date as soon as the diagnosis was made. 11 patients had CNS involvement (phenotype A), 5 did not (phenotype B) and one (no. 15) was considered intermediate (phenotype A/B) as he had mild cognitive delay and very severe somatic involvement at the age of 25.7 years (baseline of this study). He was included in group A.
3. Methods
All the patients had an at least yearly multidisciplinary evaluation according to Muenzer et al. [6]. A multi-detector computed tomography (MDCT) of the upper airways was performed in addition to the standard evaluation if clinically needed. The baseline evaluation was performed in the 6 months prior to the start of ERT and the last evaluation was carried out within 6 months from end of study (January–July 2014). The liver size was evaluated by abdominal examination and the spleen size by abdominal ultrasounds [11]; corneal clouding was scored as 1 (normal cornea), 2 (mild clouding) or 3 (severe clouding). Data about retina were scored as 1 for normal retina and 2 for retinal degeneration. Left ventricular mass index (LVMI) was calculated as the left ventricular mass normalized for body surface area according to the recommendations of the American Society of Echocardiography (ASE) [12]. Normal geometry (no hypertrophy) was considered < 115 g/m2 [13]. Functional status and Quality of Life (QoL) were tested through the MPS Health Assessment Questionnaire (MPS-HAQ) [14]. Cognitive function was evaluated through specific tests (administered by PM or FN) chosen according to the patient's age and clinical status: Griffiths Developmental Mental Scales, Bayley Scales of Infant Development (BSID-II), Wechsler scales (WISC-R, WISC-III, WPPSI), Stanford–Binet and Leiter International Performance Scale-Revised (Leiter-R). Each result was converted to a Z-score. Z-scores were classified according to Orsini and Laicardi [15] and ICD-10 Guide for Mental Retardation. (www.who.int/mental_health/media/en/69.pdf accessed on 20 August 2014; World Health Organization, Geneva, 1996). Vineland Adaptive Behavior scales were used to evaluate the most severely cognitively compromised patients [16].
3.1. Statistics
In order to analyze the impact of ERT, the Wilcoxon rank sum test was performed on the changes from baseline to 5 and 7 years of ERT, to test significant differences in the following clinical parameters: urinary GAGs, splenomegaly, hepatomegaly, heart echocardiographic parameters and Joint Range of Motion (JROM). All tests were two-sided. SAS 9.2 package (SAS Institute, Cary, NC) was used for data analysis.
4. Results
4.1. Safety
A total of 6085 infusions were administered. Infusion-related adverse reactions (IARs) occurred in 4/17 patients. The most common IARs were itching and urticaria that occurred from 1 month to 7 years after starting ERT. Anti-idursulfase antibodies (Abs) were absent or mildly increased (IgG 800–1600), with absent IgE. All reactions were treated and prevented by the usage of antihistamine and corticosteroid medications. One patient (no. 8), in agreement with the family, discontinued the treatment after 7 years due to IARs (urticaria and wheezing) and progressive CNS involvement.
4.2. Urinary GAGs
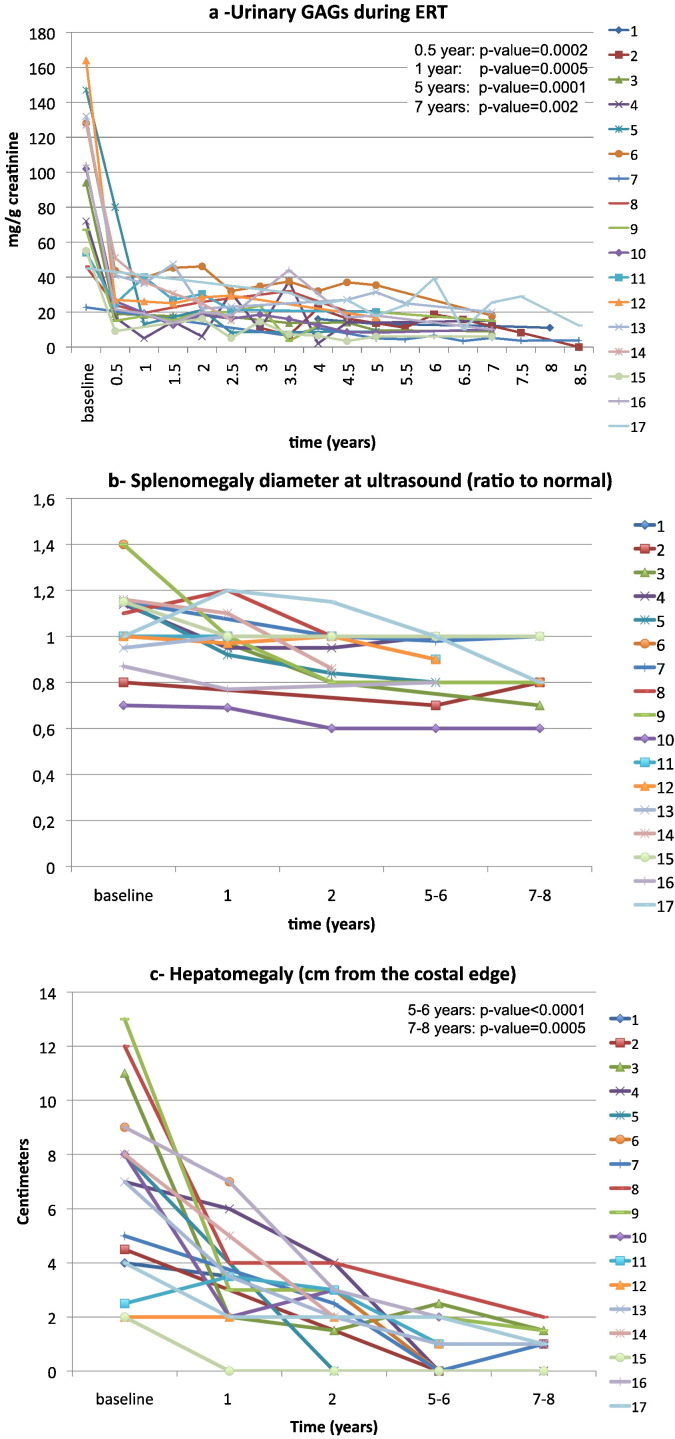
Urinary GAGs were elevated at baseline and decreased significantly during the first year of treatment (p = 0.0005). The significantly lower values compared to baseline were maintained throughout the treatment for 5 (16 subjects p = 0.0001) and 7 years (12 subjects p = 0.002) (Fig. 1a).
Fig. 1.
