Abstract
Background and aims
BH4-sensitive phenylketonuria (PKU) patients relax their phenylalanine (Phe) restricted diet due to increased Phe tolerance, while keeping dried blood Phe concentrations with in the therapeutic range. We aimed to investigate metabolic control, eating habits and nutrient supply under long-term BH4-therapy.
Patients and methods
Retrospective analysis of mean dried blood Phe concentrations and their variability, food and nutrient intake in BH4-sensitive patients (n = 8, 3f, age 6.0–16.6 y) under classical dietary treatment for one year and during the three years after initiation of BH4.
Results
Phe concentrations of BH4-sensitve PKU patients remained within therapeutic range throughout the observation period, independent of therapeutic regime. Under BH4, Phe tolerance increased significantly (493.2 ± 161.8 mg/d under classical diet vs 2021.93 ± 897.4 mg/d two years under BH4; P = 0.004). Variability of Phe concentrations remained unchanged (mean SD; P = 1.000). Patients adjust their food choice and significantly increased their intake of cereals, potatoes, dairy products and meat (P = 0.019, P = 0.016, P = 0.016 and P = 0.016, respectively). Under diet changes after implementation of BH4 a drop in micronutrient intake (vitamin D, folic acid, iron, calcium, iodine) could be revealed (P = 0.005, P < 0.001, P = 0.004, P = 0.001, P = 0.003, respectively).
Conclusions
BH4-sensitive PKU patients can achieve good metabolic control under an adjuvant BH4- or a BH4 monotherapy. The liberalized diet under BH4 seems to jeopardize the quality of patients' nutrition, and these patients require close follow-up and special nutrition education to minimize the risk for imbalanced diet and nutrient deficiencies.
Abbreviations: AAM, amino acid mixture; BH4, tetrahydrobiopterin; Phe, phenylalanine; PKU, phenylketonuria
1. Introduction
Since approval of sapropterindihydrochlorid (BH4) for the treatment of BH4-sensitive phenylketonuria (PKU, OMIM 261600), a large number of patients switched from classical dietary treatment (phenylalanine (Phe) restricted diet and Phe-free amino acid supplements enriched with micronutrients) to an additional BH4 supplementation [1]. BH4 therapy usually allows increase of daily Phe consumption by a factor of 2–3, still keeping Phe concentrations in dried blood within the therapeutic range [2]. Some BH4-sensitive PKU patients can entirely stop dietary treatment and no longer need any amino acid supplement (AAM) [2], [3], [4]. As a consequence, quality of life, therapy adherence and thus, long-term metabolic control and outcome of these patients might be improved [1], [5]. Moreover, there is some evidence for a stabilization of Phe concentrations under BH4 therapy [6], [7]. This is of importance as the stability of Phe concentrations in the organism seems to have a relevant influence on long-term cognitive outcome [6], [8], [9], [10]. Higher Phe fluctuations are associated with cognitive deficits, especially with a negative impact on working memory, inhibitory control and executive strategic processing [9].
Switching from a Phe restricted diet to a relaxed or even free diet under BH4 supply is a challenge. Due to the increased Phe tolerance, patients are allowed to extend their choice of natural food. Initially, they maintain some of their customary eating patterns such as a restricted consumption of food with high protein content e.g. dairy products, fish or meat. At the same time, they partly adopt eating habits of the general population. As a consequence, fruit and vegetable consumption markedly decreases, while the supply of potatoes, pasta and rice exceeds the average intake of the healthy peers [2]. Such an imbalanced nutrition bears a high risk for an insufficient micronutrient intake [2], [11] and may result in impaired physical and neurological development [12], [13], [14]. Changing eating habits, indeed, is difficult, as food preferences and aversions primarily develop during the first years of life resulting in stable and almost unchangeable eating patterns [15].
The study presented here aimed to evaluate metabolic control, including variability of blood Phe, as well as food and nutrient intake in BH4-sensitive PKU patients under long-term BH4 therapy.
