Abstract
Disorders caused by defects in the mitochondrial translation system are clinically and genetically heterogeneous. The elongation phase of mitochondrial protein synthesis requires, among many other components, three nuclear-encoded elongation factors: EFTu (TUFM; 602389), EFTs (TSFM; 604723), and EFG1 (GFM1; 606639). Mutations have been identified in the genes encoding all three elongation factors, and they result in combined respiratory chain deficiencies and severe phenotypes with an early fatal outcome. So far, only eleven patients have been reported with mutations in GFM1. Here we describe an additional three patients with novel GFM1 mutations. Our results confirm the tissue-specific effect of GFM1 mutations, since we found only slightly decreased respiratory chain enzyme activities in muscle and fibroblasts, but a severe deficiency in the liver. Hence, a thorough biochemical evaluation is important to guide genetic investigation in patients suspected for a mitochondrial disorder.
Abbreviations: OXPHOS, oxidative phosphorylation; RC, respiratory chain; CS, citrate synthase
Keywords: EFG1 (GFM1, 606639); Neonatal mitochondrial hepatoencephalopathy; Mitochondrial disorder
1. Introduction
Eukaryotes contain two translational systems, one in the cytosol and one in the mitochondria. The mitochondrial translation machinery comprises mitochondrial DNA (mtDNA) encoded rRNA and tRNAs as well as numerous nuclear-encoded proteins, including mitochondrial ribosomal proteins, and initiation, elongation and termination factors [1]. All these components are essential for maintaining the primary function of the mitochondria, the biosynthesis of energy (ATP) via the oxidative phosphorylation (OXPHOS) pathway. Disorders caused by defects in the mitochondrial translation system are clinically and genetically heterogeneous. The elongation phase of mitochondrial protein synthesis requires, among many other components, three nuclear-encoded elongation factors: EFTu (TUFM; 602389), EFTs (TSFM; 604723), and EFG1 (GFM1; 606639). Mutations have been identified in the genes encoding all three elongation factors, and they result in severe phenotypes with an early fatal outcome [2], [3]. Usually, a combined respiratory chain (RC) deficiency is found, i.e. decreased activity of two or more RC complexes, excluding complex II, which is the only complex where the subunits are encoded entirely by the nuclear genome. So far, only eleven patients have been reported with mutations in GFM1 [3], [4], [5], [6], [7], [8], [9]. Here we describe an additional three patients with novel GFM1 mutations.
2. Materials and methods
2.1. Patients
2.1.1. Patient 1
Patient 1 was a boy, the first of three children from healthy Danish parents. The parents were consanguineous since they had common second great grandparents. He was born at term after a normal pregnancy and delivery. All birth parameters were below average, weight was 2200 g (< 3 percentile), birth length 45 cm (< 3 percentile) and head circumference 32 cm (< 3 percentile). At birth, only a single umbilical artery was noted, and he had dysmorphic features with retrognathia, epicanthus, simply shaped external auricles, a unilateral simian crease, hypospadia, maldescensus testis and a left-sided paralysis of the facial nerve. Abnormal posturing was also noted.
Within the first 10 h he was admitted to the neonatal department due to lactic acidosis with symptoms consisting of twitching of the extremities, abnormal head movements, abnormal breathing and vomiting. There was hypoglycemia with a blood glucose of 0.6 mmol/l (normal value [NV] > 2.5 mmol/l), standard base-excess − 25.1 (NV +/− 3) and serum lactate of 23.4 mmol/l (NV < 2.0). He had a normal ECG. Initially he was treated with intravenous glucose, which was discontinued after four days. The following days his respiration normalized and he became clinically stable.
At three months, a brain CT showed bilateral temporal cortical atrophy and widespread hypodense areas in the white matter. He had poor eye contact and his mental development was markedly delayed. He developed liver failure with coagulopathy and encephalopathy and died at four months of age. A muscle biopsy showed hypotrophic muscle fibers and abnormal lipid vacuoles in the sarcoplasm. An autopsy showed severely disordered cortical migration with polymicrogyria, glandular hypospadia, micronodular cirrhosis and germ cell hypoplasia.
