Abstract
Glycogen storage disease type Ia (GSD-Ia), characterized by impaired glucose homeostasis and chronic risk of hepatocellular adenoma (HCA), is caused by a deficiency in glucose-6-phosphatase-α (G6Pase-α or G6PC) activity. In a previous 70–90 week-study, we showed that a recombinant adeno-associated virus (rAAV) vector-mediated gene transfer that restores more than 3% of wild-type hepatic G6Pase-α activity in G6pc−/− mice corrects hepatic G6Pase-α deficiency with no evidence of HCA. We now examine the minimal hepatic G6Pase-α activity required to confer therapeutic efficacy. We show that rAAV-treated G6pc−/− mice expressing 0.2% of wild-type hepatic G6Pase-α activity suffered from frequent hypoglycemic seizures at age 63–65 weeks but mice expressing 0.5–1.3% of wild-type hepatic G6Pase-α activity (AAV-LL mice) sustain 4–6 h of fast and grow normally to age 75–90 weeks. Despite marked increases in hepatic glycogen accumulation, the AAV-LL mice display no evidence of hepatic abnormalities, hepatic steatosis, or HCA. Interprandial glucose homeostasis is maintained by the G6Pase-α/glucose-6-phosphate transporter (G6PT) complex, and G6PT-mediated microsomal G6P uptake is the rate-limiting step in endogenous glucose production. We show that hepatic G6PT activity is increased in AAV-LL mice. These findings are encouraging for clinical studies of G6Pase-α gene-based therapy for GSD-Ia.
Abbreviations: AAV, adeno-associated virus; BW, body weight; ER, endoplasmic reticulum; G6Pase-α, glucose-6-phosphatase-α; G6P, glucose-6-phosphate; G6PC, glucose-6-phosphatase-α gene; G6PT, glucose-6-phosphate transporter; GPE, G6PC promoter and enhancer; GSD-Ia, glycogen storage disease type Ia; HCA, hepatocellular adenoma
Keywords: Gene therapy, Recombinant adeno-associated virus vector, Glucose homeostasis, Glucose-6-phosphate transporter
Highlights
-
•
Establish the minimal hepatic G6Pase-α activity restoration required to provide a blood glucose level that enables GSD-Ia mice grow to old age (75–90 weeks).
-
•
Establish the minimal hepatic G6Pase-α activity restoration required that enables GSD-Ia mice grow to old age (75-90 weeks).
-
•
Define the lowest dose for G6Pase-α gene therapy that provides efficacy in mice, which informs a phase I/II clinical trial design in human GSD-Ia.
1. Introduction
Glycogen storage disease type Ia (GSD-Ia, MIM232200) is an autosomal recessive monogenic disease characterized by impaired blood glucose homeostasis. It is caused by a deficiency in glucose-6-phosphatase-α (G6Pase-α or G6PC) activity, which catalyzes the hydrolysis of glucose-6-phosphate (G6P) to glucose in the liver, kidney, and intestine [1], [2]. Topological analyses show that G6Pase-α contains multiple transmembrane domains that anchor it in the endoplasmic reticulum (ER) membrane. Since the active site of G6Pase-α lies inside the ER lumen [3], G6Pase-α depends on the ER-associated transmembrane protein G6P transporter (G6PT) to translocate G6P from the cytoplasm into the ER lumen [1], [2]. This transport is the rate-limiting step in endogenous glucose production [4]. Together, the enzyme and transporter form a G6Pase-α/G6PT complex that is essential for interprandial blood glucose homeostasis.