2. Patients and methods
2.1. Patients
A retrospective analysis of metabolic control comprising variations of Phe concentrations as well as food and nutrient intake over a period of four years (one year prior BH4 and three years under BH4 treatment) in eight BH4-sensitive PKU patients (3f/5 m) was performed. All patients proved to be BH4-sensitive by a six week prolonged BH4-responsiveness test, as previously described [5]. All included PKU patients were diagnosed by newborn screening and early and continuously treated by a Phe restricted diet with essential amino acid as well as micronutrient supply by AAM until the start of BH4. They followed the BH4 therapy at least for three years. Patients received individual diet counseling at each clinical visit, at least four times a year.
The study was approved by the University of Leipzig's ethics committee (registration-number 440-12-17122012) and registered at DRKS, the International Clinical Trials Registry Platform (DRKS00004942).
3. Methods
3.1. Assessment of dried blood Phe concentrations
Dried blood Phe concentrations were regularly assessed according to the current recommendations for treatment of PKU for the German speaking countries [16]. Phe concentrations in dried blood were determined by liquid chromatography/tandem mass spectrometry (LC–MS/MS), as previously described [17].
3.2. Assessment of food and nutrient intake
Information about food consumption and nutrient intake (Phe, energy, macro- and micronutrients: iron, iodine, calcium, zinc, vitamin D, C, B1, B2, B6, B12 and folic acid) was gathered from detailed three-day dietary records. Food choice and mean nutrient intake of BH4-sensitive patients were evaluated before (under classical PKU diet) and after three months and two years of BH4-therapy.
All ingested food was allocated to food groups as previously described [2] and shown in Fig. 1. Nutritional analysis was conducted using the Food and Control Management System “Diät 2000” based on the updated version of the Bundeslebensmittelschlüssel [18]. Additional information on AAM, low protein food as well as other processed food provided by the manufacturers was added to the database.
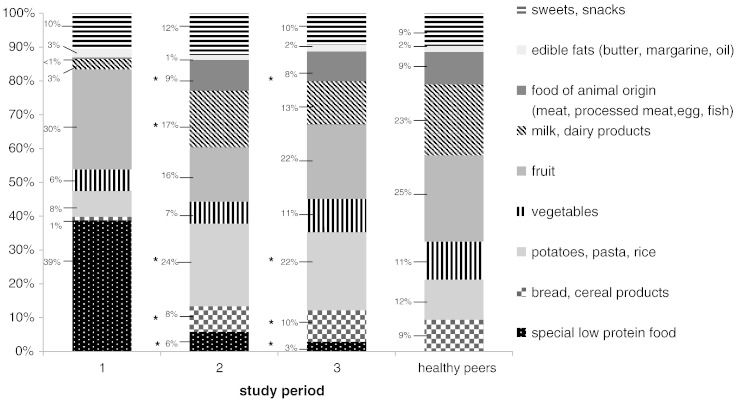
Fig. 1.
Food consumption of BH4-sensitive patients under Phe restricted diet (bar 1) compared to BH4 therapy three months and two years after its introduction (middle bars 2 and 3) compared to age-matched healthy German children (right bar). Shown are the shares of food groups (%) of total food consumption (*significant differences in BH4-sensitive patients under Phe restricted diet compared to BH4 therapy:bread: study period 1 vs. 2 P = 0.014 and 1 vs 3 P = 0.019; potatoes, pasta rice 1 vs 2 P = 0.008 and 1 vs 3 P = 0.016; milk/dairy products: 1 vs 2 P = 0.016; food of animal origin (meat, processed meat, fish, egg): 1 vs 2 P = 0.008 and 1 vs 3 P = 0.016; special low protein products: 1 vs 2 P = 0.008 and 1 vs 3 P = 0.016).
Mean nutrient intake of BH4-sensitive patients under BH4 therapy was compared to the current recommendations for nutrient intake [19]. In addition, mean nutrient as well as food intake were compared to data from a cohort of age-matched healthy German children [20].
3.3. Statistics
All procedures were performed using SPSS for Windows 20 (SPSS Inc., Chicago, Illinois).