2.1.2. Patient 2
Patient 2 was the younger sister of patient 1, born two and a half years later. She was born after a normal pregnancy and delivery at 38 weeks. The birth parameters were all < 3 SD. She developed hypoglycemia with lactic acidosis in the first day of life, and within days, signs of liver dysfunction. She had failure to thrive, with poor weight gain. She died 14 days old. Autopsy was not performed, but a postmortem muscle biopsy showed normal muscle histology.
2.1.3. Patient 3
Patient 3 was a girl, the second child of healthy non-consanguineous parents. She was born after a normal pregnancy and delivery at gestational age 41 + 5. Her birth weight and length were normal (2970 g and 51 cm, respectively), but her head circumference was only 33 cm (< 3 percentile). She was admitted to the neonatal department shortly after birth due to lethargy and attenuated respiration with lactic acidosis, the serum lactate was 25 mmol/l (NV < 2.0). During the first week liver dysfunction was found, whereas an echocardiography was normal. A cerebral ultrasound revealed two pseudocysts, but was otherwise normal. Her neurological development was severely delayed, and a cerebral MRI one month old revealed severe leukodystrophy. At two months old she was hypertonic and had onset of epileptic seizures. She died at three months of age during a febrile episode.
2.2. Molecular and biochemical analyses
Analysis of RC enzyme activity in muscle, liver and fibroblasts was performed as previously reported [10].
DNA was extracted from cultured fibroblasts and blood samples using standard methods. Based on the clinical phenotypes with hepatoencephalopathy and a combined RC enzyme deficiency, Sanger sequencing of the coding regions and exon–intron junctions of POLG, DGUOK, MPV17 and GFM1 was performed. The RefSeq for GFM1 was NM_024996.5, primer sequences are available upon request. The bioinformatic programs Sorting Intolerant From Tolerant (SIFT), Polymorphism Phenotyping (PolyPhen-2) and MutationTaster were used for in silico evaluation of the pathogenicity of the mutations. Western blot analysis was performed essentially as previously reported [11]. The membrane was incubated with an antibody against EFG1 (a kind gift from Eric A. Shoubridge, Montreal), and an antibody against porin (Proteintech, Chicago, IL, USA) was used as a loading control. The secondary antibody was goat anti-rabbit (Dako, Glostrup, Denmark).
3. Results
Analysis of RC enzyme activity in muscle tissue revealed a complex IV deficiency in patient 1 with a slightly decreased complex I activity. In patients 2 and 3, a slight decrease of complex IV was found (Table 1). Patient 1 had normal RC enzyme activity in fibroblasts, whereas patients 2 and 3 had a decreased complex IV activity (complex I was not investigated in patient 3). In patient 3, a severe combined RC defect was found in liver tissue (Table 1).
Table 1.
Results of respiratory chain enzyme analysis.
| CI/CS | CI/CS | CIII/CS | CIV/CS | CS | |
|---|---|---|---|---|---|
| Muscle | |||||
| Patient 1 | 58 (65–135) | 81 (59–141) | 106 (44–156) | 28 (43–157) | 104 (40–161) |
| Patient 2 | 89 (65–135) | 94 (59–141) | 138 (44–156) | 42 (43–157) | 65 (40–161) |
| Patient 3 | 66 (65–135) | 127 (68–133) | 128 (56–144) | 62 (65–135) | 102 (25–175) |
| Liver | |||||
| Patient 3 | 4 (43–176) | 43 (28–195) | 24 (45–208) | 3 (42–181) | 410 (51–176) |
| Fibroblasts | |||||
| Patient 3 | N.p.a | 102 (67–141) | 95 (75–130) | 36 (67–155) | 112 (44–156) |
| CI | CII | CIII | CIV | |
|---|---|---|---|---|
| Fibroblasts | ||||
| Patient 1 | 17 (11–26) | 23 (7–21) | 65 (25–65) | 85 (73–197) |
| Patient 2 | 26 (11–26) | 14 (7–21) | 59 (25–65) | 56 (73–197) |
Enzyme activities of citrate synthase (CS) and respiratory chain complexes I–IV corrected for CS activity. Values are expressed as percentage of control mean (reference ranges in parentheses). The enzyme analyses were carried out at different times, and the reference values have been changed slightly. The activities of complexes I through IV in fibroblasts from patients 1 and 2 are expressed as mU/mg protein (reference ranges in parentheses). Abnormal values are shown in bold.
