Summary
Activated human neutrophils produce a fibrillar DNA network [neutrophil extracellular traps (NETs)] for entrapping and killing bacteria, fungi, protozoa and viruses. Our results suggest that the neutrophil extracellular traps show a resistant amyloidogenic backbone utilized for addressing reputed proteins and DNA against the non‐self. The formation of amyloid fibrils in neutrophils is regulated by the imbalance of reactive oxygen species (ROS) in the cytoplasm. The intensity and source of the ROS signal is determinant for promoting stress‐associated responses such as amyloidogenesis and closely related events: autophagy, exosome release, activation of the adrenocorticotrophin hormone/α‐melanocyte‐stimulating hormone (ACTH/α‐MSH) loop and synthesis of specific cytokines. These interconnected responses in human activated neutrophils, that have been evaluated from a morphofunctional and quantitative viewpoint, represent primitive, but potent, innate defence mechanisms. In invertebrates, circulating phagocytic immune cells, when activated, show responses similar to those described previously for activated human neutrophils. Invertebrate cells within endoplasmic reticulum cisternae produce a fibrillar material which is then assembled into an amyloidogenic scaffold utilized to convey melanin close to the invader. These findings, in consideration to the critical role played by NET in the development of several pathologies, could explain the structural resistance of these scaffolds and could provide the basis for developing new diagnostic and therapeutic approaches in immunomediated diseases in which the innate branch of the immune system has a pivotal role.
Keywords: ACTH axis, amyloidogenesis, exosomes, neutrophil extracellular trap, ROS evaluation
Introduction
Neutrophils, the most abundant human leucocytes, play a pivotal role in innate immunity, constituting the first cells recruited into sites of infection. In response to numerous stimuli, neutrophils migrate from the circulating blood into inflamed tissues where they employ several strategies to contain and clear infections. These strategies include phagocytosis, degranulation, the release of lytic enzymes into the phagosome or in the extracellular space and the production of reactive oxygen intermediates with anti‐microbial potential 1, 2, 3, 4, 5. Another neutrophil response mechanism against non‐self is the production of neutrophil extracellular traps (NETs), a web‐like structure for entrapping and killing bacteria, fungi, protozoa and viruses extracellularly 6, 7, 8, 9.
The NET is formed by decondensed chromatin (DNA and histones H1, H2A, H2B, H3, H4) and is decorated by proteins from primary granules (for example, myeloperoxidase and neutrophil elastase), secondary granules (such as lactoferrin) and tertiary granules (matrix metalloproteinase 9, etc.). The NETs have been induced by internal and/or pathogen‐derived molecular signals [cytokines such as interleukin (IL)‐8, tumour necrosis factor (TNF)‐α, high mobility group box 1 (HMGB1), lipopolysaccharide (LPS), N‐formyl‐methionyl‐leucyl‐phenylalanine (fMLP) 7, 8, 9, 10, 11, 12, 13 and pharmacological agents, such as phorbol myristate acetate (PMA) 5, 7.
NETs may contribute to microbial containment by forming a scaffold functioning as a physical barrier, and enhance anti‐microbial synergy while minimizing damage to host tissues 14, 15, 16. The formation of NETs in vertebrates has been considered a strategy linked indubitably to an innate immune response and it is a typical property shared by neutrophils, eosinophils 17, monocytes/macrophages 18 and mast cells 19.
Several authors have described that freely circulating phagocytic haemocytes in protostomes and in deuterostomes, when stimulated with LPS, show dilated reticulum cisternae filled with spatially organized fibrillar material. The objective of the accumulated material within cytoplasm is to be exocytosed and assembled into a resistant scaffold to template melanin and to drive this toxic pigment towards the non‐self 20, 21, 22. As highlighted by our previous studies, the framework of phagocytic circulating cells was made of amyloid fibrils 20, 21, 22. Due to the similarity in the release of fibrillar structures by LPS‐activated phagocytic haemocytes and LPS‐stimulated neutrophils, we also hypothesized that the NET could be characterized by an amyloid structure in these human cells.
Amyloidogenesis is dependent upon cytoplasmic pH acidification due to increased reactive oxygen species (ROS), an event already described widely in activated neutrophils 2, 15, 23, 24. This process is also accompanied by an increase of IL‐18 expression. This cytokine has been associated with amyloid deposition in vertebrates and is also linked to Alzheimer's disease 25, 26, where it can be considered an early marker for amyloidogenesis and for disease progression 25.
Despite the fact that amyloidogenesis is generally associated with degenerative pathologies, amyloid fibril formation may contribute to normal physiology having different functions, such as bacterial/fungi biofilm formation, scaffolding for different molecules, regulation of melanin synthesis, epigenetic control of polyamines and information transfer in a wide range of organisms, from bacteria to mammals 22, 27, 28, 29, 30, 31.
The bi‐directional interactions between the neuroendocrine and immune systems have been examined 32, 33, focusing on adrenocorticotrophic hormone (ACTH), alpha melanocyte‐stimulating hormone (α‐MSH) and neutral endopeptidase (NEP) production. It is now recognized that in both invertebrates and vertebrates the non‐self presence is perceived and managed by the two systems, able to influence each other mutually. ACTH and α‐MSH are released by the immunocompetent cells to control immune reactivity 34, 35, 36. These factors regulate the cellular functions of the immune system locally, in an autocrine and paracrine manner, influencing the proliferation of immune mediator production and immunocyte trafficking 22, 23, 37.
Expression of the ACTH is regulated by cleavage to α‐MSH by NEP. Further NEP is also involved in the degradation of amyloid fibrils 38, 39. Taken together, these data suggest that this stress sensor/response system occurs in neutrophils.
Materials and methods
All experiments were performed in five independent replicates.
Neutrophil purification and activation
Circulating neutrophils were isolated from venous blood or buffy coat derived from healthy donors. Briefly, blood was allowed to sediment on dextran at 37°C for 30 min (from Leuconostoc spp., Mr 450 000–650 000; Sigma‐Aldrich, Milan, Italy). Supernatant was recovered and PMN were isolated by Ficoll‐Paque PLUS (GE Healthcare, Milan, Italy) density‐gradient centrifugation; contaminating erythrocytes were eliminated by hypotonic lysis for 10 min in distilled water and the following (g/l) were added: 8·25 ammonium chloride (NH4Cl), 1·00 potassium bicarbonate (KHCO3) and 0·04 ethylenediamine tetraacetic acid (EDTA). Cells were then washed three times in 0·15 M NaCl.