Patients affected by GSD-Ia are unable to maintain glucose homeostasis and present with fasting hypoglycemia, growth retardation, hepatomegaly, nephromegaly, hyperlipidemia, hyperuricemia, and lactic acidemia [1], [2]. The only therapy currently available is a dietary therapy [5], [6]. With strong compliance, dietary therapy enables patients to maintain normoglycemia but the underlying pathological processes remain uncorrected. One of the most significant chronic risks is hepatocellular adenoma (HCA) that develops in 70–80% of GSD-Ia patients over 25 year-old [7], [8]. In 10% of GSD-Ia patients, HCAs undergo malignant transformation to hepatocellular carcinoma (HCC) [9]. We have previously shown that systemic administration of rAAV-GPE, a recombinant adeno-associated virus (rAAV) pseudotype 2/8 vector expressing human G6Pase-α directed by the human glucose-6-phosphatase-α gene (G6PC) promoter/enhancer (GPE), delivers the G6Pase-α transgene to the liver and corrects hepatic G6Pase-α deficiency in G6pc−/− mice [11]. We have also shown that the rAAV-GPE-treated G6pc−/− mice expressing more than 3% of wild-type hepatic G6Pase-α activity grow normally for 70–90 weeks, exhibit a normal blood metabolite profile, display no hepatic abnormalities and have normal levels of hepatic triglyceride [11]. In contrast to GSD-Ia patients and untreated G6pc−/− mice, which cannot tolerate a short fast, the rAAV-GPE-treated G6pc−/− mice can sustain 24 h of fast [11]. Using a liver-specific G6pc-null (L-G6pc−/−) mouse strain, Mutel et al. [12] showed that L-G6pc−/− mice developed marked hepatic steatosis and 100% of the mice developed HCA 78 weeks after gene deletion. Interestingly, none of the rAAV-GPE-treated G6pc−/− mice developed hepatic steatosis or HCA over the 70–90 week study [11].
In this study, we examine the minimal hepatic G6Pase-α activity required to confer therapeutic efficacy and prevent HCA formation. We show that rAAV-GPE-treated G6pc−/− mice expressing 0.5–1.3% of normal hepatic G6Pase-α activity (AAV-LL mice) can tolerate 4–6 h of fast, grow to old age (75–90 weeks), and do not develop hepatic steatosis or HCA.
2. Materials and methods
2.1. Infusion of G6pc−/− mice with rAAV-GPE
All animal studies were conducted under an animal protocol approved by the NICHD Animal Care and Use Committee. The rAAV-GPE vector [10] was infused into 2-week-old G6pc−/− mice via the retro-orbital sinus. Age-matched G6pc+/+/G6pc+/− were used as wild type controls while 6-week-old G6pc−/− mice were used as negative controls. Since G6pc−/− mice cannot survive a fast, liver samples were collected at sacrifice without fasting. Liver samples from wild-type and AAV-LL mice were collected at sacrifice following a 4-hour fast.
2.2. Phosphohydrolase and microsomal G6P uptake assays
Microsome isolation, phosphohydrolase, and G6P uptake assays were determined essentially as described previously [13]. Reaction mixtures (100 μl) containing 50 mM cacodylate buffer, pH 6.5, 10 mM G6P and appropriate amounts of microsomal preparations were incubated at 37 °C for 10 min. Disrupted microsomal membranes were prepared by incubating intact membranes in 0.2% deoxycholate for 20 min at 0 °C. Non-specific phosphatase activity was estimated by pre-incubating disrupted microsomal preparations at pH 5 for 10 min at 37 °C to inactivate the acid labile G6Pase-α.
In G6P uptake assays, microsomes were incubated for 10 min at 37 °C in a reaction mixture (100 μl) containing 50 mM sodium cacodylate buffer, pH 6.5, 250 mM sucrose, and 0.2 mM [U-14C]G6P (50 μCi/μmol, American Radiolabeled Chemicals, St Louis, MO). The reaction was stopped by filtering immediately through a nitrocellulose membrane (BA85, Schleicher & Schuell, Keene, NH). Microsomes permeabilized with 0.2% deoxycholate, to abolish G6P uptake, were used as negative controls.
Enzyme histochemical analysis of G6Pase-α was performed by incubating 10 μm thick liver tissue sections for 10 min at room temperature in a solution containing 40 mM Tris-maleate pH 6.5, 10 mM G6P, 300 mM sucrose, and 3.6 mM lead nitrate [14]. The trapped lead phosphate was visualized following conversion to the brown colored lead sulfide [14].
2.3. Phenotype analysis
Blood levels of glucose, cholesterol, triglyceride, lactate, urate, and insulin, along with hepatic levels of glycogen, triglyceride, and G6P were determined as described previously [11].
For hematoxylin and eosin (H&E) and oil red O staining, liver sections were preserved in 10% neutral buffered formalin, and sectioned at 4–10 μm thickness. The stained sections were visualized using the Axioskop2 plus microscope and the AxioVision 4.5 software (Carl Zeiss, Thornwood, NY). For quantitative histochemical measurement of lipid accumulation, the oil red O stain was converted into pixel density units using Adobe Photoshop CS3 (Adobe System Incorporated, San Jose, CA).