Not performed.
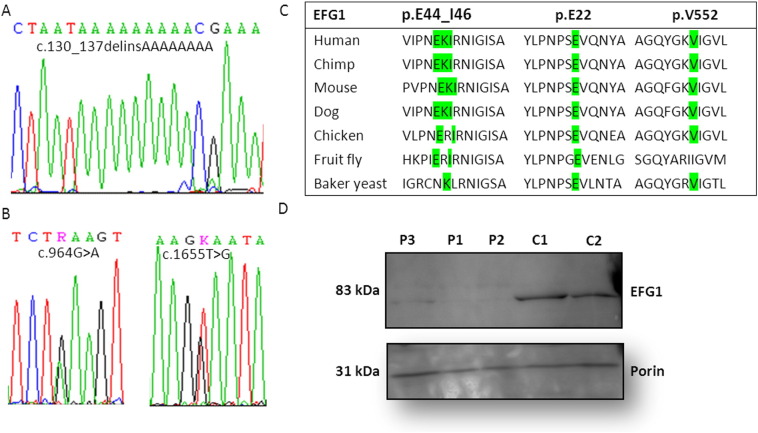
Sequencing analysis of GFM1 in patients 1 and 2 revealed a novel homozygous indel mutation, a deletion of 8 nucleotides combined with an in-frame insertion of 8 adenosine nucleotides (c.130_137delinsAAAAAAAA) (Fig. 1A). The mutation is predicted to substitute three highly conserved amino acids (EKI) starting from codon 44 with three lysine residues (p.E44_I46delinsKKK) (Fig. 1C). In silico analysis predicts substitution of the glutamic acid and isoleucine residues with lysine to be potentially pathogenic (SIFT: deleterious (score: 0, median: 3.63), MutationTaster: disease causing (p-value: 1) PolyPhen 2: probably damaging (score: 0.960)). The parents were heterozygous carriers of the mutation.
Fig. 1.
Detection and characterization of the GFM1 mutations. (A) Sequence traces of c.130_137delinsAAAAAAAA, (B) c.964G>A and c.1655T>G. (C) EFG1 sequence alignment between different species. All the affected amino acids are located in highly conserved regions across species. (D) Western blot analysis of EFG1, lanes P1–3: patients' fibroblasts, lanes C1–C2: controls' fibroblasts. The band of ~ 83 kDa corresponds to the expected size for EFG1. An antibody against porin was used as a loading control.
In patient 3, two missense mutations were identified in GFM1: c.964G>A (p.E322K) located in exon 7 and c.1655T>G (p.V552G) located in exon 14 (Fig. 1B and C). The parents were each a heterozygous carrier of either mutation. Neither mutation has been reported previously, and they are both located in conserved regions of the protein (Fig. 1C). Furthermore, in silico analysis predicts both substitutions to be disease-causing (p.E322K: SIFT: deleterious (score: 0, median: 3.63) MutationTaster: disease causing (p-value: 1), PolyPhen 2: probably damaging (score: 0.960)/p.V552G: SIFT: deleterious (score: 0, median: 3.63), MutationTaster: disease causing (p-value: 1), PolyPhen2: probably damaging (score: 0.992)). Western blot analysis showed absence of EFG1 protein in the two siblings (patients 1 and 2), whereas a very weak band (~ 83 kDa) corresponding to EFG1 was observed in patient 3 fibroblasts (Fig. 1D).