Neutrophils (8 × 104 cells/ml) were seeded on rounded glass coverslips (12 mm diameter, treated with 0·1% gelatinase) or in six‐well plates in RPMI‐1640 [2% fetal bovine serum (FBS)] and either unstimulated or stimulated with 100 ng/ml LPS (from Escherichia coli, serotype O55:B5,) for 15 or 40 min or with 5 ng/ml PMA (Sigma‐Aldrich) for 15 min. Stimulated and unstimulated neutrophils were then processed for different procedures.
Light microscopy and transmission electron microscopy (TEM)
For routine TEM, neutrophils were collected in six‐well plates and fixed with 4% glutaraldehyde in 0·1 M Na‐cacodylate buffer (pH 7·2).
Pellets were washed in 0·1 M Na‐cacodylate buffer (pH 7·2) and post‐fixed for 20 min with 1% osmic acid in cacodylate buffer (pH 7·2). After standard dehydration in an ethanol series, samples were embedded in an Epon‐Araldite 812 mixture and sectioned with a Reichert Ultracut S ultratome (Leica, Nussloch, Germany). Semithin sections were stained by conventional methods (crystal violet and basic fuchsin) and were observed with a light microscope (Eclipse Nikon, Amsterdam, the Netherlands). Thin sections were stained by uranyl acetate and lead citrate and observed with a Jeol 1010 electron microscope (Jeol, Tokyo, Japan).
Intracellular ROS evaluation
Oxidative stress can induce proteins to adopt an insoluble beta‐pleated sheet conformation 40, and according to numerous authors 41, 42, 43 oxidative damage appears to be the earliest event preceding amyloid fibril formation. Thus, it is important to evaluate the overproduction of ROS in relation to LPS activation responsible for amyloid fibril production 21. According to Grimaldi and co‐workers 21, 22, a gross identification of cells showing increased ROS is obtained with differential May–Grünwald–Giemsa staining (Bioptica, Milan, Italy). The different staining of cells depends upon cytoplasmic pH (alkaline pH increases blue while acid pH enhances a pink or reddish tinge in the stained specimens). Coverslips were mounted in Eukitt and slides were observed under an Eclipse Nikon microscope with ×50 or ×100 objectives.
ROS production and its derivation was validated by use of MitoSOX™ Red mitochondrial superoxide indicator (Molecular Probes, Eugene, OR, USA), a fluorigenic probe used to detect the degree of intracellular level of ROS obtained from mitochondrial activity. Once in the mitochondria, MitoSOX™ Red reagent is oxidized selectively by superoxide and exhibits red fluorescence. Oxidation can be detected by monitoring the increase in fluorescence. Following the manufacturer's protocol, resting and activated neutrophils were washed once in fresh medium, resuspended in phosphate‐buffered saline (PBS) and incubated in the dark with 4 μM MitoSOX prepared in Hanks's balanced salt solution (HBSS)/Ca/Mg for 10 min at 37°C. Nuclei were counterstained with 4′,6′‐diamino‐2‐phenylindole (DAPI; Sigma‐Aldrich). Fluorescence was determined by excitation/emission maxima of approximately 510/580 nm. Fluorescent images, visualized on a fluorescence Eclipse Nikon microscope, were acquired with a DS‐5 M‐L1 Nikon digital camera system.
ROS production was also evaluated by the use of 2′,7′‐dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes), a fluorigenic probe used commonly to detect the overall degree of intracellular level of ROS. H2DCFDA is a non‐fluorescent compound that readily crosses cell membranes. It is hydrolyzed to 2′,7′‐dichlorofluorescein (DCF) within cells and becomes fluorescent when it is oxidized by ROS. Oxidation can be detected by monitoring the increase in fluorescence. Resting and activated neutrophils were incubated in the dark with 10 µM 2′,7′‐dichlorodihydrofluorescein diacetate (H2DCFDA) for 30 min at 37°C; nuclei were stained with DAPI. Fluorescence was determined by excitation at 488 nm and emission at 525 nm wavelength; fluorescent images visualized on a fluorescence Eclipse Nikon microscope were acquired with a DS‐5 M‐L1 Nikon digital camera system.
In parallel, we also performed a quantitative experiment with the same probe. Freshly isolated cells were suspended at a concentration of 1 × 106 cells/ml in HBSS medium and then incubated for 1 h with H2DCFDA 2 µmol/l in the dark at 37°C; cells were then washed twice with HBSS. Fluorescence measurements were performed using a spectrofluorimeter (Perkin‐Elmer LS‐50B; Perkin‐Elmer Instruments, Bridgeport, CT, USA). Excitation wavelength was set at 488 nm and fluorescence emission was collected at 525 nm. Intracellular ROS levels were then expressed in arbitrary units (AU) as fluorescence intensity. The overall degree of cytoplasmic ROS was tested on resting cells, on cells after LPS activation (after 15 and 40 min) and on cells treated with PMA (after 15 min), the potent synthetic agonist.
Amyloid fibril characterization
Amyloid structures were identified according to Le Vine 44 by staining cells with thioflavin S (ThS) and visualizing the amyloid‐specific green/yellow fluorescence with an Eclipse Nikon microscope. Images were acquired with a DS‐5 M‐L1 Nikon digital camera system.
Using the Image J software package (http://rsbweb.nih.gov/ij/download.html), the fluorescence ratio (expressed as integrated density) between the amyloid‐associated ThS signal and the NET DNA‐associated DAPI was calculated.
Amyloid fibrils were also characterized with Congo red staining, according to published methods 45, and observed under cross‐polarized light with an Axioskop 2 microscope (Carl Zeiss, Jena, Germany), equipped with a MC 80 DX camera (Carl Zeiss).
Amyloid fibrils (see the next section) were also localized immunocytochemically using an antibody directed against (PMEL17), a protein that has amyloid characteristics and contributes to form fibrillar structures in mammals 46.
Immunocytochemistry for ACTH, α‐MSH, NEP, interleukin 18 and Pmel17 localization
The presence of amyloid fibrils was confirmed by using the primary antibody anti‐human Pmel17 (H‐300) polyclonal antibody (1 : 100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The presence of ACTH and its cleavage product (due to degrading enzyme NEP), α‐MSH and the proinflammatory IL‐18 were assessed using the following primary antibodies: anti‐human ACTH polyclonal antibody (1 : 100 dilution; Abcam, Cambridge, UK); anti‐human α‐MSH polyclonal antibody (1 : 100 dilution; Abcam); anti‐human IL‐18 polyclonal antibody (1 : 100 dilution; Abnova, Taipei City, Taiwan); and anti‐human CD10/CALLA (NEP) monoclonal antibody (1 : 100 dilution; GeneTex, Hsinchu City, Taiwan). Incubations with suitable secondary antibodies conjugated with cyanin 5 (Cy5, 1 : 50 dilution; Abcam) were performed for 1 h in a dark humid chamber at room temperature. Nuclei were stained with DAPI (Sigma‐Aldrich). The PBS buffer used for washing steps and antibody dilutions contained 2% bovine serum albumin (BSA; Sigma‐Aldrich) and 0·1% Tween20.