Results of treatment with idursulfase in 17 MPS II patients. In the right upper corner of some figures the p-value is given. 1a shows the decrease of urinary glycosaminoglycans from baseline during ERT; 1b: individual ratio to normal of splenomegaly at baseline and at 2, 5–6 and 7–8 years of ERT are reported: after 5 years of treatment no patient has splenomegaly; and 1c: hepatomegaly decreased over time: only very mild hepatomegaly was found at 7–8 years of ERT.
4.3. Organomegaly
Organomegaly decreased progressively and steadily throughout the years of treatment. Difference from baseline was significant both for the spleen (at 5–6 years 15 pts: p = 0.0005; at 7–8 years 12 pts: p = 0.004) and for the liver (at 5–6 years 16 pts: p < 0.0001; at 7–8 years 12 pts: p = 0.0005) (Fig. 1b and c).
4.4. Anthropometry
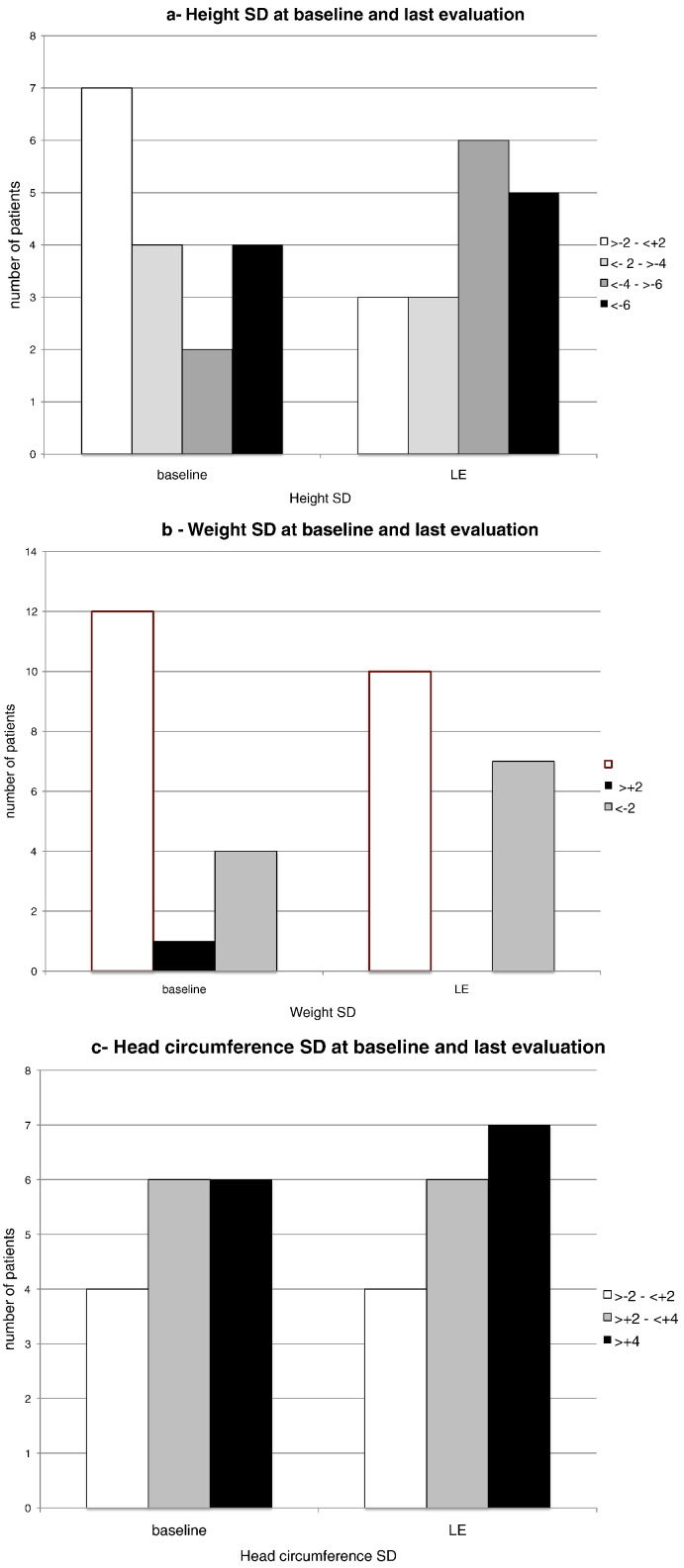
Fig. 2a shows that at baseline 7 patients had height within normal Standard Deviations (SD) (age 2.3–8.7 years, mean 5.9) and 10 of them (age 7.2–25.5 years, mean 13.2) had height below − 2 SD (between − 2.3 and − 7.4 SD). At the last evaluation (from 3 to 10.3 years of treatment) only 3 patients were within normal range and the other 14 were below − 2 SD (between − 2.3 and − 11.3 SD). Two brothers (no. 11 and no. 12), who started the therapy respectively at 4 and 2 years of age, had heights in the normal range at the last follow-up but interestingly the youngest (age 7.2 y 0 SD) was taller than his brother (age 9.6 years − 2 SD). Most of these patients had normal weight in the first years of life and became underweight during adolescence (Fig. 2b). Macrocrania was a common finding that worsened or remained stable over the years (Fig. 2c).
Fig. 2.
Anthropometry during ERT in 17 Hunter subjects. 2a, 2b and 2c show the number of subjects with normal (>− 2–< 2 SD, white bars) or abnormal (gray or black bars) anthropometric parameters (height, weight, head circumference respectively) at baseline (BL) and at last evaluation (LE) (5–9 years of ERT).