4. Discussion
Mutations in GFM1 are associated with a recessively inherited mitochondrial disorder displaying a severe phenotype leading to an early fatal outcome [3], [4], [5], [6], [7], [8], [9], [10]. We here report three patients with GFM1 mutations, two siblings who are homozygous for an in frame mutation (c.130_137delinsAAAAAAAAA) and a patient who is compound heterozygous for two missense mutations (c.964G>A and c.1655T>G). The pathogenicity of these three novel mutations is supported by in silico analysis, segregation analysis, and Western blot analysis showing severely decreased or absent EFG1 protein and decreased RC activity, in concordance with the findings in previously reported patients with GFM1 mutations.
The clinical phenotype of patients with GFM1 mutations is summarized in Table 2.
Table 2.
Summary of 14 patients diagnosed with a defect in EFG1: GFM1 mutations, respiratory chain enzyme analysis and clinical presentation.
| ID |
GFM1 mutations (NM_024996.5) |
RC activity of the five complexes |
Clinical presentation | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | I | II | III | IV | V | ||||
| P1 | Homozygous: c.521A>G p.N174S |
Muscle Fib. |
52% 40% |
69% |
54% 18% |
Female, first cousin parents, cesarean section, IUGR, microcephaly, stiffness, few movements. From day 10, severe metabolic acidosis. From day 7, progressive liver dysfunction. Died 27 days old. Postmortem liver examination: cholestasis and necrosis. Brain: hypoplasia of corpus callosum, cystic lesion in the basal ganglia | Coenen et al. | ||
| P2 | Homozygous: c.521A>G p.N174S |
Fib. | 13% | 31% | Male sibling of P1. Blood lactate up to 9.3 mmol/l. Brain US: generalized atrophy, small corpus callosum. Delayed growth and development, increased muscle tone in upper extremities. Signs of liver failure from week 7. Died five months old. |
||||
| P3 | Compound heterozygous: c.961T>Ca/ c.1765-2_1765-1delb p.S321P/p.G589 |
Muscle Fib. |
38%c | 51%c | 50%c | 10%c 20%c |
Unrelated parents. IUGR. Metabolic acidosis, blood lactate 22 mmol/l, hyperbilirubinemia, hypoalbuminemia soon after birth. Mild facial dysmorphism. Large patent ductus arteriosus. Brain MRI normal, MRS showed elevated lactate. Developed ascites, coagulopathy, respiratory failure. Died 9 days old. Autopsy: liver steatosis, microvesicular cholestasis, pesudoacinar formation, bile duct proliferation, iron deposition. Kidney lipid accumulation. Neuropathology: porencephaly, microcephaly, dysgenesis of cingulated gyri. |
Antonicka et al. | |
| P4 | Compound heterozygous: c.961T>Ca/ c.1765-2_1765-1delb p.S321P/p.G589 |
Fib. | 26%c | Female sibling of P3. IUGR, oligohydramnios. Induced delivery week 24, died 45 min later. Liver: excess iron. |
|||||
| P5 | Compound-heterozygous: c.139C>T/c.1487T>Gd p.47X/M496R |
Muscle Fib. |
~ 59% ~ 40% |
~ 109% ~ 106% |
Np ~ 48% |
~ 53% ~ 20% |
Np ~ 47% |
Female, unrelated parents. Respiratory stress, metabolic acidosis 2 days old. Episodes with hyper- and opisthotonus. Serum lactate 10.2 mmol/l. Elevated transaminases. From age six months, episodic metabolic acidosis, with lactate up to 86 mM. Psychomotor regression, microcephaly, hypotonia, limb spasticity, nystagmus. Enlarged liver. Brain MRI: polymicrogyria, diffuse signal abnormalities in the cerebral, cerebellar and pontine WM, multiple cystic lesions. Died 14 months old. |
Valente et al. |
| P6 | Homozygous: c.748C>T p.R250W |
Muscle Fib. |
~ 113% ~ 34% |
~ 145% ~ 115% |
~ 98% ~ 79% |
~ 136% ~ 25% |