In co‐localization experiments, the cells were first stained with ThS (as described above) and then incubated with anti‐Pmel17 antibody (as described above).
In control samples, primary antibodies were omitted and samples were treated with BSA/Tween20 containing PBS. Coverslips were mounted in CitiFluor (CitiFluor Ltd, London, UK). Slides were observed under an Eclipse Nikon microscope.
Statistical analysis
Data are presented as mean ± standard error of the mean (s.e.m.), with n indicating the number of observations. Parametric continuous variables were compared by means of Student's t‐test. Analysis of the correlation between functional responses of neutrophils was performed by linear regression analysis (for continuous variables) and statistical significance for correlations was set at P < 0·05. Calculations were performed using commercial software (GraphPad Prism version 5·00 for Windows; GraphPad Software, San Diego, CA, USA; www.graphpad.com).
Results
Morphology and behaviour of neutrophils
In order to study the morphofunctional modifications that occur during the activation of neutrophils, we examined these cells after stimulation with LPS at 15 min (corresponding to the starting point of the event) and 40 min according to Jaillon et al. 47 to observe NET formation. The rapid activation of these cells can be achieved utilizing different (natural or chemical) stressors such as LPS and PMA, respectively, with a final result of massive degranulation and NET formation 7. Even if PMA is generally considered the most widely used stimulus to induce NET formation 48 (Fig. 1d), we preferred to focus on the activation gained with a natural (LPS) rather than a chemical (PMA) agonist. In addition, LPS provides initially slower and more reliable kinetics, allowing us to describe the modulated responses leading to NET formation step by step.
Figure 1.
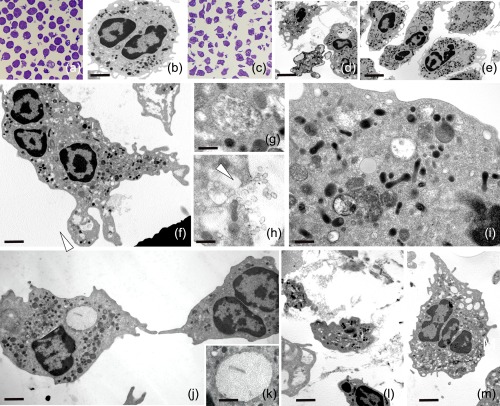
Unstimulated and stimulated human neutrophils. Thin and semithin sections of resting (a,b) and activated neutrophils (c–m). The unstimulated cells (a,b) are roundish, but after stimulation (c) with both phorbol myristate acetate (PMA) (d) and lipopolysaccharide (LPS) (e–m) they lose their globoid shape. Starting from 15 min after stimulation, neutrophils show a transformed phenotype characterized by irregular profile, due to the presence of protrusions and pseudopodia (d,e). In a time lapse between 15 and 40 min from LPS activation (e–m), a series of events are detectable due to the presence of multi‐vesicular bodies (f,g), released exosomes (f,h) and autophagosomes containing portions of the cytosol (i). Activated cells show large dilated reticulum cisternae (j,k) (arrowheads) filled with spatially organized fibrillar material (k) or empty vacuoles (l,m) and material among cells (m). Scale bars. (a) 6 μm; (b) 5 μm; (d) 9 μm; (e) 3·5 μm; (f) 4·5 μm; (g) 1·9 μm.
Human neutrophils, in resting condition, were spheroidal cells easily distinguishable under optical and electron microscopy by their compressed, multi‐lobed nuclei and different types of granules embedded in homogeneous cytoplasm, while very few neutrophils showed cytoplasmic projections (Fig. 1a,b). Within 15 min after LPS administration, optical and TEM analysis indicated that most of the neutrophils were flattened with irregular profiles due to cytoplasmic projections and with evident multi‐lobular ‘opened’ nuclei (Fig. 1c,e). From 15 to 40 min several specific events could be detected. In the cytoplasm, the presence of multi‐vesicular bodies, exocytosis events of exosomes (Fig. 1f–h) as well as premature autophagosomes, engulfing cytoplasm portions, were identified (Fig. 1i). Concomitantly, the presence of empty vacuoles or rough reticulum cisternae containing fibrillar material were observed (Fig. 1j,k).
Finally, 40 min after LPS stimulation, neutrophils were characterized by a massive cellular vacuolization accompanied, although not in all activated cells, by NET formation (Fig. 1l,m).
Amyloid fibrils production
The fibrillar material observed in the human neutrophils showed staining properties typical of amyloid fibrils 49, 50. First Congo red (CR) positivity, evaluated by the characteristic yellow‐green birefringence under crossed polarization, has been used commonly to test the presence of amyloid fibrils 44. With regard to cells in the resting condition (Fig. 2a,b), the activated cells (Fig. 2c–f) were CR‐positive, showing the typical apple‐green birefringence of amyloid under polarized light (Fig. 2d,f). To confirm amyloid presence, the neutrophils were also stained by ThS (Fig. 2g–n). The characteristic bright yellow‐green fluorescence, indicating amyloid presence, was visible both in cytoplasm (Fig. 2h–n) and in correspondence with the NETs (Fig. 2k–n), more readily evident in the stimulated cells compared to controls (Fig. 2g). Semiquantitative analysis of the concomitant amyloid fibril presence (ThS) and DNA (DAPI) of NETs showed a ratio of 0.78 ± 0.03. Immunolocalization with a specific antibody directed against Pmel17, a basic mammalian protein involved in amyloidogenesis 27, further supported the presence of amyloid. In comparison to resting neutrophils, activated cells (Fig. 2o) showed increased Pmel17 expression consistent with the degree of activation (Fig. 2p–r). In particular, at approximately 40 min of stimulation, the signal, first present especially in the cytoplasm, became detectable in the NETs, typically DAPI‐positive (Fig. 2q,r). In activated neutrophils, Pmel17 immunoreactivity was found co‐localized to a cytoplasmic area that is also ThS‐positive (Fig. 2s–u).
Figure 2.