4.5. Eyes, ENT and respiratory assessment
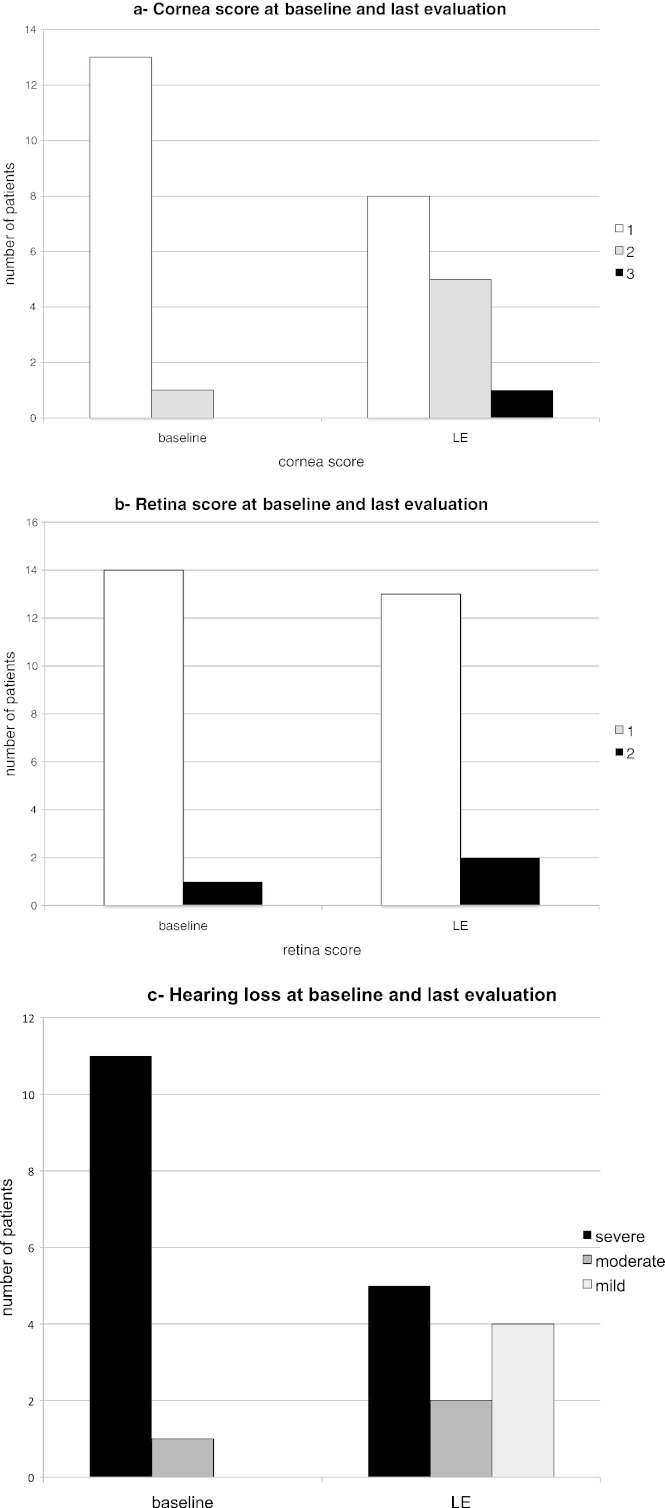
The corneal clouding and the retinal degeneration showed a tendency to worsen (Fig. 3a and b).
Fig. 3.
Eyes and hearing evaluation during ERT in 17 MPS II patients (baseline—BL- and last evaluation—LE- at 5–9 years of ERT). 3a and b shows the stratification of severity of cornea and retina scores respectively. Higher scores at the last evaluation reflect more severe ocular damage. 3c: hearing deficit stratification shows an improvement with ERT.
The hearing was tested at baseline in 12 patients. 11 had a severe (70–110 dB) hearing impairment (between 2000 and 4000 Hz) and one (no. 17) had a moderate hearing loss. On ERT, 6 patients improved with a gain of 20–50 dB, 4 stabilized and one patient (no. 17) worsened (Fig. 3c). One patient was never tested after baseline.
Cardiorespiratory monitoring and/or continuous nocturnal oxygen saturation were performed in 10 patients. Only two patients (nos. 3 and 7) had severe obstructive apneas prior to and following 7 and 5 years of ERT, respectively. Patient no. 14 underwent a tracheotomy 3 years after starting ERT, while suffering from a severe acute bronchopulmonary infection. Spirometry was performed in all 5 patients belonging to group B: patient no. 2 had a normal result both at baseline and at last examination. The other 4 patients had a severe obstructive airway disease at the last evaluation. Patient no. 7 and patient no. 17 worsened from baseline. MDCT of the airways was carried out in patients nos. 1, 3, 4, 6, 7 and 9, if signs or symptoms suggested tracheal stenosis, or as part of their anesthesiological evaluation before a surgical procedure. These patients had tracheal stenosis in the medium (2 patients) or in the distal tract (1 patient) or in both (3 patients) 4–6 years after starting ERT.
4.6. Heart
Patient no. 15 had undergone mitral and aortic mechanical valve substitution at the age of 13 and at the time of this study he had tricuspidal insufficiency. One patient had all valves involved, 2 other patients had 3 valves damaged; all the others had 2 valves involvement with the prevalence of mitral and aortic. At the last follow-up the severity of valve disease had worsened in 4 patients and was unchanged in the others.
At baseline, ejection fraction (EF) was reduced in 2 patients (nos. 14 and 15) who improved at the last evaluation. Only patient no. 10 worsened, developing a very mild EF reduction.
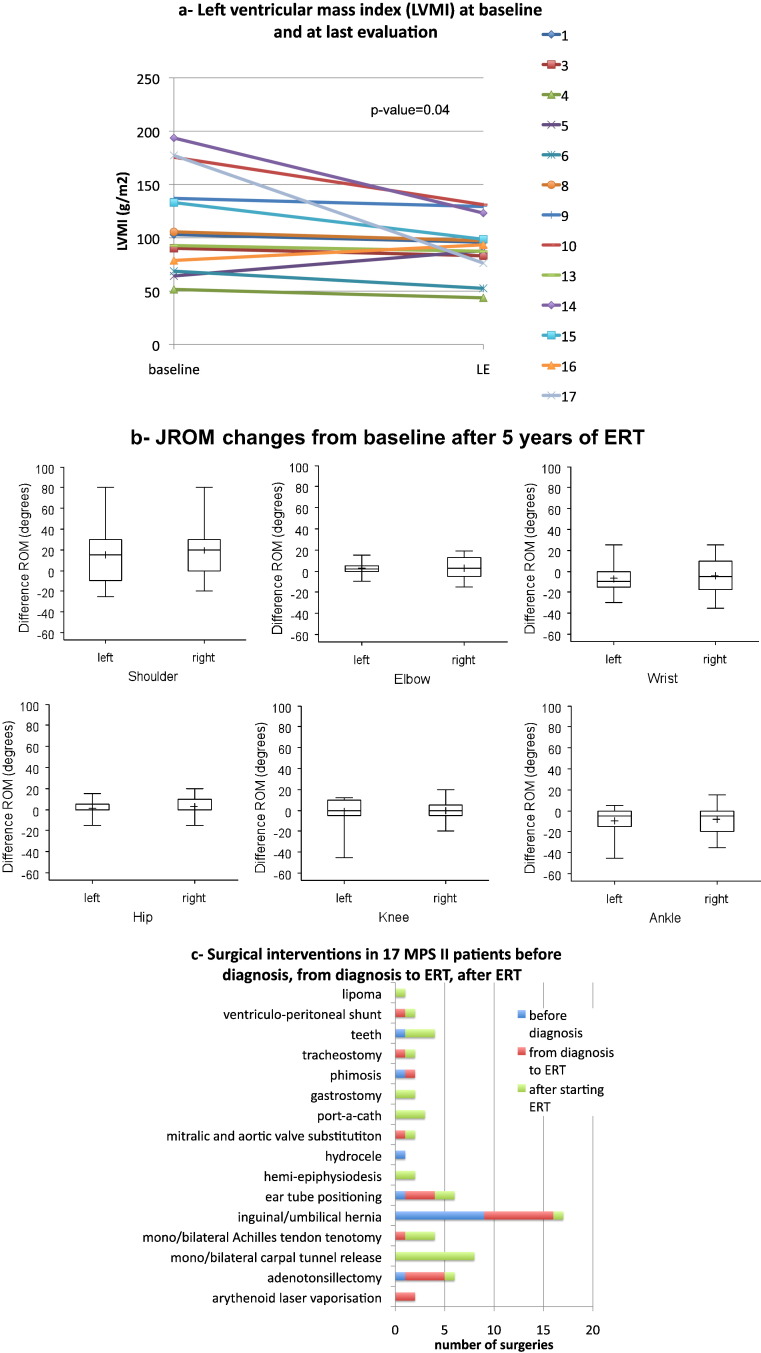
12/13 patients showed a significant reduction of LVMI at the last evaluation compared to baseline (5–8 years of treatment, Fig. 4a). A clear improvement of LVMI (g/m2) was particularly seen in 4/13 patients who had an overt hypertrophy at baseline (136.9–193.6, g/m2 to 76.3–131.1 g/m2).