Mean and standard deviation (SD) of Phe concentrations and amount of Phe concentrations above the age specific therapeutic range (given as percentage of total the number of Phe concentrations) were computed per evaluated year (one year prior BH4 treatment, years one, two, and three after BH4 initiation) of each single patient. Laboratory and nutritional data from each study period were averaged and used for analyses. If normality of distribution could be assured, longitudinal changes in mean Phe concentrations and nutrient supply over the different study periods were analyzed by Wilk's multivariate analysis of variance (MANOVA) with “time” as the within-subject factor with four levels (study periods 1 through 4). If Wilk's analysis yielded a significant effect of “time”, this was followed by repeated (sequential) contrasts to locate pairs with significant changes over time. In analogy, data which did not follow a normal distribution were analyzed by Friedman test and Wilcoxon test. Phe variability was evaluated by averaging the SDs from the single patients. Significance was accepted for P < 0.05. Data are given as mean ± standard deviation unless otherwise stated.
4. Results
4.1. Patient characteristics
Patients' mean age at introduction of BH4 was 10.5 ± 3.8 years (range 6.0–16.6 years). Under classical PKU diet and even under BH4 all except two patients showed normal height and body weight development with a BMI between the 3rd and the 97th percentile. One patient had a BMI above the 97th percentile, another one below the 3rd percentile, already before starting BH4 therapy.
BH4 was administered as Kuvan® (Merck Serono) with an initial median dosage of 18 mg/kg KG (range 10–19 mg/kg KG). Under BH4 therapy, five of the eight BH4-sensitive patients could give up their Phe restricted diet entirely and stopped any AAM supply because of adequate protein intake from natural food. In the other three BH4-sensitive patients AAM dosage could be reduced, they could only partly liberate their diet. However, in one patient AAM supply had to be reintroduced due to severe atopic skin lesions, improving thereafter.
4.2. Metabolic control and Phe tolerance
All investigated BH4-senstive patients showed good metabolic control over the complete evaluation period. Mean dried blood Phe concentrations rose significantly over time (Table 1), but stayed within therapeutic range [16].
Table 1.
Metabolic control of eight BH4-sensitive PKU patients throughout the evaluation period.
| Before BH4 treatment/under Phe restricted diet (A) | 1. year under BH4 (B) | 2. year under BH4 (C) | 3. year under BH4 (D) | Pa | |
|---|---|---|---|---|---|
| Mean Phe concentration in dried blood (μmol/l) (SD) | 262.2 (129.4) | 337.1 (129.6) | 382.7 (148.1) | 371.7 (119.8) | A vs B P = 0.016b A vs C P = 0.016b A vs D P = 0.008b B vs D P = 0.039b |
| Mean percentage of Phe concentrations above the therapeutic range (SD) | 13.2 (23.7) | 26.2 (28.4) | 14.3 (28.1) | 15.1 (28.1) | n.s. |
| Mean variability of Phe concentrations (μmol/l) (SD) | 77.9 (35.9) | 87.0 (24.7) | 84.4 (24.9) | 80.6 (29.3) | n.s. |
Analysis by Friedman test, and Wilcoxon test.
Significant differences.
Simultaneously, the patients could significantly increase their mean Phe consumption under BH4 treatment by a factor 4 (Phe tolerance at baseline 493.2 ± 161.8 mg/d vs. 2208.9 ± 1336.4 mg/d and 2021.9 ± 897.4 mg/d after three months and two years, respectively; P = 0.018 and P = 0.004).
During the evaluation period the frequency of Phe concentrations above the therapeutic range declined in all patients as they got older and in some, a higher upper therapeutic value was allowed according to the current recommendations for treatment of PKU for the German speaking countries [16]. However, the differences were not significant. The variability of Phe concentrations in dried blood did not change over time in BH4-sensitive patients (Table 1).
4.3. Food consumption by BH4-sensitive patients
Fig. 1 shows the food consumption by BH4-sensitive PKU patients under classical dietary treatment compared to BH4 therapy three months and two years after its initiation. In addition, these data are compared to data from aged-matched healthy German children [20].