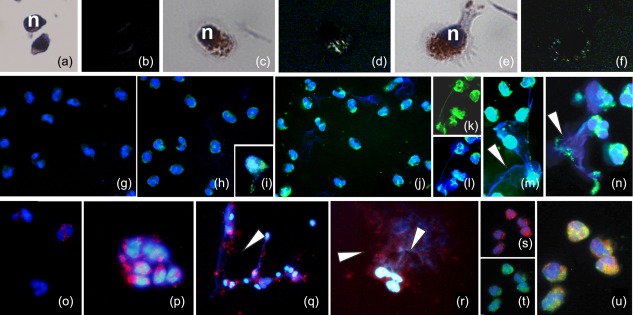
Amyloidogenesis in lipopolysaccharide (LPS)‐stimulated neutrophils. (a–n) Detection of amyloid fibril presence with Congo red (CR) and thioflavin S (ThS) stainings. (a–f) CR staining: unstimulated neutrophils (a,b) in comparison with LPS‐stimulated cells (c–f) at the starting point of activation. The CR positive cells show the typical apple green birifrangence evidencing the spotted presence of amyloid structures in the cytoplasm. Nuclei are counterstained with haematoxylin (n). (g–n) Identification of amyloid fibrils with ThS: unstimulated neutrophils (g) in comparison with stimulated cells at 15 min (h–j) and 40 min (k–n) from LPS administration. Within cells (h–j) and extracellularly (k–n), amyloid fibrils are localized by ThS bright fluorescence. Nuclei and DNA material in neutrophil extracellular traps (NETs) are stained with 4′,6′‐diamino‐2‐phenylindole (DAPI) and marked in brilliant blue. Both single staining, for ThS and DAPI (k,l) and the two overlaid signals are proposed to better identify the presence of amyloid and DNA materials. Note the co‐localization of blue (DAPI) and green (ThS) in NETs released on stimulation (arrowheads). The semi‐quantitative analysis of the concomitant amyloid fibril presence (ThS) and DNA (DAPI) of NETs showed a ratio of 0·78 ± 0·03. (o–u) (PMEL17) immunolocalization: immunofluorescence staining showing, in comparison to control (o), the increased expression of Pmel17 that is present in the cytoplasm of stimulated neutrophils (p–r) and in the NETs (q,r). DAPI (blue) staining for DNA shows distinct cores indicating nuclei, as well as diffuse patterns evidencing extracellular DNA in NETs (arrowheads). (s–u) Double‐labelling of neutrophils with ThS (green) and antibody against Pmel17 (red). The co‐localization of two signals (merge in yellow, u) is quite good. Nuclei (blue) are stained with DAPI.
Intracellular ROS evaluation
Previous studies have suggested that in different types of phagocytic/circulating cells, from invertebrates to vertebrates, amyloid scaffold formation occurs in relation to a redox status/cytoplasmic pH modification 21, 22. In addition, further information suggests that in humans, ROS influence the formation of extracellular traps (ETs) in a process termed ‘ETosis’ 24, 51. During the early stages of immune response, neutrophil activation, leading to ROS generation and resulting in pH acidification, is a process linked closely to NET production 15, 52, 53. We showed that a growing number of LPS‐stimulated neutrophils were characterized by pink cytoplasm in May–Grünwald–Giemsa differential staining (Fig. 3a–e), giving a gross indication of a lower intracellular pH.
Figure 3.
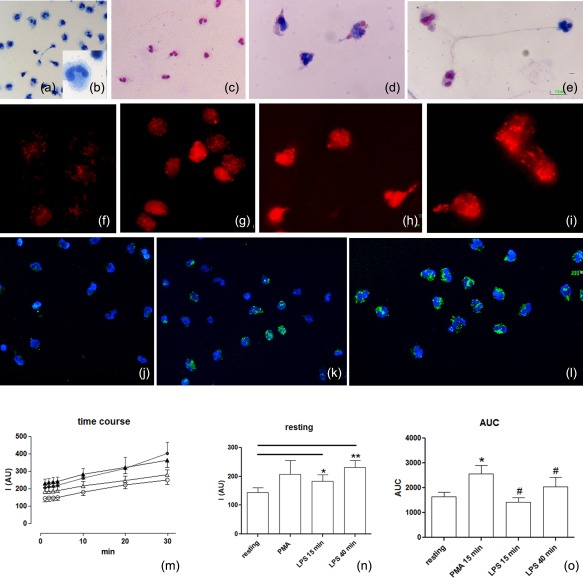
Evaluation of intracellular reactive oxygen species (ROS). (a–e) Light microscopy: resting (a,b) and stimulated cells (c–e) are stained using the May–Grünwald–Giemsa technique. The differential staining of neutrophils depends on cytoplasmic pH (the pink mark indicates acid pH while the blue mark shows an alkaline pH). (f–o) ROS evaluation was obtained using fluorescence dyes: the MitoSOX red used as a selective indicator of mitochondrial superoxide and 2′,7′‐dichlorodihydrofluorescein diacetate (H2DCFDA) to detect the overall degree of cytoplasmic ROS. Most ROS (red signal) were generated in mitochondria (f–i) starting from the resting condition (f) up to 15 min (g) and 40 min (h,i) after lipopolysaccharide (LPS) stimulation. The increase of the overall degree of intracellular ROS (j–l), using H2DCFDA, is localized by fluorescence microscopy: (j) cells in resting condition (k,l) 15 and 40 min after LPS stimulation, respectively. In neutrophils, from the resting condition to neutrophil extracellular trap (NET) formation, the concentration of cellular ROS levels increased following appropriate [LPS and phorbol myristate acetate (PMA)] stimulation on a rapid time‐scale. (m) Spectrofluorimetric evaluation of the time–course of ROS generation in human neutrophils under resting conditions (empty circle), prestimulation for 15 min with PMA (filled circles) for 15 min (empty triangles) or 40 min (filled triangles) with LPS. (n) Delta variations of values measured in neutrophils under the different conditions. (o) Area under the curve (AUC) of fluorescence measured for detection of neutrophil ROS generations during the 30 min of detection. Data are presented as mean ± standard error of at least three to five separate experiments. *P < 0·05 versus resting PMN; #P < 0·05 versus PMA.
Using the redox‐sensitive dye MitoSOX and the fluorigenic probe H2DCFDA, we detected the increased degree of the intracellular free radical production attributable to mitochondrial activity (Fig. 3f–i) and the overall degree of intracellular level of ROS (Fig. 3j–l), respectively, in relation to the administration time.