Fig. 4.
Other results of ERT in 17 MPS II patients. 4a: Left ventricular mass index (LVMI) (mg/m2) at baseline and last evaluation (LE): significant decrease (p = 0.04). 4b shows the changes from baseline of last joint range of motion (JROM) evaluation: only mild improvements not reaching significance were obtained for all the examined joints except for right shoulder (p = 0.04). 4c: number and type of surgeries in 17 MPS II Hunter patients before and during ERT.
4 patients (nos. 2, 7, 11 and 12) underwent baseline evaluation in other hospitals and their baseline LVM assessment was not comparable or not available. No. 7 had LVM hypertrophy (143.4 g/m2) after 9 years of ERT, the other 3 were normal, at the last evaluation.
All 17 patients' median and mean LVMI at the last evaluation were 93.45 and 94.4 g/m2 respectively (range of 43.7–143.4 g/m2; SD 26.3).
4.7. JROM, 6 minute walking test and QoL assessment
Most of the improvement of JROM was seen in the first two years of ERT for shoulders, elbows, hips and knees. Change from baseline at 5 years of ERT (13 patients) was statistically significant only for the right shoulder (p = 0.03). No patient worsened from baseline (Fig. 4b). 6 Minute Walking Test (6MWT) was performed at baseline and then repeated yearly. In group B, the walked distance increased or remained stable (change from baseline 0–174 m, mean 70.8) after 7 years. In group A, no patient improved and almost all patients became poorly testable due to hyperactive behavior or because wheel-chair bound.
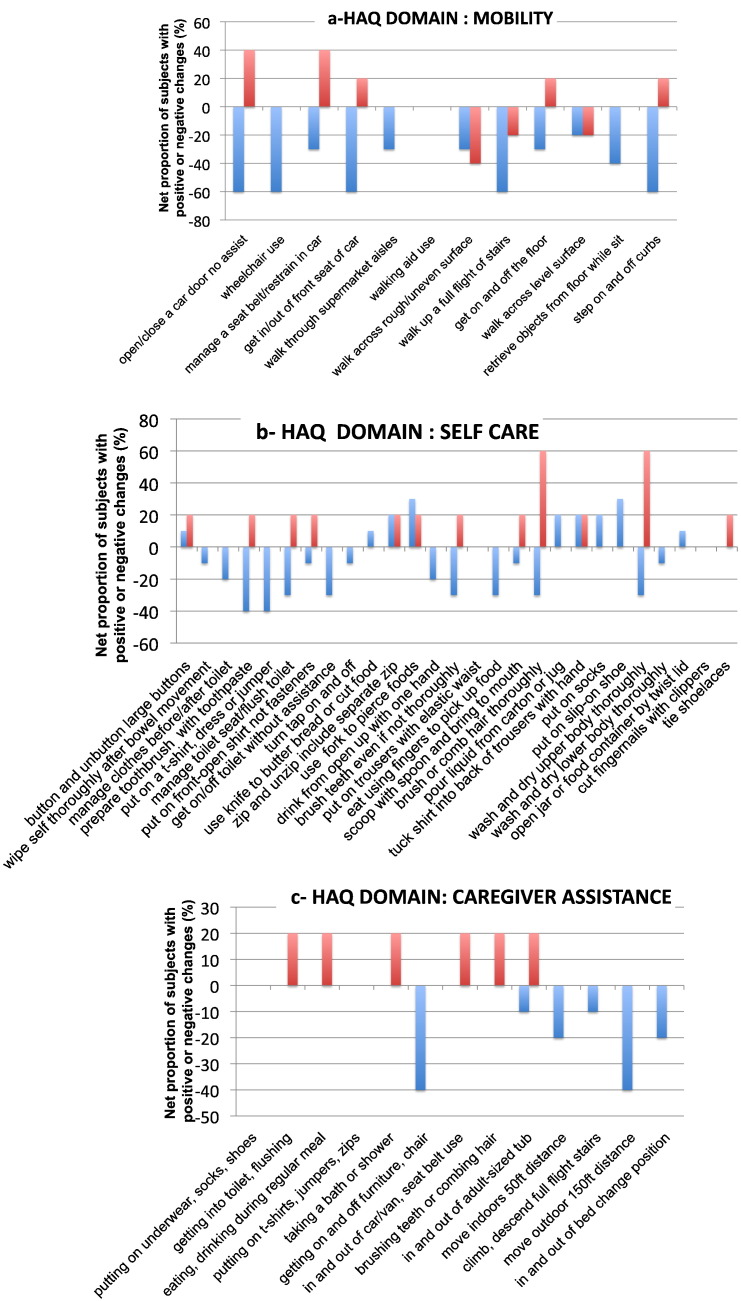
15 patients (5 MPS II B and 10 MPS II A) underwent MPS HAQ at baseline and at last evaluation (5–9 years of ERT). Fig. 5 a, b and c shows the net proportion of subjects in both groups (MPS IIA and MPS II B) with positive or negative changes from baseline for each MPS-HAQ item. The MPS II B patients improved for 23/52 items and worsened for 3/52. MPS IIA patients improved for only 9/52 and worsened for 32/52 items. The other items (27 for MPS IIB and 11 for MPS IIA) remained stable during the years. MPS II B patients were all walking, both at the baseline and last evaluation. In group A, only patients nos. 10 and 15 were wheelchair-bound for some hours a day at baseline; conversely only 2 were still deambulating at last evaluation (nos. 11 and 12).