Before introduction of BH4 therapy, all BH4-sensitive patients followed a Phe restricted diet, having some characteristics of a vegan diet. Due to the partially very low Phe tolerance these patients spent important shares of total food consumption on fruit and vegetables and on special low protein products, mainly bread, pasta and milk substitute. Additionally, they consumed relatively large amounts of sweets. Only very small amounts of normal bread, milk, dairy products, processed meat or fish were consumed.
As a result of the increased Phe tolerance under BH4 these patients markedly increased their intake of normal, protein rich food, primarily bread, potatoes, pasta and rice during short-term follow-up over a three month period (Fig. 1). Their mean consumption of milk, dairy products, meat and processed meat rose significantly, but with marked interindividual differences. The consumption of fish or egg products remained very low in all patients. No change over long-term follow-up could be observed. The markedly reduced fruit consumption after initiation of BH4 re-increased slightly, but not significantly over the two following years. The mean consumption of special low protein products significantly declined further in long-term follow-up. No changes over the complete study course could be detected regarding the consumption of edible fats as well as sweets and snacks.
Compared to age-matched healthy German children, BH4-sensitive PKU patients still show remarkable differences in nutrition habits. Over a period of two years the consumption of fruit, vegetables, meat and sweets became similar to this of the healthy peers. However, even after two years of adaptation their consumption of milk and dairy products was markedly lower. In contrast, BH4-sensitive patients nearly consumed twice as much potatoes, pasta and rice compared to their peers.
4.4. Macronutrient intake of BH4-sensitive patients
Under classical dietary treatment total protein intake (sum of synthetic protein from AAM and intact protein from natural food) was markedly above the recommended range (171.5 ± 41.8% of DACH). Total protein intake remained stable throughout the evaluation period, but the proportions of synthetic and natural protein changed. Whereas under classical PKU diet patients ingest 123.9 ± 30.7% of current recommendation for protein intake [19] as synthetic protein from AAM, this amount declined to 26.6 ± 45.6% and 40.0 ± 50.7% under BH4 therapy in short- and long-term follow-up, respectively. In contrast, the ingested amount of natural protein increased from 47.5 ± 17.1% of the current recommendations under classical dietary treatment to 146.6 ± 80.2% and 121.6 ± 47.4% three months and two years after initiation of BH4 therapy.
Whereas under classical PKU diet BH4-sensitive patients' mean carbohydrate intake was adequate, this dropped below the current recommendations after three-month BH4 therapy (81.8 ± 20.4%; [19]), remaining stable during long-term follow-up (82.7 ± 21.4%). However, due to high variability, these differences were not significant. The mean fat intake as well as total caloric intake remained unchanged over the complete time of investigation (P = 1.000 and P = 0.527).
4.4.1. Micronutrient intake by BH4-sensitive patients
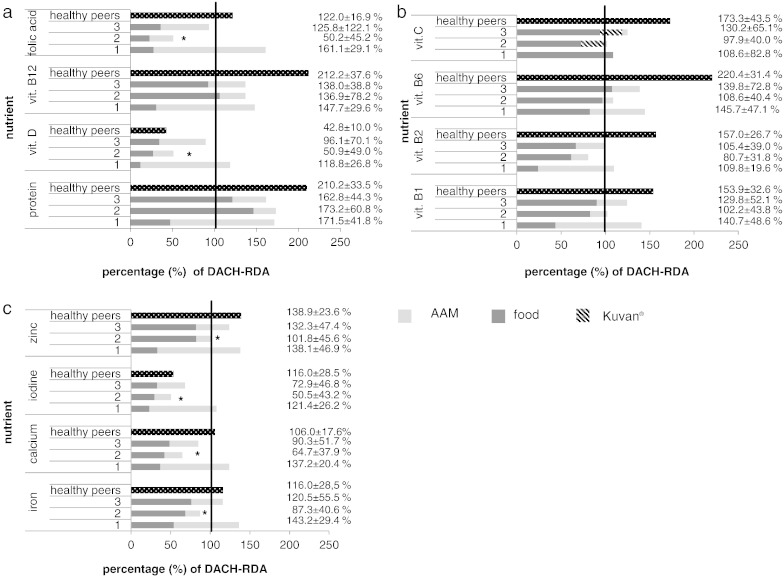
Fig. 2a–c displays the mean micronutrient intake of BH4-sensitive patients under BH4 compared to classical dietary treatment and additionally, in comparison to data from aged-matched healthy German children [19].