Female, SGA, second cousin parents. Onset two days old with feeding problems and reduced consciousness. Psychomotor retardation, hypotonia, poor eye contact. Onset of seizures 8 weeks old. Borderline microcephaly, wandering eye movements. Hypertonia of extremities, brisk reflexes. Blood lactate 4.9, CSF lactate 2.5. Brain MRI: small frontal cortex, thin corpus callosum, delayed myelination. Hepatomegaly from age 8 months. Died 2 years old due to pneumonia. |
Smits et al. | |
| P7 | Compound heterozygous: c.539delG/c.688G>A p.G180Afs*11/p.G230S |
Fib. | 68% | 47% | Female, non-consanguineous parents. IUGR. Microcephaly. Onset two days old with lactic acidosis, lactate up to 25 mmol/l, CSF lactate 12 mmol/l. Abnormal liver biochemistry. Recurrent episodes of lactic acidosis. Hypotonia, myopathic facies, slight facial dysmorphism. Progressive liver dysfunction with hepatomegaly. FTT, psychomotor retardation. Nystagmus, ptosis. Tube feeding. Died 8 months old. Brain MI: global cystic changes in subcortical WM, lesions in putamen and globus pallidus, enlarged ventricles. |
Balasubramaniam et al. | |||
| P8 | Homozygous: c.2011C>T p.R671C |
Muscle Fib. |
~ 84% ~ 62% |
~ 86% ~ 428% |
~ 80% ~ 401% |
~ 30% ~ 183% |
~ 113% ~ 154% |
Male, consanguineous parents. Feeding difficulties and vomiting immediately after birth. Microcephaly, coarse facies, hypotonia, dystonia, polyneuropathy, psychomotor retardation. Tube feeding. No seizures, but abnormal eeg. Died 4 years old. Brain MRI: vermis hypoplasia, pontine atrophy. Supratentorial atrophy, parenchymal loss of WM, cortex, basal ganglia. |
Galmiche et al. |
| P9 | Homozygous: c.1193T>C p.L398P |
Muscle Fib. |
~ 93% | ~ 74% ~ 200% |
~ 33% ~ 38% |
Male, consanguineous parents. Low birth weight. Spasticity with hyperreflexia and contractures. Developed hypoglycemia, metabolic acidosis, elevated lactate 30 h old. Abnormal liver biochemistry. Recurrent episodes of lactic acidosis. Developmental delay, microcephaly, dysmorphism, hypospadias. Died 20 months old. | |||
| P10 | Compound heterozygous: c.720delT/c.2011C>T p.E241NfsX1/ p.R671C |
Muscle Fib. |
↓↓ ↓ |
Onset before 1 week of age. Hypsarrhythmia, failure to thrive, dystonia, squint. | Calvo et al. | ||||
| P11 | Compound heterozygous: c.720delT/ c.910A>G p.E241NfsX1/p.K304E |
Liver | ↓↓ | ↓↓ | Onset before one year of age. Developmental delay, seizures, hypotonia, episodic metabolic acidosis. | ||||
| P12 | Homozygous: c.130_137delins p.E44_I46delinsKKK |
Muscle Fib. |
~ 89% ~ 155% |
~ 137% ~ 329% |
~ 241% ~ 260% |
~ 65% ~ 116% |
Male, parents related. Small birth parameters. Dysmorphic facial features. Onset on day 1 with lactic acidosis and hypoglycemia. Lactate up to 23 mmol/l. Brain CT: cortical atrophy, hypodense areas in WM. Psychomotor retardation and liver failure. Died four months old. Brain autopsy showed polymicrogyria. Liver histology: cirrhosis. |
This paper | |
| P13 | Homozygous: c.130_137delins p.E44_I46delinsKKK |
Muscle Fib. |
~ 137% ~ 236% |
~ 159% ~ 200% |
~ 314% ~ 236% |
~ 98% ~ 77% |
Younger sister of patient 12. Small for GA. Onset day 1 with hypoglycemia and lactic acidosis. Liver failure and FTT. Died 14 days old. | ||
| P14 | Compound heterozygous: c.964G>A/ c.1655T>G p.E322K/p.V552G |
Liver Muscle Fib. |
~ 9% ~ 102% Np |
~ 154% ~ 187% ~ 152% |
~ 53% ~ 229% ~ 126% |
~ 7% ~ 95% ~ 54% |
Female, non-consanguineous parents. Microcephaly from birth. Neonatal onset with lactic acidosis, serum lactate 25 mmol/l. Liver dysfunction in the first week. Epilepsy and hypertonia form 2 months. Brain MRI: severe leukodystrophy. Died 3 months old. |