Fig. 5.
Net proportion of subjects (%) with positive or negative changes in the three domains of MPS HAQ (a) Mobility, b) Self care, and c) Care giver assistance) at last evaluation compared to baseline in 17 MPS II patients treated with idursulfase. Blue bars represent cognitively impaired subjects (10 MPS II patients from group A); and red bars represent cognitively normal subjects (5 MPS II patients from group B).
4.8. Cognitive function
In all group B patients the Z-score ranged from − 2.1 (Mild Mental Retardation/Borderline) to + 1.5 (Superior) and was maintained or improved during the follow-up. Patient no. 15 was Borderline at baseline (25.7 years) and worsened to moderate mental retardation at the last examination after 63 months of ERT. All other 11 MPS II A patients soon became not testable due to a fast decline of cognitive functions associated with hyperactive behavior. At last evaluation, only 2 group A patients were still testable (nos. 11 and 12), the others were examined through Vineland Adaptive Behavior Scales and had a mental age ranging from < 1 y 6 m to 1 y 9 m at a mean age of 14.9 years (median 15 and range of 6–21).
4.9. Epilepsy
8/17 (47.1%) patients, all belonging to group A, developed epilepsy at an age ranging 7–15.2 years regardless of ERT commencing timing. They are now treated with antiepileptic drugs (sodium valproate, carbamazepine, levetiracetam, topiramate, fenitoin, oxcarbazepine) in mono or multi-therapy, with partial benefit.
4.10. Orthopedic/X-ray and brain and spine MRI features
Orthopedic/X-ray and brain and spine MRI features' proportions at end of study are reported in Table 2 for both groups (A and B). A relevant burden of signs and symptoms at starting of ERT was maintained or worsened throughout the treatment.
Table 2.
Orthopedic/X-ray features and brain and spine MRI abnormalities of 17 MPS II patients after treatment with idursulfase for 5–9 years. CSF = cerebrospinal fluid and PVSs = peri-vascular spaces.
| Patients (n = 17) | Phenotype A (n = 12) % | Phenotype B (n = 5) % | Total % | |
|---|---|---|---|---|
| Orthopedic/X-ray features | ||||
| Dens dysplasia | 13 | 91.7 | 40 | 76.5 |
| Platyspondilia | 13 | 66.7 | 100 | 76.5 |
| Intervertebral disc abnormalities | 9 | 41.7 | 80 | 52.9 |
| Kyphosis | 11 | 58.3 | 80 | 64.7 |
| Scoliosis | 11 | 58.3 | 80 | 64.7 |
| Lordosis | 1 | – | 20 | 5.9 |
| Carpal tunnel syndrome | 10 | 50 | 80 | 58.8 |
| Hip dysplasia | 10 | 58.3 | 60 | 58.8 |
| Genu valgum | 8 | 33.3 | 80 | 47.1 |
| Foot abnormalities | 9 | 50 | 60 | 52.9 |
| MRI features | ||||
| Enlargement of Virchow-Robin PVSs | 12 | 83.3 | 40 | 70.6 |
| Subarachnoid CSF space enlargement and 3rd ventricle enlargement | 10 | 75 | 20 | 58.8 |
| White matter signal abnormalities | 9 | 58.3 | 40 | 52.9 |
| Periodontoid tissue thickening | 6 | 41.7 | 20 | 35.3 |
| Pituitary sella abnormalities | 6 | 41.7 | 20 | 35.3 |
| Enlarged cisterna magna | 3 | 25 | 0 | 17.6 |
| Craniosynostosis | 2 | 16.7 | 0 | 11.8 |
| Spinal stenosis | 2 | 8.3 | 20 | 11.8 |
| Hyperostosis | 2 | 16.7 | 0 | 11.8 |
4.11. Surgery and other treatments with anesthesia
All together, our 17 patients underwent 62 surgical interventions or treatments with anesthesia, 14 of which prior to diagnosis, 21 in-between diagnosis and commencement of ERT, and 27 following ERT commencement. Individually the number of interventions ranged from 0 (nos. 11, 12) to 7 (no. 9) (Fig. 4c).
5. Discussion
The majority of our patients were affected by the severe form of MPS II for which clinical trial data are lacking [17]. Tomanin et al. [10] recently analyzed data from 27 MPS II subjects involved in a multicenter Italian study, 17 of whom were cognitively impaired. Those patients were treated for 3.5 years with ERT and no significant changes were found from baseline except for urinary GAGs and hepatomegaly. Another study [17] showed improvement in at least 4 signs/symptoms in 22 MPS II neuronopathic subjects from 5 centers following at least 2 years of ERT. Our study is the longest single center prospective independent study showing detailed clinical data of 17 Hunter patients treated with idursulfase for 5–9 years.
In this study idursulfase showed a safe profile with only few mild and easy manageable IARs.
5.1. GAGs and organomegaly
Muenzer et al. [5], [18] reported a significant improvement in 94 cognitively normal patients who participated in phase III trial and its extension, up to 3 years of treatment. Our data show that these parameters improve with ERT even in the more severe Hunter phenotype, and this improvement is sustained at 7 years of treatment.
5.2. Growth
The natural history of growth in MPS II patients has been described in several papers [19], [20], [21]. After the first few years of age, patients start losing centiles and reach a final mean height invariably below − 2 SD. Jones et al. [22], Schulze-Frenking et al. [23], Patel et al. [24], and Cho et al. [25] showed height improvement with HSCT or ERT. On the contrary, Zuber et al. [26] did not see any significant difference in his 13 ERT-treated patients. In our cohort, it is difficult to evaluate the effect of ERT on growth due to the small number of subjects and to the advanced age of most of them at the beginning of ERT. Probably attributable to ERT is the fact that the youngest of a couple of brothers who started ERT at the same time, was taller than the elder at the last evaluation.