Spectrofluorimetric evaluation of the time–course of ROS generation in human neutrophils under resting conditions or stimulated with LPS in comparison with PMA validated the data of immunocytochemical localizations. In neutrophils, from the resting condition to NET formation, the ROS level concentration increased rapidly following LPS stimulation. ROS production was modulated not only to the administration time, but was also connected strictly to the type of activator (i.e. LPS or PMA).
The experimental profile of ROS levels in unstimulated neutrophils and of changes induced by LPS or PMA stimuli are shown in Fig. 3m–o. In particular, samples preincubated with LPS or PMA showed higher resting values in comparison to unstimulated samples (Fig. 3m–o).
Closely related events during NET formation: amyloidogenesis, IL‐18 production and ACTH/α‐MSH axis activation
Neutrophil activation is accompanied by proinflammatory cytokine production 4. Among the plethora of these cytokines we have examined IL‐18, because it is generally known as a link with stress‐associated responses and amyloid deposition 54; LPS‐activated neutrophils showed increased production in this cytokine (Fig. 4a–d).
Figure 4.
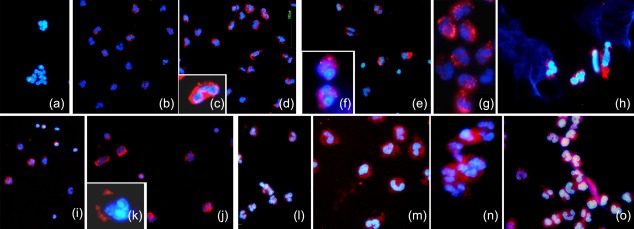
Characterization of lipopolysaccharide (LPS)‐stimulated neutrophils. (a–o) Immunocytochemical characterizations. Interleukin (IL)‐18 (a–d), adrenocorticotrophin hormone (ACTH) (e–h), alpha melanocyte‐stimulating hormone (α‐MSH) (i–j) and neutral endopeptidase (NEP) (l–o) expressions show high levels of positivity in activated neutrophils (b–d, f–h, j–k, m–o), while in resting cells the expression of IL‐18 (a), ACTH (e), α‐MSH (i) and NEP (l) is basal.
Activation of stress circuits resulting in the extensive production of ACTH and α‐MSH was also observed in LPS‐activated neutrophils. The production of ACTH (Fig. 4e–h) and its cleavage product α‐MSH (Fig. 4I,j) were observed by immunocytochemical localizations.
NEP is an enzyme associated with cleavage of ACTH to α‐MSH 22, 55, 56, 57, as well as amyloid degradation and removal 58, 59, 60, 61, 62, 63.
In stimulated neutrophils, concomitant with the increase in ACTH and amyloid fibril synthesis, an increment in production of NEP (Fig. 4l–o) was also observed by immunocytochemistry both in the cytoplasm (Fig. 4m) and on NETs (Fig. 4o).
Discussion
The results presented aim to provide evidence that, after LPS stimulation, neutrophils show tuned events, culminating with the production of amyloid fibrils that, in turn, made a backbone structure for addressing reputed proteins and DNA against the non‐self. The NET formation is regulated by the imbalance of reactive oxygen species in the cytoplasm.
NETs released against bacteria, fungi, viruses or protozoa have been described extensively from morphological, biochemical and molecular viewpoints 2, 4, 5, 14, 15, 16, 64 and it has long been debated whether or not these web‐like structures were produced in a controlled process, rather than in a disintegration cellular event 48.
Here we provide evidence concerning the modulated events that take place during the activation phase of neutrophils showing that the LPS‐stimulated cells are the reservoir of amyloid fibrillar structures that, in turn, form NETs. These fibrils show all the characteristics of amyloid: birefringence upon Congo red staining 45 and ThS positivity 44, and harbour a basic mammalian protein, Pmel17 27, 28, as validated by the co‐localization of signals. Few naive neutrophils show amyloid fibrils that are compartmentalized by membranes of rough reticulum cisternae, while upon LPS challenge most of the activated neutrophils exhibit the production of amyloid material. In a sequence of events, ultrastructural and immunocytochemical analysis revealed that neutrophils start to synthesize and harbour fibrillar materials that are released from the intracellular repositories to support NET formation. We suggest that the amyloid fibrils, charged cationically, may co‐operate to form the scaffold for addressing those numerous proteins and nuclear DNA, charged anionically, has already been well identified and described by several authors 4, 7, 8, 47.
In stimulated neutrophils, the association of amyloid fibrils with NET formation is supported by several events, starting from REDOX cytoplasmic changes where ROS is immunodetected and measured quantitatively by spectrofluorimetric evaluation (Fig. 3). To strengthen the idea that amyloid production in neutrophils is a possible and common event, it is important to bear in mind that the change in cytoplasmic pH linked to ROS generation is considered as the earliest event that induces proteins to adopt a β‐sheet conformation preceding the formation of amyloid fibrils 40, 41, 62. ROS production has been reported as an absolute requisite to NET formation and has been demonstrated extensively in neutrophils stimulated by bacteria and fungi 2, 5, 7, 8, 15, 19, 52, 53, 65. Moreover, in a recent paper, Zawrotniak and co‐workers reported that the treatment of neutrophils with N‐acetyl‐cysteine (NAC), a potent anti‐oxidant, influences the production of NET. This drug decrease the ROS level in a dose‐dependent manner 66.