Fig. 2.
(a–c). Protein and micronutrient intake of BH4-sensitive patients under Phe restricted diet (left bar;1) and under BH4 therapy 3 months and 2 years after its introduction (middle bars 2 and 3) in compared to age-matched healthy German children (right bar) as percent of DACH-RDA. The vitamin C content of Kuvan® was included into the calculation (5 mg vitamin C per 100 mg Kuvan®). Under the Phe restricted diet all these patients took an AAM, while under BH4 only 3 and 4 patients took some, respectively (*significant differences in total micronutrient intake in BH4-sensitive patients under Phe restricted diet compared to BH4 therapy: Pvitamin D = 0.005; Pfolic acid < 0.001; Piron = 0.004; Pcalcium = 0.001; Piodine = 0.003, Pzinc = 0.022).
Under classical dietary treatment all BH4-sensitive patients showed a sufficient micronutrient supply. The highest percentage of vitamins and minerals was derived from AAM and only a small amount from natural food. Three months after introduction of BH4 therapy the patients' micronutrient intake decreased compared to the classic dietary treatment and partially dropped markedly below the current recommendations [19] as the patients reduced or even stopped AAM supply. The differences were significant for vitamin D, folic acid, iron, calcium, iodine and zinc. After two-year BH4 therapy, the mean intake of these micronutrients slightly improved, mainly driven by one patient in whom AAM was reintroduced. However, even after two years the patients do not meet the recommendation for vitamin D, folic acid, iron, calcium and iodine supply. Including the vitamin C content of Kuvan® (5 mg vitamin C per 100 mg sapropterin dihydrochloride), total vitamin C supply did not differ between classical PKU diet and BH4 therapy (P = 1.000).
In comparison to our data, healthy German children aged 6 to 17 years meet the recommendations for almost all investigated micronutrients except for vitamin D and iodine (Fig. 2a–c).
5. Discussion
Classical PKU is one of the most common inborn errors of amino acid metabolism [16]. Patients depend on a very strict lifelong Phe restricted diet. About 20% of the patients prove to be BH4-sensitive [21], therefore enjoying a significant better residual enzyme activity under BH4 supplementation. Under an adjuvant BH4 therapy these patients can liberalize their dietary restrictions and most of them adapt eating habits of their healthy peers.
The study presented here investigated metabolic control and eating habits, including food and nutrient intake of eight BH4-sensitive PKU patients under long-term therapy with BH4. All these patients benefit from a significant increase of their Phe tolerance by a factor of four under BH4, while keeping Phe concentrations in the therapeutic range. No changes regarding Phe fluctuations could be revealed.
Consumption of special low protein food and fruits declined in favor of more protein rich food e.g. normal bread, dairy products and meat. In four BH4-sensitive patients Phe restricted diet and AAM supply could be terminated, the other four further needed some Phe restriction and AAM, even during long-term follow-up. Micronutrient supply declined under BH4 therapy and dropped below the recommended range, even over the long-term course of follow-up.
Whereas the BH4-sensitive PKU patients showed a significant decrease in Phe concentration during the BH4-responsiveness-test [5], in long-term course their mean Phe concentration under BH4 therapy was higher compared to one year prior the introduction of BH4 under classical PKU-diet. Nevertheless, the mean Phe concentration of the single patient remained within the age-dependent therapeutic range and the mean percentage of Phe concentrations above the therapeutic range declined [16]. This is caused by the patients' change in age during the 3-year evaluation period.