5.3. ENT and respiratory function
Improvement of hearing on ERT has been reported in MPS II mice [27]. No specific data in MPS II patients are published in the literature. The hearing threshold improved in 6 of our patients during ERT. This result may be mainly attributed to decreased mucous production and reduction of thickness of the mucosa.
In this study, 4 of the 5 patients who performed respiratory function studies did show severe obstructive dysventilatory syndrome which was not cured by ERT; in 2 of them (no. 1 and no. 7) MDCT showed tracheal deformities.
ERT had a significant positive impact on absolute forced vital capacity in 94 attenuated Hunter patients who had been treated for 3 years [18]. An improvement of percent predicted forced vital capacity has also been shown in other 10 attenuated adult patients [7]. These effects are more easily attributed to improved musculo-skeletal and cardiovascular functions [28] or to the decreased redundancy of soft tissues in the upper airways, rather than to the improvement of the laryngeal—tracheal deformities and skeletal abnormalities contributing to restrictive lung disease, which do not improve on ERT [29], [30]. This may be the reason why our patients did not improve on ERT. However it is reasonable to expect that ERT might have a preventative role for tracheal deformities only when started precociously [31], [32].
5.4. Heart
Progressive cardiac abnormalities are a common finding in MPS II like in other MPS types [33]. Only few reports with a limited number of patients are available on the cardiac evolution of MPS II patients on ERT [7], [34]. In our patients, ERT appeared to be effective on heart hypertrophy with significant reduction of LVM. Systolic ventricular function also improved in 2 patients and did not worsen in most of the others who had normal EF at baseline. These heart data are quite new for MPS II and strongly suggest that ERT is effective in improving heart function in MPS II similarly to what has been observed in MPS I [35].
5.5. JROM, 6MWT and QoL
Passive JROMs improved in many joints but a mild statistical significance was obtained only for the right shoulder. Curiously an increased shoulders' improvement in comparison to the other joints was also reported in the study by Muenzer et al. [18]. In our patients the increase of ROM for the shoulders was 15–30° but 10 or 5° were obtained for many joints. A clear improvement of the 6MWT was also seen in 4 out of the 5 cognitively normal patients. Taken collectively, these are important results that contributed to reduce disability in cognitively normal patients; it allowed easier accomplishment of living activities such as walking, combing, brushing teeth, dressing, and using cutlery. This was also confirmed by the improved results of the QoL questionnaire in those 5 patients who were free of cognitive involvement. On the contrary, although JROMs also improved in the 12 MPS IIA patients, they could not benefit from that because most of daily functions were hampered by their severe neuropsychological decline.
5.6. Neurological assessment
As expected, the neuropsychological evolution and onset of epilepsy were independent from ERT in our cohort. The lack of efficacy of ERT in the CNS is the main limitation for choosing this treatment in patients with the severe form of MPS II. For these cases, the recent report by Escolar et al. [36] on HSCT efficacy in early treated severe Hunter patients encourages hope for the future.
5.7. Orthopedic, radiological and neuroradiological findings
Orthopedic, radiological and neuroradiological findings showed an apparent progression of the disease, despite ERT, with a significant burden of residual illness. This has already been reported in ERT treated MPS II patients [18] and in other MPS subjects [37]. However, increasing number of reports describe an improved orthopedic evolution in younger siblings of MPS affected couples when the second brother/sister was treated very early with ERT [38], [39], [40] in a pseudo-pre-symptomatic condition. These observations strongly support the opinion that very early diagnosis is needed for those MPS patients who might have access to ERT.
5.8. Surgery
Besides the inevitable progression of cognitive dysfunction in patients with the severe form, the burden of illness in MPS II patients is still very elevated, if one considers all different kinds of surgeries these patients undergo in their life as reported for our patients and for the other patients in the literature [41]. Although it is well known, it must be reminded that in these patients surgery is associated with increased anesthesiological risks; for this reason it is suggested that any surgery should be performed by a well-trained anesthesiologists and surgeons team who know how to use specific intubation/extubation techniques suitably recommended for patients with MPS [41].
In summary, as pointed out by Glamuzina et al. [42], parameters used to assess ERT efficacy in clinical trials may not be used in all patients due to their utmost young age or mental delay. We acknowledge that the limitations of the study are mainly represented by lack of control patients and by the unfeasibility to test those patients who were severely retarded. Nevertheless we were able to demonstrate a positive impact of ERT on urinary GAGs, organomegaly, hearing, heart function, and JROM. An evidence of improvement of 6MWT and QoL was clear only in cognitively adequate patients.
Two issues in this paper merit to be more extensively commented: the first one concerns findings of very significant residual skeletal disease in all patients despite ERT. It is widely recognized that bone disease starts very early in the life of MPS patients and that a regression with ERT of established orthopedic signs is not expected. Conversely, a complete normalization of all bone tissue parameters has recently been demonstrated in MPS I mice after neonatal bone marrow transplantation [43]. An improvement in MPS II patients' skeletal outcome could probably be obtained with a very early, possibly pre-symptomatic treatment which might become available only with diagnosis through neonatal screening. The second issue is the fact that the QoL of those patients with the most common neuronopathic form of MPS II is not substantially improved by ERT while cognitive skillfulness progressively gets worse. This might bring about all the questions and doubts related to the administration of ERT in neuronopathic MPS II patients, despite the lack of efficacy on CNS, that have been extensively discussed in a recent paper [44]. The authors concluded that the right to initiate ERT must be given to all newly diagnosed patients. They usually gain clinical advantages from ERT consisting mainly in diminished macroglossia, organomegaly and hospital admission for respiratory infections. The same paper suggests that the decision to continue or stop ERT when the disease becomes more advanced has to be taken in agreement with parents and by weighing pros and cons for each single case. In fact, the lack of efficacy of ERT on CNS is an important limitation carrying doubts about the real usefulness of ERT in MPS patients with CNS involvement. It is thus correct to expect that some of these patients will stop ERT at a certain time of their life when the positive effect is no more evident. This is not in contrast with the introduction of the neonatal screening for MPS II. Neonatal screening would allow treatment of all MPS II patients who will benefit of pre-symptomatic ERT and will have less somatic consequences. Later on, should ERT advantages be no more evident in those patients with CNS involvement, treatment could possibly be stopped in agreement with the parents and on an individual basis. Should treatments crossing the blood–brain barrier (intrathecal ERT, hematopoietic stem cell transplantation) be available in the future, there is a possibility that not all the patients addressed to CNS treatments will be precociously identified. Some will be recognized through molecular analysis, others from early neurological signs, but it is reasonable to foresee that a percentage of patients will not be early allocated to cognitively competent or not competent groups. Further studies are needed to identify specific biochemical markers in order to early differentiate the two groups.