ROS, despite their potential destructive activity, can be considered as a promoter of a variety of cellular processes 42, 43, 62. In LPS‐activated neutrophils, the ‘identity’ and intensity of the ROS signal are of paramount importance: the site of ROS production is initially mitochondrial (as demonstrated by analysis with Mitosox) and the ROS level is lower compared to that measured after 40 min from activation, when it can be considered cytoplasmatic (as demonstrated by H2DCFDA positivity). The changes in ROS intensity from 15 to 40 min are important regulators and sustainers of several crucial cellular events: (i) activation of stress‐sensoring circuits to produce and release molecules including ACTH, α‐MSH and NEP; (ii) promotion of the salvific autophagic process; and (iii) exocytosis of exosomes. ACTH, produced by numerous types of cells at many sites in the body 67 with autocrine/paracrine activation, can alter the immune function significantly, provoking cell shape changes and chemotaxis 38, 39, 68. Furthermore, it is also recognized as a regulator of amyloidogenesis. ACTH is converted to α‐MSH by NEP, resulting finally in ACTH inactivation and cessation of cell stimulation ( see the schematic overwiev in Fig. 5). The interactions between the immune and neuroendocrine systems are quite complex 69, 70, 71. The relationship between ACTH/α‐MSH production and melanogenesis in stressed areas such as the skin is well known 37, 72, 73, 74. In cells in which melanin synthesis, a potent immune response against stressful input, occurs pigment production is controlled by both the concentration of ACTH that depends, in turn, on the rate of its cleavage to α‐MSH by NEP enzyme and by the amyloid scaffold produced physiologically to template the pigment 20, 21, 22, 27. It is important to emphasize that NEP activity is implicated not only in ending ACTH signalling, but is also critical to catabolize the amyloid fibrils; the two different systems share the same enzyme to regulate their presence. More provocative is that the ACTH/α‐MSH loop regulates immune and inflammatory reactions, but these factors are also linked to pathologies such as Alzheimer's disease, characterized by an important inflammatory component 36, 50. Apart from these immunomodulators, the dialogue between the immune and neuroendocrine systems is also mediated by numerous cytokines, as demonstrated in lymphocytes 75. In particular, the enhanced production of IL‐18, a representative proinflammatory cytokine activating innate immune responses, is also involved in vertebrate amyloidogenesis 25, 26 and is generally transferred by released exosomes 76. Interestingly, several authors show the relationship between multi‐vesicular bodies and autophagy that is considered to be a salvific process that contributes to the maintenance of cellular homeostasis 77, 78. The results presented here indicate that in LPS‐stimulated neutrophils there is also a correlation between amyloidogenesis and ROS generation, change in cytoplasmic pH, enhanced expression of the ACTH/α‐MSH loop, synthesis of specific cytokine, autophagy and exosome release. These closely related partners appear to play a key role in the management of the amyloid fibrils that constitute the backbone of NET. Taken together, these interconnected responses represent primitive but potent innate defence weapons in invertebrates and vertebrates. In neutrophils, as in innate immunocompetent cells, amyloidogenesis ensues upon recognition of the non‐self by pathogen‐associated molecular pattern receptors (PAMPs). Amyloid fibrils from activated neutrophils in human, phagocytes/haemocytes and/or granulocytes in several invertebrates (all cellular types categorizing as circulating immunocytes) achieve the same objective of trapping, isolating the non‐self, and favours killing of pathogens by acting as a vehicle to concentrate toxic products. Unlike other types of immune cells, those mentioned above do not arrive in close contact with the non‐self, and their amyloid scaffold is utilized as a bridge towards the invader. Furthermore, these innate immune cells are able to optimize a primitive simple response that is assumed, from a biochemical viewpoint, to be a general mechanism to form amyloid fibrils 79.
Figure 5.
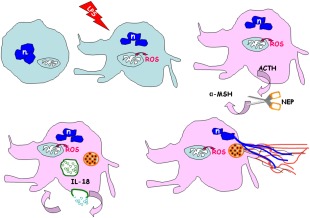
Schematic overview explaining the possible behaviour of lipopolysaccharide (LPS)‐activated neutrophils. LPS activation of neutrophils results in an increase of reactive oxygen species (ROS) levels. Concomitant cross‐talk between immune and neuroendocrine systems induces an activation of stress‐sensoring circuits to produce adrenocorticotrophin hormone (ACTH), neutral endopeptidase (NEP), alpha melanocyte‐stimulating hormone (α‐MSH) and release of interleukin (IL)‐18. In the cell‐linked events, such as production of exosomes, autophagocytosis and amyloidogenesis take place. The reticulum cisternae become an amyloid fibril reservoir. Subsequently, the exocytosed amyloid lattice co‐operates to form the backbone structure of NET. n = nucleus.
In conclusion, in invertebrates from lower groups to primitive Chordata, stimulated circulating cells produce an amyloid scaffold to convey melanin close to the non‐self, isolating it from other host tissues and focusing toxic pigment near the non‐self invader 2, 20, 21, 22, 27. In vertebrates, neutrophils work in an analogous manner and NETs are used to harbour and concentrate cytotoxic compounds against bacteria or fungi, thus enhancing killing effects 5, 15.
As a final comment, considering the importance of NET formation that is involved directly or indirectly in the pathogenesis of different severe disorders (neutrophils are thought responsible for inflammatory profile in many diseases such as systemic lupus erythematosus, where they are not able to dismantle NETs, etc.) 80, 81, the relevance of these findings (i.e. the presence of amyloid fibrils organized in a resistant scaffold) for clinical use needs consideration.
Disclosure
The authors declare that they have no disclosures.
Author contributions
L. P., B. B., E. G., P. D. and A. L. performed the experiments. L. P., M. de E., F. M. and R. V. analysed the data. L. P., M. de E., F. M., G. T., A. G. and D. N. conceived or designed the experiments. L. P., M. de E., F. M. and R. V. wrote the manuscript. L. P. was a participant in the cellular and molecular biology doctoral program of the University of Insubria and B. B. and E. G. are participants in the biotechnology, biosciences and surgical technologies doctoral programmes.
Acknowledgements
This work was supported by a grant from CARIPLO Foundation 2012 to Roberto Valvassori. Grant number: CUPJ31J11004830003 URLs: http://www.fondazionecariplo.it (Fondazione Cassa di Risparmio Delle Province Lombarde) (bando 2011, N.2011‐2092) and the Ministero dell'Istruzione dell'Università e della Ricerca PRIN (Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale), 20109XZEPR_004 and 2010NECHBX_003. This study was supported technically by Centro Grandi Attrezzature (CGA) core facilities of University of Insubria. We thank Dr Ben Atter for editing the manuscript and Dr Angela Scanzano for technical support.