||
Residual activities expressed as a percent of the lowest control value stated. Arrow indicate significance at decreased levels RC activity.
Fib. = fibroblasts.
Previously reported as c.1068T>C.
Previously reported as c.1872_delAG.
Residual activities expressed as a percent of control mean value.
Previously reported as c.1478T>G.
In a few patients (4/14) intrauterine growth retardation was found. Most patients were born at term, but half of the patients were small for gestational age. All patients had neonatal onset of disease, usually in the first days of life, with e.g. feeding problems or metabolic acidosis. All patients had psychomotor retardation, and most had hypo- and/or hypertonia. Microcephaly was found in seven of the 14 patients. In addition, polyneuropathy, dystonia, facial dysmorphism and epilepsy were found in a variable number of patients, and, as previously discussed, most had liver failure (11/14). Death was from nine days to four years. Neuroimaging showed bilateral basal ganglia hyperintensities, polymicrogyria, cerebral atrophy, corpus callosum atrophy, delayed myelination or cystic lesions [3], [4], [5], [6], [7], [8], [9]. Hence the clinical phenotype is quite specific, and a defect in EFG1 should be suspected in a patient with neonatal onset of lactic acidosis, psychomotor retardation, hepatic dysfunction and RC deficiency.
EFG1 is a GTPase, which catalyzes the translocation step of mitochondrial protein synthesis. The process involves the movement of peptidyl-tRNA from the ribosomal-acceptor (A) site to the peptidyl (P) site following removal of a deacylated tRNA from the peptidyl (P) site to the ribosomal exit (E) site. This movement results in advancement of the mRNA by one codon and subsequently leaves the ribosomal-acceptor (A) site available for a new elongation cycle [1]. The biochemical deficiency in patients with GFM1 mutations is a combined OXPHOS deficiency, without any quantitative or qualitative mtDNA abnormalities. Interestingly, the deficiency has been shown to be tissue-specific, although GFM1 is ubiquitously expressed [4], [8]. Smits et al. reported a patient with a combined RC enzyme deficiency in fibroblasts, and only a slight decrease of complex III activity in muscle, a rather uncommon finding in mitochondrial disorders [8]. The authors speculated that adaptive processes, e.g. compensatory changes in the levels of other translation factors, may be involved. Antonicka et al. investigated the molecular basis for tissue specificity in two siblings with GFM1 mutations [4]. They found that the severity of the OXPHOS defect in the patients correlated with the amount of mutant protein; EFG1 protein was undetectable in the liver, where BN-PAGE showed less than 10% residual assembly of complexes I and IV, and a 50% decrease in the assembly of complex V. EFG1 was barely detectable in skeletal muscle and fibroblasts, where reductions in RC amount of 50–80% were found, whereas heart tissue showed only a decrease of complex IV of 50%, where EFG1 was present at almost 60% of control levels. They also found that the relative ratios of the levels of the translation factors EFG1, EFTu and EFTs were strikingly different between tissues, with an ~ 4-fold increase of EFTu in patient heart tissue, which could explain the tissue specific defects found in the patients. The RC enzyme analysis in our patient 3 also illustrates a tissue-specific defect, with a severe combined RC deficiency identified in the liver and a much milder defect in muscle and fibroblasts.