Insulin tolerance testing of mice consisted of a 4-hour fast, prior to blood sampling, followed by intraperitoneal injection of insulin at 0.25 IU/kg, and repeated blood sampling via the tail vein for 1 h.
2.4. Statistical analysis
The unpaired t test was performed using the GraphPad Prism Program, version 4 (San Diego, CA). Values were considered statistically significant at P < 0.05.
3. Results
3.1. The minimal hepatic G6Pase-α activity required to confer therapeutic efficacy in G6pc−/− mice
To examine the minimal hepatic G6Pase-α required to confer therapeutic efficacy, 2-week-old G6pc−/− mice were infused with doses of rAAV-GPE titrated to restore and maintain 0.2–3.0% [1.8 × 1012 to 3.6 × 1012 viral particles (vp)/kg] of wild-type hepatic G6Pase-α activity. Metabolic profiles of the infused animals were monitored up to age 90 weeks.
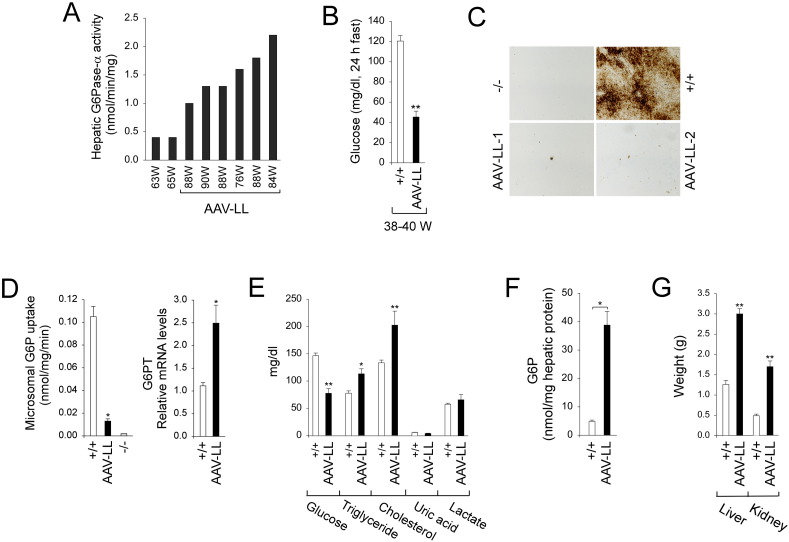
Hepatic microsomal G6Pase-α activity in old (75–90 week-old) wild-type mice (n = 18) averages 171.8 ± 7.1 nmol/min/mg. Untreated G6pc−/− mice suffer from hypoglycemic seizures and rarely live to age 3 weeks. Of the 8 rAAV-GPE-treated G6pc−/− mice expressing more than 0.2% of wild-type hepatic G6Pase-α activity at sacrifice, only two developed hypoglycemic seizures at ages 63 and 65 weeks, and were prematurely sacrificed. The hepatic G6Pase-α activity of these prematurely sacrificed mice was 0.4 nmol/min/mg, which is just 0.2% of wild-type activity, and the lowest activity restored in the treatment group (Fig. 1A). The 6 remaining mice that were unaffected by seizures, expressed 0.8 to 2.2 nmol/min/mg of hepatic G6Pase-α activity, equating to 0.5–1.3% of wild-type activity at sacrifice (75–90 weeks). Mice expressing 0.5–1.3% of wild type activity were designated AAV-LL mice (Fig. 1A). At age 38–40 weeks, AAV-LL mice could sustain a 24-hour fast, although their fasted glucose levels fell to the low end of the normal range (45.3 ± 5.8 mg/dl) (Fig. 1B). However, by age 75–90 weeks their tolerance to fasting had dropped to only 4–6 h. The rAAV vectors rarely integrate into the host genome, remaining predominantly as circular episomal elements [15]. Therefore, a gradual loss of the episomal rAAV vector genomes or their expression could account for the change seen in the AAV-LL mice whose initial tolerance of a 24 hour fast at age 38–40 weeks dropped to a 4–6 hour tolerance of fasting at age 75–90 weeks.
Fig. 1.