References
- 1. Borregaard N, Cowland J. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997; 89:3503–21. [PubMed] [Google Scholar]
- 2. Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 2007; 5:577–82. [DOI] [PubMed] [Google Scholar]
- 3. Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol 2009; 30:513–21. [DOI] [PubMed] [Google Scholar]
- 4. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptative immunity. Nat Rev Immunol 2011; 11:519–31. [DOI] [PubMed] [Google Scholar]
- 5. Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ 2011; 18:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res 2011; 343:237–49. [DOI] [PubMed] [Google Scholar]
- 7. Brinkmann V, Reichard U, Goosmann C et al Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–5. [DOI] [PubMed] [Google Scholar]
- 8. Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 2006; 8:668–76. [DOI] [PubMed] [Google Scholar]
- 9. Saitoh T, Komano J, Saitoh Y et al Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus‐1. Cell Host Microbe 2012; 12:109–16. [DOI] [PubMed] [Google Scholar]
- 10. Keshari RS, Jyoti A, Dubey M et al Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLOS ONE 2012; 7:e48111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL‐8 and their presence in preeclampsia. Hum Immunol 2005; 66:1146–54. [DOI] [PubMed] [Google Scholar]
- 12. Sangaletti S, Tripodo C, Chiodoni C et al Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 2012; 120:3007–18. [DOI] [PubMed] [Google Scholar]
- 13. Maugeri N, Campana L, Gavina M et al Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost 2014; 12:2074–88. [DOI] [PubMed] [Google Scholar]
- 14. Wartha F, Beiter K, Normark S, Henriques‐Normark B. Neutrophil extracellular traps: casting the NET over pathogenesis. Curr Opin Microbiol 2007; 10:52–6. [DOI] [PubMed] [Google Scholar]
- 15. Yousefi S, Milhalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ 2009; 16:1438–44. [DOI] [PubMed] [Google Scholar]
- 16. Zawrotniak M, Rapala‐Kozik M. Neutrophil extracellular traps (NETs) – formation and implications. Acta Biochim Pol 2013; 60:277–84. [PubMed] [Google Scholar]
- 17. Yousefi S, Gold JA, Andina N et al Catapult‐like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 2008; 14:949–53. [DOI] [PubMed] [Google Scholar]
- 18. Chow OA, von Kockritz‐Blickwede M, Bright AT et al Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 2010; 8:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Kockritz‐Blickwede M, Nizet V. Innate immunity turned inside‐out: antimicrobial defence by phagocyte extracellular traps. J Mol Med 2009; 87:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Falabella P, Riviello L, Pascale M et al Functional amyloids in insect immune response. Insect Biochem Mol Biol 2012; 42:203–11. [DOI] [PubMed] [Google Scholar]
- 21. Grimaldi A, Tettamanti G, Congiu T et al The main actors involved in parasitization of Heliothis virescens larva. Cell Tissue Res 2012; 350:491–502. [DOI] [PubMed] [Google Scholar]
- 22. Grimaldi A, Girardello R, Malagoli D et al Amyloid/melanin distinctive mark in invertebrate immunity. Invert Survival J 2012; 9:153–62. [Google Scholar]
- 23. Kirchner T, Möller S, Klinger M, Solbach W, Laskay T, Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm 2012; 2012:849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stoiber W, Obermayer A, Steinbacher P, Krautgartner WD. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules 2015; 5:702–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bossù P, Ciaramella A, Salani F et al Interleukin‐18, from neuroinflammation to Alzheimer's disease. Curr Pharm Des 2010; 16:4213–24. [DOI] [PubMed] [Google Scholar]
- 26. Alboni S, Montanari C, Benatti C et al Constitutive and LPS‐regulated expression of interleukin‐18 receptor beta variants in the mouse brain. Brain Behav Immun 2011; 25:483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fowler DM, Koulov AV, Alory‐Jost C, Marks M, Balch W, Kelly J. Functional amyloid formation within mammalian tissue. PLOS Biol 2006; 4:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fowler DM, Koulov AV, Balch WE, Kelly JF. Functional amyloid from bacteria to humans. Trends Biochem Sci 2007; 32:217–24. [DOI] [PubMed] [Google Scholar]
- 29. Watt B, van Niel G, Fowler DM et al N‐terminal domains elicit formation of functional Pmel17 amyloid fibrils. J Biol Chem 2009; 284:35543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maury CPJ. The emerging concept of functional amyloid. J Intern Med 2009; 265:329–34. [DOI] [PubMed] [Google Scholar]
- 31. Iconomidou VA, Hamodrakas SJ. Natural protective amyloids. Curr Protein Pept Sci 2008; 9:291–309. [DOI] [PubMed] [Google Scholar]
- 32. Weigent DA, Carr DJ, Blalock JE. Bidirectional communication between the neuroendocrine and immune systems. Common hormones and hormone receptors. Ann NY Acad Sci 1990; 579:17–27. [DOI] [PubMed] [Google Scholar]
- 33. ThyagaRajan S, Priyanka HP. Bidirectional communication between the neuroendocrine system and the immune system: relevance to health and diseases. Ann Neurosci 2012; 19:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scholzen TE, Luger TA. Neutral endopeptidase and angiotensin‐converting enzyme – key enzymes terminating the action of neuroendocrine mediators. Exp Dermatol 2004; 13:22–6. [DOI] [PubMed] [Google Scholar]
- 35. Ottaviani E, Malagoli D, Franceschi C. Common evolutionary origin of the immune and neuroendocrine systems: from morphological and functional evidence to in silico approaches. Trends Immunol 2007; 28:497–502. [DOI] [PubMed] [Google Scholar]
- 36. Caruso C, Carniglia L, Durand D, Scimonelli TN, Lasaga M. Melanocortins: anti‐inflammatory and neuroprotective peptides In Neurodegeneration, eds. LM Martins. InTech, 2012:93–121. [Google Scholar]
- 37. Slominski A, Zbytek B, Szczesniewski A et al CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab 2005; 288:E701–6. [DOI] [PubMed] [Google Scholar]
- 38. Marr RA, Rockenstein E, Mukherjee A et al Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci 2003; 23:1992–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Turner AJ, Nalivaeva NN. New insights into the roles of metalloproteinases in neurodegeneration and neuroprotection. Int Rev Neurobiol 2007; 82:113–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposits of Alzheimer's disease amyloid β‐peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging 2003; 24:415–20. [DOI] [PubMed] [Google Scholar]
- 41. Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr 2000; 71:621S–9S. [DOI] [PubMed] [Google Scholar]
- 42. Zhu X, Perry G, Smith MA. Two hits and you're out? A novel mechanistic hypothesis of Alzheimer disease. Lancet Neurol 2004; 3:219–26. [DOI] [PubMed] [Google Scholar]
- 43. Chen Q, Ding Q, Keller JN. The stationary phase model of aging in yeast for the study of oxidative stress and age‐related neurodegeneration. Biogerontology 2005; 6:1–13. [DOI] [PubMed] [Google Scholar]