With the markedly increased Phe tolerance under BH4, patients immediately changed their eating habits, which remained relatively stable over the long-term follow-up. Fortunately, the initially decreased fruit intake after BH4-introduction recovers after two years of BH4-treatment and is comparable to the group of healthy German children [20]. This is important as fruit and vegetables provide vitamins, minerals, trace elements, phytochemicals and dietary fiber and therefore being essential in a healthy nutrition.
The main difference in food choice between long-term BH4 treated patients and their healthy peers consists in a markedly lower consumption of fish among food of animal origin and dairy products while the missing energy of this food group is replaced by a doubled intake of potatoes and pasta. This is mirrored in the insufficient supply of their dependent micronutrients: iodine, calcium and vitamin D [19]. These long-term data confirm the results revealed in our earlier study about food consumption and nutrient intake in BH4-sensitive patients on a relaxed diet due to BH4 supplementation over a short-term period [2]. In one of the investigated patients, however, AAM had to be reintroduced for development of atopic skin rashes while simultaneously showing a drop of serum zinc concentrations below the normal range. In addition, his protein intake dropped below 80% of the recommended amount. Especially the first time after implementation of a BH4 therapy seems to be a critical period with respect to patient's nutrient intake as the switching from a strict to a liberalized diet regime is difficult for many of them. Nonetheless, nutrition dependent diseases should be considered in the long-term follow-up of these patients [12], [13], [14] as for all PKU patients with a relatively high Phe tolerance [11]. Improvement of micronutrient intake by adjusting food choice would be desirable. Despite repeated specific dietary training, this could not be achieved over a two year period. The factors influencing food choice are very complex, including socio-economic components as well as psychological aspects [22], [23]. Furthermore, parents' eating habits are an important factor influencing their children's choice of food [24]. Changing eating habits, in general, is difficult and needs time. Food preferences and aversions primarily develop during the first years of life resulting in stable eating patterns [15]. With regard to this aspect, an early start of BH4 therapy, even within the first weeks of life after neonatal testing of BH4-sensitivity would be desirable. The efficient and safe application of BH4 even in children under four years of age could already be shown [25].
In conclusion, with an adjuvant BH4 therapy or a BH4 monotherapy, BH4-sensitive PKU patients can maintain good metabolic control. Switching from a strict Phe restricted diet to a relaxed or even free diet under BH4 still seems to be a challenge regarding patients' nutrition. Especially patients who stop AAM supply are at a higher risk for nutrient deficiencies as seen in short- as well as long-term follow-up. Therefore, these patients need close follow-up.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors gratefully acknowledge Prof. Markus Scholz (Institute for Medical Informatics, Statistics and Epidemiology (IMISE), University of Leipzig) for statistically counseling.
This work was partly supported by an unrestricted investigator initiated research grant by Nutricia GmbH, Nutricia metabolics.
We acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.
Contributor Information
Alena G. Thiele, Email: Alena.Thiele@medizin.uni-leipzig.de.
Carmen Rohde, Email: Carmen.Rohde@medizin.uni-leipzig.de.
Ulrike Mütze, Email: Ulrike.Muetze@medizin.uni-leipzig.de.
Maria Arelin, Email: Maria.Arelin@medizin.uni-leipzig.de.
Uta Ceglarek, Email: Uta.Ceglarek@medizin.uni-leipzig.de.
Joachim Thiery, Email: Joachim.Thiery@medizin.uni-leipzig.de.
Christoph Baerwald, Email: Christoph.Baerwald@medizin.uni-leipzig.de.
Wieland Kiess, Email: Wieland.Kiess@medizin.uni-leipzig.de.
Skadi Beblo, Email: Skadi.Beblo@medizin.uni-leipzig.de.