Biochemical and phenotypic analyses. (A) Hepatic microsomal G6Pase-α activity is shown at the indicated ages in weeks (W). Hepatic microsomal G6Pase-α activity in 75–90 week-old wild-type mice (n = 18) averaged 171.8 ± 7.1 nmol/min/mg (100%). The AAV-LL mice (n = 6) expressing 0.5–1.3% of wild-type hepatic G6Pase-α activity are grouped and named based on their hepatic G6Pase-α activity restored relative to wild-type activity as described previously [11]. (B) Blood glucose levels following a 24-hour fast in 38-40 week-old wild-type (n = 8) and AAV-LL (n = 6) mice. (C) Histochemical analysis of hepatic G6Pase-α activity. Freshly sectioned liver specimens were analyzed for G6Pase-α activity using the method of lead trapping of phosphate generated by G6P hydrolysis [14]. Each image represents an individual mouse. (D) Hepatic microsomal G6P uptake activity and G6PT mRNA expression in 75–90 week-old wild-type (n = 8), AAV-LL (n = 6), and 6-week old G6pc−/− (n = 4) mice. (E) Serum metabolite profiles of 75–90 week-old wild-type (n = 18) and AAV-LL (n = 6) mice. (F) Hepatic G6P levels in 75–90 week-old wild-type (n = 23) and AAV-LL (n = 6) mice. (G) Liver and kidney weights of 75–90 week-old wild-type (n = 8) and AAV-LL (n = 6) mice. (−/−), 6-week-old untreated G6pc−/− mice; (+/+), 75–90 week-old wild type mice. Data are mean ± SEM. *P < 0.05, **P < 0.005.
Enzyme histochemical analysis showed that G6Pase-α activity in wild-type liver was distributed throughout the liver but staining was not uniform (Fig. 1C). As expected, there was no stainable G6Pase-α activity in the liver sections of untreated G6pc−/− mice (Fig. 1C). The G6Pase-α activity in the AAV-LL liver section was in a few foci with a substantial proportion of activity, while most areas had little or no detectable G6Pase-α activity (Fig. 1C), consistent with previous data [11].
Hepatic endogenous glucose production is controlled by the functional coupling of G6Pase-α and G6PT [1], [2]. As expected, the G6pc−/− hepatic microsomes exhibited no significant G6P uptake activity, despite containing an intact G6PT [13] (Fig. 1D). In contrast, hepatic microsomes from AAV-LL mice showed G6P uptake activities 12% of wild-type activity (Fig. 1D). We have previously shown that reduced G6Pase-α activity stimulates G6PT mRNA expression [11]. Indeed, restoration of 0.5–1.3% of wild-type G6Pase-α activity in AAV-LL mice led to a 2.4-fold increase in hepatic G6PT mRNA expression over that in wild-type mice (Fig. 1D). Since G6P transport is the rate limiting component of the G6Pase-α/G6PT complex [4], increasing G6PT expression under conditions of low G6Pase-α activity appears to act as a compensatory mechanism to maintain glucose homeostasis.
The AAV-LL mice displayed reduced serum levels of glucose, increased levels of serum triglyceride and cholesterol but normal serum profiles of uric acid and lactate (Fig. 1E). However, with less than 1.3% of wild-type hepatic G6Pase-α activity restored, hepatic G6P levels in AAV-LL mice were 8-fold higher than that the old control mice (Fig. 1F). AAV-LL mice also developed hepatomegaly and nephromegaly (Fig. 1G) which were significantly more prominent than in mice titrated to express more than 3% of wild-type hepatic G6Pase-α activity [11].
3.2. Insulin tolerance profiles
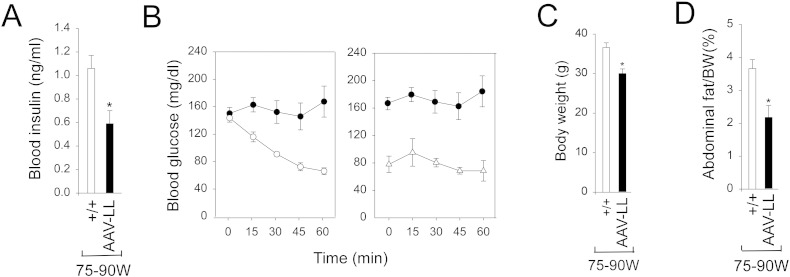
Insulin signaling regulates hepatic glucose and lipid metabolism [16]. After 4 h of fasting, blood insulin levels in 75–90-week-old AAV-LL mice (n = 6) were significantly lower than the values in wild type (n = 8) mice (Fig. 2A).