Conflict of interest
RP, MR, LB, SG, and PR have received reimbursement of expenses and honoraria for lectures on the management of lysosomal storage diseases, including Hunter disease, from Genzyme, Shire, and BioMarin. RP, MR, SG, MG, and ES in the last 2 years have been involved in clinical trials on MPS patients. SB, AB, ADL, PDL, DG, CG, MG, DG, PM, GM, FN, MR, RG, PR, LT, GV, and AB declare to have no conflicts of interest.
Acknowledgments
The authors wish to thank Fondazione Pierfranco and Luisa Mariani, Milano, for providing financial support for clinical assistance to metabolic patients, Vera Marchetti for her work in organizing patients' evaluations, all patients and their families and the doctors and nurses who provided weekly administration of ERT at the local sites of infusion or at home: dr Maria Pia Villa, Vercelli; dr Silvia Fungi, Savigliano (Cn);dr Alberto Martelli, Garbagnate Milanese (Mi); dr Claudio Zanacca, Sassuolo (Modena); dr Massimiliano Filosto, Brescia; dr Cesare Antonio Ghitti, Alzano Lombardo (Mi); dr Giosuè Ghilardi, Piario (Bg); dr Giancarlo Izzi, Parma; dr Francesca Brambillasca, ERT@home; prof Marco Zecca, Pavia; prof Gianni Bona, Novara; prof Guido Grassi and dr Maria Teresa Pozzi, Monza; prof Riccardo Longhi, Como; and dr Alfredo Caminiti, Cantù.
References
- 1.Scarpa M., Almassy Z., Beck M., Bodamer O., Bruce I., De Meirleir L. Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease. Orphanet J. Rare Dis. 2011;6:72. doi: 10.1186/1750-1172-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer L., Langford-Smith K., Bigger B., Fildes J. Mucopolysaccharide diseases: a complex interplay between neuroinflammation, microglial activation and adaptive immunity. J. Inherit. Metab. Dis. 2014;37:1–12. doi: 10.1007/s10545-013-9613-3. [DOI] [PubMed] [Google Scholar]
- 3.Martin R., Beck M., Eng C., Giugliani R., Harmatz P., Muñoz V. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008;121:e377–e386. doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 4.Scarpa M. Mucopolysaccharidosis type II. In: Pagon R.A., Bird T.D., Dolan C.R., editors. GeneReviews™ [Internet] 2007. (Seattle (WA), USA) [Google Scholar]
- 5.Muenzer J., Wraith J.E., Beck M., Giugliani R., Harmatz P., Eng C.M. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet. Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 6.Muenzer J., Beck M., Eng C.M., Escolar M.L., Giugliani R., Guffon N.H. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009;124:e1228–e1239. doi: 10.1542/peds.2008-0999. [DOI] [PubMed] [Google Scholar]
- 7.Okuyama T., Tanaka A., Suzuki Y., Ida H., Tanaka T., Cox G.F. Japan Elaprase® Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (mucopolysaccharidosis II, MPS II) Mol. Genet. Metab. 2010;99:18–25. doi: 10.1016/j.ymgme.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Guffon N., Bertrand Y., Forest I., Fouilhoux A., Froissart R. Bone marrow transplantation in children with Hunter syndrome: outcome after 7 to 17 years. J. Pediatr. 2009;154:733–737. doi: 10.1016/j.jpeds.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka A., Okuyama T., Suzuki Y., Sakai N., Takakura H., Sawada T. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol. Genet. Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Tomanin R., Zanetti A., D'Avanzo F., Rampazzo A., Gasparotto N., Parini R. Clinical efficacy of enzyme replacement therapy in paediatric Hunter patients, an independent study of 3.5 years. Orphanet J. Rare Dis. 2014;9:129. doi: 10.1186/s13023-014-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megremis S.D., Vlachonikolis I.G., Tsilimigaki A.M. Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology. 2004;231:129–134. doi: 10.1148/radiol.2311020963. [DOI] [PubMed] [Google Scholar]
- 12.Schiller N.B., Shah P.M., Crawford M., DeMaria A., Devereux R., Feigenbaum H. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J. Am. Soc. Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Pastores G.M., Arn P., Beck M., Clarke J.T.R., Guffon N., Kaplan P. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with mucopolysaccharidosis type I. Mol. Genet. Metab. 2007;91:37–47. doi: 10.1016/j.ymgme.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Orsini A., Laicardi C. Traduzione e adattamento italiano a cura di Caterina Laicardi e Arturo Orsini, Organizzazioni Speciali. 1997. WAIS-R, Wechsler Adult Intelligence Scale Revised: Manuale David Wechsler. (Firenze) [Google Scholar]
- 16.Sparrow S., Balla D., Cicchetti D. Vineland adaptive behaviour scales. In: Giunti O.S., editor. Adattamento Italiano a cura di Giulia Balboni e Luigi Predabissi. 2003. [Google Scholar]
- 17.Lampe C., Bosserhoff A.-K., Burton B., Giugliani R., de Souza C., Bittar C. Long-term experience with enzyme replacement therapy (ERT) in MPS II patients with a severe phenotype: an international case series. J. Inherit. Metab. Dis. 2014;1–7 doi: 10.1007/s10545-014-9686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muenzer J., Beck M., Eng C., Giugliani R., Harmatz P., Martin R. Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet. Med. 2011;13:95–101. doi: 10.1097/GIM.0b013e3181fea459. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz I.V., Ribeiro M.G., Mota J.G., Toralles M.B., Correia P., Horovitz D. A clinical study of 77 patients with mucopolysaccharidosis type II. Acta Paediatr. 2007;96:63–70. doi: 10.1111/j.1651-2227.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 20.Parini R., Jones S.A., Harmatz P., Giugliani R., Larroque S., Mendelsohn N.J. The natural history of growth in patients with Hunter syndrome: data from the Hunter Outcome Survey (HOS) J. Inehrited Metablic Dis. 2014;37:S131. doi: 10.1016/j.ymgme.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Patel P., Suzuki Y., Maeda M., Yasuda E., Shimada T., Orii K.E. Growth charts for patients with Hunter syndrome. Mol. Genet. Metab. Rep. 2014;1:5–18. doi: 10.1016/j.ymgmr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones S.A., Parini R., Harmatz P., Giugliani R., Fang J., Mendelsohn N.J. The effect of idursulfase on growth in patients with Hunter syndrome: data from the Hunter Outcome Survey (HOS) Mol. Genet. Metab. 