References
- 1.Keil S., Anjema K., van Spronsen F.J., Lambruschini N., Burlina A., Bélanger-Quintana A. Long-term follow-up and outcome of phenylketonuria patients on sapropterin: a retrospective study. Pediatrics. 2013;131:e1881–e1888. doi: 10.1542/peds.2012-3291. [DOI] [PubMed] [Google Scholar]
- 2.Thiele A.G., Weigel J.F., Ziesch B., Rohde C., Mütze U., Ceglarek U., Thiery J., Müller A.S., Kiess W., Beblo S. Nutritional Changes and micronutrient supply in patients with phenylketonuria under therapy with tetrahydrobiopterin (BH(4)) JIMD Rep. 2013;9:31–40. doi: 10.1007/8904_2012_176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambruschini N., Pérez-Dueña B., Vilaseca M.A., Mas A., Artuch R., Gassió R., Gómez L., Gutiérrez A., Campistol J. Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol. Genet. Metab. 2005;86(Suppl 1):S54–S60. doi: 10.1016/j.ymgme.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Burlina A., Blau N. Effect of BH(4) supplementation on phenylalanine tolerance. J. Inherit. Metab. Dis. 2009;32:40–45. doi: 10.1007/s10545-008-0947-1. [DOI] [PubMed] [Google Scholar]
- 5.Ziesch B., Weigel J., Thiele A., Mütze U., Rohde C., Ceglarek U., Thiery J., Kiess W., Beblo S. Tetrahydrobiopterin (BH4) in PKU: effect on dietary treatment, metabolic control, and quality of life. J. Inherit. Metab. Dis. 2012;35:983–992. doi: 10.1007/s10545-012-9458-1. [DOI] [PubMed] [Google Scholar]
- 6.Burton B.K., Bausell H., Katz R., Laduca H., Sullivan C. Sapropterin therapy increases stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU) Mol. Genet. Metab. 2010;101:110–114. doi: 10.1016/j.ymgme.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey M., Nation J., Francis I., Boneh A. Effect of tetrahydrobiopterin on Phe/Tyr ratios and variation in Phe levels in tetrahydrobiopterin responsive PKU patients. Mol. Genet. Metab. 2011;104:89–92. doi: 10.1016/j.ymgme.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Anastasoaie V., Kurzius L., Forbes P., Waisbren S. Stability of blood phenylalanine levels and IQ in children with phenylketonuria. Mol. Genet. Metab. 2008;95:17–20. doi: 10.1016/j.ymgme.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Hood A., Grange D.K., Christ S.E., Steiner R., White D.A. Variability in phenylalanine control predicts IQ and executive abilities in children with phenylketonuria. Mol. Genet. Metab. 2014;111:445–451. doi: 10.1016/j.ymgme.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleary M., Trefz F., Muntau A.C., Feillet F., van Spronsen F.J., Burlina A., Bélanger-Quintana A., Giżewska M., Gasteyger C., Bettiol E., Blau N., MacDonald A. Fluctuations in phenylalanine concentrations in phenylketonuria: a review of possible relationships with outcomes. Mol. Genet. Metab. 2013;110:418–423. doi: 10.1016/j.ymgme.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Rohde C., von Teeffelen-Heithoff A., Thiele A.G., Arelin M., Mütze U., Kiener C., Gerloff J., Baerwald C., Schultz S., Heller C., Müller A.S., Kiess W., Beblo S. PKU patients on a relaxed diet may be at risk for micronutrient deficiencies. Eur. J. Clin. Nutr. 2014;68:119–124. doi: 10.1038/ejcn.2013.218. [DOI] [PubMed] [Google Scholar]
- 12.Healton E.B., Savage D.G., Brust J.C., Garrett T.J., Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore) 1991;70:229–245. doi: 10.1097/00005792-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Black R.E., Williams S.M., Jones I.E., Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am. J. Clin. Nutr. 2002;76:675–680. doi: 10.1093/ajcn/76.3.675. [DOI] [PubMed] [Google Scholar]