Fig. 2.
Phenotype and insulin tolerance profiles of wild-type and AAV-LL mice. (A) Blood insulin levels. (B) Insulin tolerance profiles of 12-week-old wild-type (○, n = 8), 75–90 week-old wild-type mice (●, n = 8) and AAV-LL (Δ, n = 6) mice treated with 0.25 IU/kg of insulin. (C) Body weights. (D) Abdominal fat content. (+/+), 75–90 week-old wild type mice (n = 8). The AAV-LL mice (n = 6) are rAAV-GPE-treated G6pc−/− mice expressing 0.5–1.3% of wild-type hepatic G6Pase-α activity. Data are mean ± SEM. *P < 0.05, **P < 0.005.
Because blood glucose levels in the AAV-LL mice are significantly lower than the levels in wild-type mice (Fig. 1E), a reduced insulin dose of 0.25 IU/kg was chosen to evaluate insulin tolerance profiles. Following an intraperitoneal insulin injection, blood glucose levels in 12-week-old wild-type (n = 8) mice decreased with time (Fig. 2B). Blood glucose levels in the 75–90 week-old wild-type and AAV-LL mice failed to decrease following insulin injection (Fig. 2B), reflecting the previously reported age-related decrease in insulin sensitivity [17].
3.3. Absence of hepatic histological abnormalities, steatosis, or HCA in AAV-LL mice
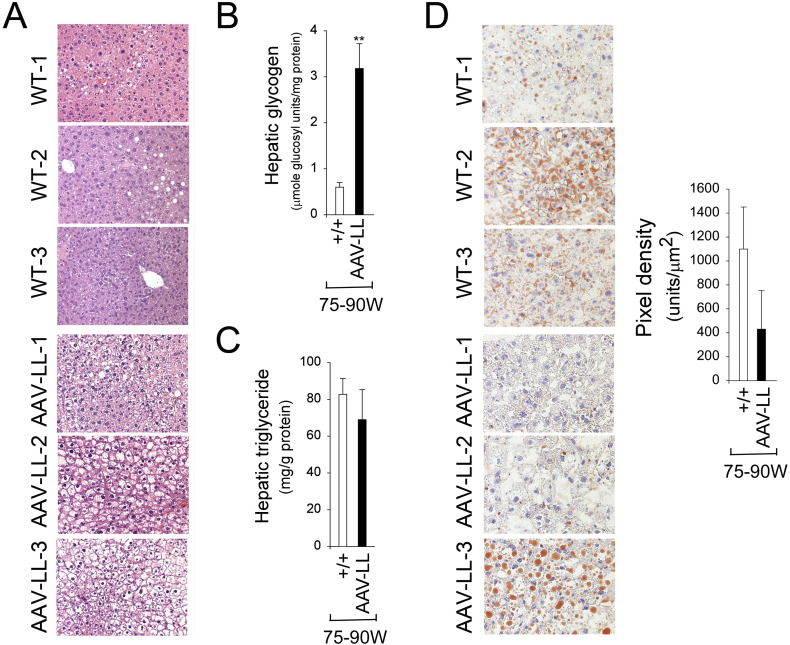
At age 75–90 weeks, the body weight (BW) of AAV-LL mice was on average 78.5% of their wild-type controls (Fig. 2C). The lower BW of the old AAV-LL mice is reflected in an overall reduction in their abdominal fat contents (Fig. 2D), suggesting that these mice do not develop age-related obesity. Histological analysis of liver biopsy samples revealed no hepatic nodules or histological abnormalities in any of the AAV-LL or wild-type mice that lived to age 75–90 weeks (Fig. 3A). While the AAV-LL mice exhibited a marked elevation of hepatic glycogen storage (Fig. 3B), hepatic triglyceride contents in the AAV-LL were not statistically different from those in wild-type mice (Fig. 3C). Oil red O staining confirmed that lipid contents in the AAV-LL mice were lower than that in wild-type controls, although the decrease was not statistically significant (Fig. 3D).