- 44. H Le Vine III. Quantification of β‐sheet amyloid fibril structures with thioflavin T. Methods Enzymol 1999; 309:274–84. [DOI] [PubMed] [Google Scholar]
- 45. Wu C, Scott J, Shea JE. Binding of Congo red to amyloid protofibrils of the Alzheimer Aβ9–40 peptide probed by molecular dynamics simulations. Biophys J 2012; 103:550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harper DC, Theos AC, Herman KE, Tenza D, Raposo G, Marks MS. Premelanosome amyloid‐like fibrils are composed of only Golgi‐processed forms of Pmel17 that have been proteolytically processed in endosomes. J Biol Chem 2008; 283:2307–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jaillon S, Peri G, Delneste Y et al The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 2007; 204:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cooper PR, Palmer LJ, Chapple ILC. Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe? Periodontology 2000 2013; 63:165–97. [DOI] [PubMed] [Google Scholar]
- 49. Vowles GH, Francis RJ. Amyloid In: Bancroft JD, Gamble M, eds. Theory and practice of histological techniques. London: Churchill Livingstone, 2002:303e324. [Google Scholar]
- 50. Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 2006; 75:333–66. [DOI] [PubMed] [Google Scholar]
- 51. Goldmann O, Medina E. The expanding world of extracellular traps: not only neutrophils but much more. Front Immunol 2013; 3:420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guimarães‐Costa AB, Nascimento MTC, Froment GS et al Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci USA 2009; 106:6748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wardini AB, Guimarães‐Costa AB, Nascimento MTC et al Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J Gen Virol 2010; 91:259–64. [DOI] [PubMed] [Google Scholar]
- 54. Sutinen EM, Pirttila T, Anderson G, Salminen A, Ojala J. Pro‐inflammatory interleukin‐18 increases Alzheimer's disease‐associated amyloid‐β production in human neuron‐like cells. J Neuroinflammation 2012; 9:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Painter RG, Dukes R, Sullivan J, Carter R, Erdös EG, Johnson AR. The down‐regulation of NEP activity on the cell membrane may modulate the function of these cells in inflammation. J Biol Chem 1988; 263:9456–61. [PubMed] [Google Scholar]
- 56. Ottaviani E, Franchini A, Franceschi C. Pro‐opiomelanocortin‐derived peptides, cytokines and nitric oxide in immune responses and stress: an evolutionary approach. Int Rev Cytol 1997; 170:79–141. [DOI] [PubMed] [Google Scholar]
- 57. Turner AJ. Exploring the structure and function of zinc metallopeptidases: old enzymes and new discoveries. Biochem Soc Trans 2003; 31:723–7. [DOI] [PubMed] [Google Scholar]
- 58. Iwata N, Mizukami H, Shirotani K et al Presynaptic localization of neprilysin contributes to efficient clearance of amyloid‐beta peptide in mouse brain. J Neurosci 2004; 24:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang DS, Dickson DW, Malter JS. β‐Amyloid degradation in Alzheimer's disease. J Biomed Biotechnol 2006; 2006:58406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. El‐Amouri S, Zhu H, Yu J, Marr R, Verma IM, Kindy MS. Neprilysin: an enzyme candidate to slow the progression of Alzheimer's disease. Am J Pathol 2008; 172:1342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meilandt WJ, Cisse M, Ho K et al Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci 2009; 29:1977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hafez D, Huang JY, Huynh AM et al Neprilysin‐2 is an important β‐amyloid degrading enzyme. Am J Pathol 2011; 178:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saido T, Leissring MA. Proteolytic degradation of amyloid β‐protein. Cold Spring Harb Perspect Med 2012; 2:a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Knight JS, Carmona‐Rivera C, Kaplan MJ. Proteins derived from neutrophil extracellular traps may serve as self‐antigens and mediate organ damage in autoimmune diseases. Front Immunol 2012; 3:380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell 2005; 121:667–70. [DOI] [PubMed] [Google Scholar]
- 66. Zawrotniak M, Kozik A, Rapala‐Kozik M. Selected mucolytic, anti‐inflammatory and cardiovascular drugs change the ability of neutrophils to form extracellular traps (NETs). Acta Biochim Pol 2015; 62:465–73. [DOI] [PubMed] [Google Scholar]
- 67. Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Ann NY Acad Sci 2010; 1192:110–6. [DOI] [PubMed] [Google Scholar]
- 68. Ottaviani E, Franchini A, Genedani S. ACTH and its role in immune‐neuroendocrine functions. A comparative study. Curr Pharm Des 1999; 5:673–81. [PubMed] [Google Scholar]
- 69. Dores RM, Lecaude S. Trends in the evolution of the proopiomelanocortin gene. Gen Comp Endocrinol 2005; 142:81–93. [DOI] [PubMed] [Google Scholar]
- 70. Huising MO, Flik G. The remarkable conservation of corticotropin‐releasing hormone‐binding protein (CRH‐BP) in the honeybee (Apis mellifera) dates the CRH system to a common ancestor of insects and vertebrates. Endocrinology 2005; 146:2165–70. [DOI] [PubMed] [Google Scholar]
- 71. Lovejoy DA, Jahan S. Phylogeny of the corticotropin‐releasing factor family of peptides in the metazoa. Gen Comp Endocrinol 2006; 146:1–8. [DOI] [PubMed] [Google Scholar]
- 72. Slominski A, Wortsman J, Pisarchik A et al Cutaneous expression of corticotropin‐releasing hormone (CRH), urocortin, and CRH receptors. FASEB J 2001; 15:1678–93. [DOI] [PubMed] [Google Scholar]
- 73. Tsatmali M, Ancans J, Thody A. Melanocyte function and its control by melanocortin peptides. J Histochem Cytochem 2002; 50:125–33. [DOI] [PubMed] [Google Scholar]
- 74. Rousseau K, Kauser S, Pritchard LE et al Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J 2007; 21:1844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dixit VD, Parvizi N. Pregnancy stimulates secretion of adrenocorticotropin and nitric oxide from peripheral bovine lymphocytes. Biol Reprod 2001; 64:242–8. [DOI] [PubMed] [Google Scholar]
- 76. Gulinelli S, Salaro E, Vuerich M et al IL‐18 associates to microvesicles shed from human macrophages by a LPS/TLR‐4 independent mechanism in response to P2X receptor stimulation. Eur J Immunol 2012; 42:3334–45. [DOI] [PubMed] [Google Scholar]
- 77. Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ 2009; 16:70–8. [DOI] [PubMed] [Google Scholar]
- 78. Hubbard VM, Valdor R, Macian F, Cuervo AM. Selective autophagy in the maintenance of cellular homeostasis in aging organisms. Biogerontology 2012; 13:21–35. [DOI] [PubMed] [Google Scholar]
- 79. Schnabel J. Amyloid: little proteins, big clues. Nature 2011; 475:S12–4. [DOI] [PubMed] [Google Scholar]
- 80. Hakkim A, Furnrohr BG, Amann K et al Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 2010; 107:9813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fuchs TA, Brill A, Duerschmied D et al Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci 2010; 107:15880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