- 14.Santiago-Fernandez P., Torres-Barahona R., Muela-Martínez J.A., Rojo-Martínez G., García-Fuentes E., Garriga M.J., León A.G., Soriguer F. Intelligence quotient and iodine intake: a cross-sectional study in children. J. Clin. Endocrinol. Metab. 2004;89:3851–3857. doi: 10.1210/jc.2003-031652. [DOI] [PubMed] [Google Scholar]
- 15.Mennella J.A., Griffin C.E., Beauchamp G.K. Flavor programming during infancy. Pediatrics. 2004;113:840–845. doi: 10.1542/peds.113.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremer H.J., Bührdel P., Burgard P., Clemens P.C., Leupold D., Mönch E., Przyrembel H., Trefz F.K., Ullrich K. Therapie von Patienten mit Phenylketonurie, Empfehlungen der Arbeitsgemeinschaft für Pädiatrische Stoffwechselstörungen (APS) (German) Monatsschr. Kinderheilkd. 1997;145:961–962. [Google Scholar]
- 17.Ceglarek U., Müller P., Stach B., Bührdel P., Thiery J., Kiess W. Validation of the phenylalanine/tyrosine ratio determined by tandem mass spectrometry: sensitive newborn screening for phenylketonuria. Clin. Chem. Lab. Med. 2002;40:693–697. doi: 10.1515/CCLM.2002.119. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann B, Bundeslebensmittelschlüssel. Max-Rubner-Institut. (2014). (Available from URL: http://www.bls.nvs2.de/).
- 19.Germany (D), Austrian (A), Swiss (CH)DACH-Reference values for the intake of nutritive substances (Referenzwerte für die Nährstoffzufuhr, DGE 2000) Deutsche Gesellschaft für Ernährung e.V. (DGE), Österreichische Gesellschaft für Ernährung (ÖGE), die Schweizerische Gesellschaft für Ernährungsforschung SGE, Schweizerische Vereinigung für Ernährung: Referenzwerte für die Nährstoffzufuhr. Frankfurt am Main: DACH; 2000.
- 20.Mensink G.B.M., Heseker H., Richter A., Stahl A., Vohmann C. 2007. Ernährungsstudie als KiGGS-Modul (EsKiMo) (Available from: URL: http://www.bmelv.de/cae/servlet/contentblob/378624/publicationFile/25912/EsKiMoStudie.pdf) [Google Scholar]
- 21.Fiege B., Blau N. Assessment of tetrahydrobiopterin (BH4) responsiveness in phenylketonuria. J. Pediatr. 2007;150:627–630. doi: 10.1016/j.jpeds.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Zabinski M.F., Daly T., Norman G.J., Rupp J.W., Calfas K.J., Sallis J.F., Patrick K. Psychosocial correlates of fruit, vegetable, and dietary fat intake among adolescent boys and girls. J. Am. Diet. Assoc. 2006;106:814–821. doi: 10.1016/j.jada.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Elfhag K., Tholin S., Rasmussen F. Consumption of fruit, vegetables, sweets and soft drinks are associated with psychological dimensions of eating behaviour in parents and their 12-year-old children. Public Health Nutr. 2008;11:914–923. doi: 10.1017/S1368980008002371. [DOI] [PubMed] [Google Scholar]
- 24.de Bourdeaudhuij I., te Velde S., Brug J., Due P., Wind M., Sandvik C., Maes L., Wolf A., Perez Rodrigo C., Yngve A., Thorsdottir I., Rasmussen M., Elmadfa I., Franchini B., Klepp K.I. Personal, social and environmental predictors of daily fruit and vegetable intake in 11-year-old children in nine European countries. Eur. J. Clin. Nutr. 2008;62:834–841. doi: 10.1038/sj.ejcn.1602794. [DOI] [PubMed] [Google Scholar]
- 25.Leuret O., Barth M., Kuster A., Eyer D., de Parscau L., Odent S., Gilbert-Dussardier B., Feillet F., Labarthe F. Efficacy and safety of BH4 before the age of 4 years in patients with mild phenylketonuria. J. Inherit. Metab. Dis. 2012;35:975–981. doi: 10.1007/s10545-012-9464-3. [DOI] [PubMed] [Google Scholar]