Fig. 3.
Histological, glycogen, and lipid analyses in the liver of 75–90 week-old wild type and AAV-LL mice. (A) H&E stained liver sections at original magnifications of × 200. Each plate represents an individual mouse. (B) Hepatic glycogen contents. (C) Hepatic triglyceride contents. (D) Oil red O staining at original magnifications of × 400 and quantification of oil red O staining. Each plate represents an individual mouse. (+/+), wild type mice (n = 8); AAV-LL (n = 6) are rAAV-GPE-treated G6pc−/− mice expressing 0.5–1.3% of normal hepatic G6Pase-α activity. Data are presented as mean ± SEM. **P < 0.005.
4. Discussion
We have previously shown that rAAV-GPE-treated G6pc−/− mice expressing more than 3% of wild-type hepatic G6Pase-α activity grow normally for 70–90 weeks, exhibit a lean phenotype, display normalized blood metabolite, sustain a 24 hour fast, and develop no hepatic abnormalities or HCA/HCC [11]. In this study, we show that mice expressing 0.2% of wild-type hepatic G6Pase-α activity suffer hypoglycemic seizures as they age, while AAV-LL mice expressing 0.5–1.3% of normal hepatic G6Pase-α activity do not experience seizures out to at least age 75–90 weeks. If the mouse is an accurate model of human glucose metabolism, this sets a lower acceptable limit of 0.5% wild-type hepatic G6Pase-α activity for any clinical gene therapy for GSD-Ia seeking to restore blood glucose homeostasis. While untreated G6pc−/− mice can tolerate a short fast of 60–75 min [11], the AAV-LL mice can sustain 4–6 h of fasting. However, as they age, the AAV-LL mice do display hyperlipidemia and marked hepatomegaly, which may lead to complications in the longer term.
During a fast, endogenous glucose is primarily produced in the liver via hydrolysis of G6P catalyzed by the G6Pase-α/G6PT complex [1], [2]. We have previously shown that rAAV-GPE-treated G6pc−/− mice expressing more than 3% of normal hepatic G6Pase-α activity are capable of hepatic glucose production averaging 61–90% of wild-type levels and also exhibit increased hepatic G6PT mRNA expression and G6P uptake activity [11]. Because the AAV-LL mice cannot sustain a 24 hour fast, we were unable to measure the levels of endogenous glucose produced in their livers. However, hepatic G6PT mRNA expression in the AAV-LL mice increased 2.4-fold over wild-type littermates, leading to increased hepatic microsomal G6P uptake activity, which averaged 12% of wild-type levels. This suggests that increases in the rate-limiting microsomal G6P uptake activity enables AAV-LL mice to sustain 4–6 h of fasting and grow to old age.
Mutel et al. [12] have shown that mice with a liver-specific knockout of G6Pase-α activity displayed marked hepatic steatosis and all mice developed HCA 78 weeks following gene deletion. In contrast, 75–90 week-old AAV-LL mice are lean, exhibit no hepatic abnormalities, no hepatic steatosis, have normal levels of hepatic triglyceride, and do not develop HCA. Insulin signaling promotes de novo lipogenesis in the liver and turns off β-oxidation of fatty acids which is essential for the maintenance of energy homeostasis [16], [18] but may contribute to the development of hepatic steatosis seen in GSD-Ia patients. Both wild-type and AAV-LL mice develop age-related insulin resistance at age 75–90 weeks. However, blood insulin levels in the AAV-LL mice were markedly lower than those in their wild-type controls. The low blood insulin levels, along with a lack of hepatic steatosis, may explain why AAV-LL mice do not develop HCA.
In summary, we have demonstrated that G6pc−/− mice expressing 0.5–1.3% of normal hepatic G6Pase-α activity exhibit normalized interprandial blood glucose homeostasis, can grow to old age without a chronic risk of HCA, but lose the ability to sustain long fasts and are still at risk of hyperlipidemia, hepatomegaly, and nephromegaly. Therefore for a gene therapy to address both short and long term consequences in GSD-Ia, restoration of at least 3% of wild-type liver G6Pase-a activity is required. These data should be valuable in informing the planning of phase I/II clinical trials for GSD-Ia gene therapy to study safety at doses that may offer efficacy.
Conflict of interest
The authors reported no potential conflicts of interest.