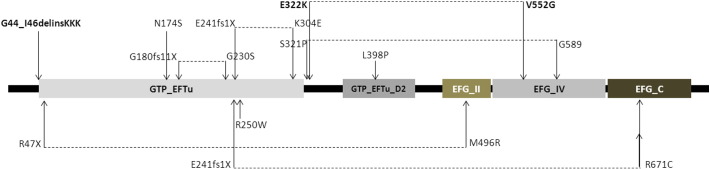
EFG1 consists of 751 amino acids and includes five Pfam domains (http://pfam.janelia.org) (Fig. 2). The novel amino acid substitutions identified in patients 1 and 2 are located in the beginning of the GTP_EFTU domain, a domain which is exposed to conformational changes mediated by the hydrolysis of GTP to GDP, whereas the E322K substitution of patient 3 is located just downstream from this domain. The V552G substitution is located in the EFG_ IV domain, which appears to be essential for the structural transition to take place (Fig. 2).
Fig. 2.
EFG1 protein structure with its functional domains (http://pfam.janelia.org). The mutations shown above EFG1 indicate the patients who suffered from liver failure. Dotted lines indicate patients who are compound heterozygous for GFM1 mutations. Bold texts indicate novel mutations reported in this paper.
Western blot analysis showed no visible EFG1 protein in fibroblasts from patients with the indel mutation, whereas a small amount was seen in patient 3 with two missense mutations. Although the patient could theoretically have some residual EFG1 activity, this is not reflected in the phenotype, which is as severe as that seen in other patients with GFM1 mutations. It has been discussed whether the clinical phenotype in patients with GFM1 mutations could be related to the localization of the mutations in EFG1 (Fig. 2). Most of the patients described display a severe phenotype; lactic acidosis shortly after birth, signs of liver failure within the first weeks of life and encephalopathy with a fatal course within the first year of life (Fig. 2: mutations illustrated above the EFG1) [4], [5], [6], [7]. However, a few patients, two patients homozygous for p.R250W and p.R671C, respectively, and one patient compound heterozygous for p.R47X/p.M496R, had a slightly milder course without affection of the liver and longer survival, up until 4 years [3], [7], [8] (Fig. 2, mutations illustrated below EFG1). Interestingly, in silico 2D modeling of the location of the three apparent milder missense mutations in EFG1 showed an association to the peripheral regions of the protein, in contrast to three other missense mutations located in the central part of the protein, where the clinical phenotype included hepatic failure. The authors suggested that the location of these mutations could explain the tissue-specific involvement observed in these patients [7].
In summary, we report three additional patients with novel GFM1 mutations, and phenotypes similar to those of previously reported patients. Our results confirm the tissue-specific effect of GFM1 mutations, since we found not only slightly decreased RC enzyme activities in muscle and fibroblasts, but also a severe deficiency in the liver. Hence, a thorough biochemical evaluation is important to guide genetic investigation in patients suspected for a mitochondrial disorder.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank the families for their participation.