2013;109:41–48. doi: 10.1016/j.ymgme.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Schulze-Frenking G., Jones S.A., Roberts J., Beck M., Wraith J.E. Effects of enzyme replacement therapy on growth in patients with mucopolysaccharidosis type II. J. Inherit. Metab. Dis. 2011;34:203–208. doi: 10.1007/s10545-010-9215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel P., Suzuki Y., Tanaka A., Yabe H., Kato S., Shimada T. Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with Hunter syndrome. Mol. Genet. Metab. Rep. 2014;1:184–196. doi: 10.1016/j.ymgmr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho S.Y., Huh R., Chang M.S., Lee J., Kwun Y., Maeng S.H. Impact of enzyme replacement therapy on linear growth in Korean Patients with mucopolysaccharidosis type II (Hunter Syndrome) J. Korean Med. Sci. 2014;29:254–260. doi: 10.3346/jkms.2014.29.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Żuber Z., Różdżyńska-Świątkowska A., Jurecka A., Tylki-Szymańska A. The effect of recombinant human iduronate-2-sulfatase (idursulfase) on growth in young patients with mucopolysaccharidosis type II. PLoS ONE. 2014;9:e85074. doi: 10.1371/journal.pone.0085074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong S.H., Chu H., Kim K.R., Ko M.H., Kwon S.Y., Moon I.J. Auditory characteristics and therapeutic effects of enzyme replacement in mouse model of the mucopolysaccharidosis (MPS) II. Am. J. Med. Genet. A. 2012;158A:2131–2138. doi: 10.1002/ajmg.a.35498. [DOI] [PubMed] [Google Scholar]
- 28.White K.K., Hale S., Goldberg M.J. Musculoskeletal health in Hunter disease (MPS II): ERT improves functional outcomes. J. Pediatr. Rehabil. Med. 2010;3:101–107. doi: 10.3233/PRM-2010-0112. [DOI] [PubMed] [Google Scholar]
- 29.Malik V., Nichani J., Rothera M.P., Wraith J.E., Jones S.A., Walker R. Tracheostomy in mucopolysaccharidosis type {II} (Hunter's syndrome) Int. J. Pediatr. Otorhinolaryngol. 2013;77:1204–1208. doi: 10.1016/j.ijporl.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Muenzer J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014;111:63–72. doi: 10.1016/j.ymgme.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Tylki-Szymanska A., Jurecka A., Zuber Z., Rozdzynska A., Marucha J., Czartoryska B. Enzyme replacement therapy for mucopolysaccharidosis II from 3 months of age: a 3-year follow-up. Acta Paediatr. 2012;101:e42–e47. doi: 10.1111/j.1651-2227.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 32.Tajima G., Sakura N., Kosuga M., Okuyama T., Kobayashi M. Effects of idursulfase enzyme replacement therapy for mucopolysaccharidosis type II when started in early infancy: comparison in two siblings. Mol. Genet. Metab. 2013;108:172–177. doi: 10.1016/j.ymgme.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Braunlin E., Harmatz P., Scarpa M., Furlanetto B., Kampmann C., Loehr J. Cardiac disease in patients with mucopolysaccharidosis: presentation, diagnosis and management. J. Inherit. Metab. Dis. 2011;34:1183–1197. doi: 10.1007/s10545-011-9359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brands M.M.G., Frohn-Mulder I., Hagemans M.C., Hop W.J., Oussoren E., Helbing W. Mucopolysaccharidosis: cardiologic features and effects of enzyme-replacement therapy in 24 children with MPS I, II and VI. J. Inherit. Metab. Dis. 2012:1–8. doi: 10.1007/s10545-011-9444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braunlin E.A., Berry J.M., Whitley C.B. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am. J. Cardiol. 2006;98:416–418. doi: 10.1016/j.amjcard.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 36.Escolar M., Poe M., Rajan D., Szabolcs P. Longterm outcomes of patients receiving umbilical blood stem cell transplantation for MPS II. Mol. Genet. Metab. 2013;108:S37–S38. [Google Scholar]
- 37.White K.K. Orthopaedic aspects of mucopolysaccharidoses. Rheumatology. 2011;50:v26–v33. doi: 10.1093/rheumatology/ker393. [DOI] [PubMed] [Google Scholar]
- 38.Gabrielli O., Clarke L.A., Bruni S., Coppa G.V. Enzyme-replacement therapy in a 5-month-old boy with attenuated presymptomatic MPS I: 5-year follow-up. Pediatrics. 2010;125:e183–e187. doi: 10.1542/peds.2009-1728. [DOI] [PubMed] [Google Scholar]
- 39.Lampe C., Atherton A., Burton B., Descartes M., Giugliani R., Horovitz D.G. Enzyme replacement therapy in mucopolysaccharidosis II patients under 1 year of age. JIMD Rep. 2014;1–15 doi: 10.1007/8904_2013_289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGill J., Inwood A., Coman D., Lipke M., De Lore D., Swiedler S. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age—a sibling control study. Clin. Genet. 2010;77:492–498. doi: 10.1111/j.1399-0004.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 41.Mendelsohn N.J., Harmatz P., Bodamer O., Burton B.K., Giugliani R., Jones S.A. Importance of surgical history in diagnosing mucopolysaccharidosis type II (Hunter syndrome): data from the Hunter Outcome Survey. Genet. Med. 2010;12:816–822. doi: 10.1097/GIM.0b013e3181f6e74d. [DOI] [PubMed] [Google Scholar]
- 42.Glamuzina E., Fettes E., Bainbridge K., Crook V., Finnegan N., Abulhoul L. Treatment of mucopolysaccharidosis type II (Hunter syndrome) with idursulfase: the relevance of clinical trial end points. J. Inherit. Metab. Dis. 2011;34:749–754. doi: 10.1007/s10545-011-9280-1. [DOI] [PubMed] [Google Scholar]
- 43.Pievani A., Azario I., Antolini L., Shimada T., Patel P., Remoli C. Neonatal bone marrow transplantation prevents bone pathology in a mouse model of mucopolysaccharidosis type I. Blood. 2014;125:1662–1671. doi: 10.1182/blood-2014-06-581207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muenzer J., Bodamer O., Burton B., Clarke L., Frenking G., Giugliani R. The role of enzyme replacement therapy in severe Hunter syndrome—an expert panel consensus. Eur. J. Pediatr. 2012;171:181–188. doi: 10.1007/s00431-011-1606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]