Acknowledgment
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and The Children's Fund for Glycogen Storage Disease Research.
References
- 1.Chou J.Y., Matern D., Mansfield B.C., Chen Y.T. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr. Mol. Med. 2002;2:121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- 2.Chou J.Y., Jun H.S., Mansfield B.C. Glycogen storage disease type I and G6Pase-β deficiency: etiology and therapy. Nat. Rev. Endocrinol. 2010;6:676–688. doi: 10.1038/nrendo.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan C.J., Lei K.J., Annabi B., Hemrika W., Chou J.Y. Transmembrane topology of glucose-6-phosphatase. J. Biol. Chem. 1998;273:6144–6148. doi: 10.1074/jbc.273.11.6144. [DOI] [PubMed] [Google Scholar]
- 4.Arion W.J., Lange A.J., Ballas L.M. Quantitative aspects of relationship between glucose 6-phosphate transport and hydrolysis for liver microsomal glucose-6-phosphatase system. Selective thermal inactivation of catalytic component in situ at acid pH. J. Biol. Chem. 1976;251:6784–6790. [PubMed] [Google Scholar]
- 5.Greene H.L., Slonim A.E., O'Neill J.A., Jr., Burr I.M. Continuous nocturnal intragastric feeding for management of type 1 glycogen-storage disease. N. Engl. J. Med. 1976;294:423–425. doi: 10.1056/NEJM197602192940805. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.T., Cornblath M., Sidbury J.B. Cornstarch therapy in type I glycogen storage disease. N. Engl. J. Med. 1984;310:171–175. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- 7.Labrune P., Trioche P., Duvaltier I., Chevalier P., Odievre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J. Pediatr. Gastroenterol. Nutr. 1997;24:276–279. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Rake J.P., Visser G., Labrune P., Leonard J.V., Ullrich K., Smit G.P. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. results of the European Study on Glycogen Storage Disease Type I (ESGSD I) Eur. J. Pediatr. 2002;161(Suppl 1):S20–S34. doi: 10.1007/s00431-002-0999-4. [DOI] [PubMed] [Google Scholar]
- 9.Franco L.M., Krishnamurthy V., Bali D., Weinstein D.A., Arn P., Clary B., Boney A., Sullivan J., Frush D.P., Chen Y.T., Kishnani P.S. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J. Inherit. Metab. Dis. 2005;28:153–162. doi: 10.1007/s10545-005-7500-2. [DOI] [PubMed] [Google Scholar]
- 10.Yiu W.H., Lee Y.M., Peng W.T., Pan C.J., Mead P.A., Mansfield B.C., Chou J.Y. Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy. Mol. Ther. 2010;18:1076–1084. doi: 10.1038/mt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y.M., Jun H.S., Pan C.J., Lin S.R., Wilson L.H., Mansfield B.C., Chou J.Y. Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy. Hepatology. 2012;56:1719–1729. doi: 10.1002/hep.25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutel E., Abdul-Wahed A., Ramamonjisoa N., Stefanutti A., Houberdon I., Cavassila S., Pilleul F., Beuf O., Gautier-Stein A., Penhoat A., Mithieux G., Rajas F. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J. Hepatol. 2011;54:529–537. doi: 10.1016/j.jhep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Lei K.J., Chen H., Pan C.-J., Ward J.M., Mosinger B., Jr., Lee E.J., Westphal H., Mansfield B.C., Chou J.Y. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type 1a mouse. Nat. Genet. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- 14.Teutsch H.F. Chemomorphology of liver parenchyma. Qualitative histochemical distribution patterns and quantitative sinusoidal profiles of G6Pase, G6PDH and malic enzyme activity and of glycogen content. Prog. Histochem. Cytochem. 1981;14:1–92. [PubMed] [Google Scholar]
- 15.Nakai H., Yant S.R., Storm T.A., Fuess S., Meuse L., Kay M.A. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leavens K.F., Birnbaum M.J. Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. Biol. 2011;46:200–215. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- 17.Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee E.J., Lee W.Y., Cho Y.K., Kim B.I., Sung K.C. Hyperinsulinemia and the development of nonalcoholic fatty liver disease in nondiabetic adults. Am. J. Med. 2011;124:69–76. doi: 10.1016/j.amjmed.2010.08.012. [DOI] [PubMed] [Google Scholar]