References
- 1.Smits P., Smeitink J., van den Heuvel L. Mitochondrial translation and beyond: processes implicated in combined oxidative phosphorylation deficiencies. J. Biomed. Biotechnol. 2010;2010:737385. doi: 10.1155/2010/737385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smeitink J.A., Elpeleg O., Antonicka H., Diepstra H., Saada A., Smits P., Sasarman F., Vriend G., Jacob-Hirsch J., Shaag A., Rechavi G., Welling B., Horst J., Rodenburg R.J., van den Heuvel B., Shoubridge E.A. Distinct clinical phenotypes associated with a mutation in the mitochondrial translation elongation factor EFTs. Am. J. Hum. Genet. Nov 2006;79(5):869–877. doi: 10.1086/508434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valente L., Tiranti V., Marsano R.M., Malfatti E., Fernandez-Vizarra E., Donnini C., Mereghetti P., De G.L., Burlina A., Castellan C., Comi G.P., Savasta S., Ferrero I., Zeviani M. Infantile encephalopathy and defective mitochondrial DNA translation in patients with mutations of mitochondrial elongation factors EFG1 and EFTu. Am. J. Hum. Genet. Jan 2007;80(1):44–58. doi: 10.1086/510559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonicka H., Sasarman F., Kennaway N.G., Shoubridge E.A. The molecular basis for tissue specificity of the oxidative phosphorylation deficiencies in patients with mutations in the mitochondrial translation factor EFG1. Hum. Mol. Genet. Jun 1 2006;15(11):1835–1846. doi: 10.1093/hmg/ddl106. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramaniam S., Choy Y.S., Talib A., Norsiah M.D., van den Heuvel L.P., Rodenburg R.J. Infantile progressive hepatoencephalomyopathy with combined OXPHOS deficiency due to mutations in the mitochondrial translation elongation factor gene GFM1. JIMD Rep. 2012;5:113–122. doi: 10.1007/8904_2011_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coenen M.J., Antonicka H., Ugalde C., Sasarman F., Rossi R., Heister J.G., Newbold R.F., Trijbels F.J., van den Heuvel L.P., Shoubridge E.A., Smeitink J.A. Mutant mitochondrial elongation factor G1 and combined oxidative phosphorylation deficiency. N. Engl. J. Med. Nov 11 2004;351(20):2080–2086. doi: 10.1056/NEJMoa041878. [DOI] [PubMed] [Google Scholar]
- 7.Galmiche L., Serre V., Beinat M., Zossou R., Assouline Z., Lebre A.S., Chretien F., Shenhav R., Zeharia A., Saada A., Vedrenne V., Boddaert N., de L.P., Rio M., Munnich A., Rotig A. Toward genotype phenotype correlations in GFM1 mutations. Mitochondrion. Mar 2012;12(2):242–247. doi: 10.1016/j.mito.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Smits P., Antonicka H., van Hasselt P.M., Weraarpachai W., Haller W., Schreurs M., Venselaar H., Rodenburg R.J., Smeitink J.A., van den Heuvel L.P. Mutation in subdomain G′ of mitochondrial elongation factor G1 is associated with combined OXPHOS deficiency in fibroblasts but not in muscle. Eur. J. Hum. Genet. Mar 2011;19(3):275–279. doi: 10.1038/ejhg.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvo S.E., Compton A.G., Hershman S.G., Lim S.C., Lieber D.S., Tucker E.J., Laskowski A., Garone C., Liu S., Jaffe D.B., Christodoulou J., Fletcher J.M., Bruno D.L., Goldblatt J., Dimauro S., Thorburn D.R., Mootha V.K. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. Jan 25 2012;4(118):118ra10. doi: 10.1126/scitranslmed.3003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wibrand F., Jeppesen T.D., Frederiksen A.L., Olsen D.B., Duno M., Schwartz M., Vissing J. Limited diagnostic value of enzyme analysis in patients with mitochondrial tRNA mutations. Muscle Nerve. May 2010;41(5):607–613. doi: 10.1002/mus.21541. [DOI] [PubMed] [Google Scholar]
- 11.Ostergaard E., Hansen F.J., Sorensen N., Duno M., Vissing J., Larsen P.L., Faeroe O., Thorgrimsson S., Wibrand F., Christensen E., Schwartz M. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain. Mar 2007;130(Pt 3):853–861. doi: 10.1093/brain/awl383. [DOI] [PubMed] [Google Scholar]