Summary
Podocytes, the main target of immune complex, participate actively in the development of glomerular injury as immune cells. Dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN) is an innate immune molecular that has an immune recognition function, and is involved in mediation of cell adhesion and immunoregulation. Here we explored the expression of DC‐SIGN on podocytes and its role in immune and inflammatory responses in lupus nephritis (LN). Expression of DC‐SIGN and immunoglobulin (Ig)G1 was observed in glomeruli of LN patients. DC‐SIGN was co‐expressed with nephrin on podocytes. Accompanied by increased proteinuria of LN mice, DC‐SIGN and IgG1 expressions were observed in the glomeruli from 20 weeks, and the renal function deteriorated up to 24 weeks. Mice with anti‐DC‐SIGN antibody showed reduced proteinuria and remission of renal function. After the podocytes were stimulated by serum of LN mice in vitro, the expression of DC‐SIGN, major histocompatibility complex (MHC) class II and CD80 was up‐regulated, stimulation of T cell proliferation was enhanced and the interferon (IFN)‐γ/interleukin (IL)‐4 ratio increased. However, anti‐DC‐SIGN antibody treatment reversed these events. These results suggested that podocytes in LN can exert DC‐like function through their expression of DC‐SIGN, which may be involved in immune and inflammatory responses of renal tissues. However, blockage of DC‐SIGN can inhibit immune functions of podocytes, which may have preventive and therapeutic effects.
Keywords: DC‐SIGN, immunoregulation, lupus nephritis, podocytes
Introduction
Lupus nephritis (LN) secondary to systemic lupus erythematosus is the most common secondary glomerular disease. The pathogenesis of LN is still unclear. Currently, the main characterizations of LN include deposition of immune complex (IC) on glomeruli, inflammatory cell infiltration and local immune inflammation, which are regulated by multiple factors. Podocytes, which are localized on the outer basement membrane, are the main targets of IC. As the most important resident cells in the kidney, podocytes participate actively in the development of glomerular injury mediated by different causes as immune cells.
Recently, the regulatory mechanism of innate immune molecules that link innate and adaptive immune responses have gained more attention. Dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN), which is a C‐type lectin, expresses on podocytes and mediates internalization of HIV‐1 into human podocytes in HIV‐associated nephropathy 1. As an innate immune molecule, DC‐SIGN has the function of immune recognition, mediating cell adhesion and regulating positive and negative immune responses 2, 3, 4, 5, 6. Few studies on DC‐SIGN are related to inflammatory diseases. Our previous study established that DC‐SIGN plays an important role in renal tubulointerstitial injury of primary glomerular nephritis 7. Recently, studies on the role of immune cells in LN have received increased attention. However, only a few studies have focused on the immune regulation of podocytes, the resident renal cells that are directly attacked by IC, in injuries caused by LN. This may limit the illustration of LN pathogenesis and treatment effectiveness. In the present study, we investigated DC‐SIGN expression on podocytes and its regulatory function in LN local inflammatory immune response.
Materials and methods
Pathological specimens of LN patients
Pathological specimens of 15 LN patients diagnosed by our department from 2010 to 2013 were chosen (four were class III LN, six class IV LN and five class V LN). Normal renal tissues from eight cases of renal transplantation mismatched or renal tumour patients were used as control. The study of human renal tissues was approved by Ethics Review Committee of Shanghai General Hospital, Shanghai Jiao Tong University. All participants provided written informed consent to participate in this study. The methods were carried out in accordance with the approved guidelines.
Animals and treatment protocol
Forty‐eight female Murphy Roths Large/lymphoproliferation (MRL/lpr) mice and eight C57BL/6J female mice were purchased from Model Animal Research Center of Nanjing University. MRL/lpr mice were provided adaptive feeding for 1 week. MRL/lpr mice were assigned randomly (1 : 1) to two groups, experimental (n = 24) and intervention (n = 24) groups. C57BL/6J mice were used as the control group (n = 8). Mice in the intervention group were injected with anti‐DC‐SIGN antibody (2 mg/kg) via the tail vein at 6 weeks. Mice were anaesthetized with ketamine and killed at 16, 20, 24 and 28 weeks of age. Twenty‐four‐h urine samples of mice were collected before they were killed. Blood samples were collected (16, 20, 24 and 28 weeks) to detect renal function. The kidneys were isolated quickly and fixed in 10% buffered formaldehyde. The protocol for animal experiments was approved by the Laboratory Animal Research Center of Shanghai General Hospital, Shanghai Jiao Tong University. All surgical procedures involved anaesthesia with ketamine, and all efforts were made to minimize suffering. The methods were carried out in accordance with the approved guidelines.
Renal function studies
For urine sample collection, mice were housed in metabolic cages, and 24‐h urine protein was detected by Beckman automatic biochemical analyser. Blood samples for serum creatinine measurement were obtained at the time of killing. Serum creatinine was measured by standard laboratory methods.
Periodic acid‐Schiff (PAS) staining
To evaluate glomerular and tubulointerstitial injury, formalin‐fixed mice renal tissue was embedded in paraffin, sectioned at 4 μm, and stained with PAS for histological analysis.
Immunohistochemistry analysis and immunofluorescence staining
An immunohistochemistry assay was used to determine DC‐SIGN expression in human and mouse renal tissues and immunoglobulin (Ig)G1 expression in mouse renal tissues. The 3‐μm thick sections were blocked with 0·3% bovine serum album for 20 min. The sections were incubated with goat anti‐DC‐SIGN antibody (Santa Cruz Biotechnology, Sana Cruz, CA, USA) or rat anti‐mouse IgG1 monoclonal antibody (mAb) (Santa Cruz Biotechnology) at 4°C overnight, then incubated with biotinylated secondary antibodies for 30 min at room temperature, and subsequently incubated with streptavidin–peroxidase for 30 min at room temperature. Diaminobenzidine (DAB) was used to develop the diffuse colour staining. The primary antibody was replaced by phosphate‐buffered saline (PBS) in the negative control.
An immunofluorescence assay was used to determine IgG1 expression and location of DC‐SIGN expression in human renal tissues. Renal tissue sections from humans were blocked with 0·3% bovine serum album for 20 min, and then incubated with 1 : 100 mouse anti‐human IgG1 mAb (Santa Cruz Biotechnology) or co‐incubated with 1 : 100 anti‐DC‐SIGN antibody (Santa Cruz Biotechnology) and mouse anti‐human nephrin antibody (Santa Cruz Biotechnology ) at 4°C overnight and followed by a subsequent incubation with 1 : 100 anti‐mouse IgG‐fluorescein isothiocyanate (FITC) antibody (Santa Cruz Biotechnology) and 1 : 100 anti‐goat IgG‐phycoerythrin (PE) antibody (Santa Cruz Biotechnology). The sections were incubated for 1 h at 37°C, washed with PBS and then mounted. The primary antibody was replaced by PBS as a negative control. Images were taken with a fluorescence microscope or confocal laser scanning microscope (Olympus, Center Valley, PA, USA).
Podocytes
Mouse podocytes were kindly gifted by Professor Peter Mundel of Mount Sinai School of Medicine. Immortalized murine podocytes were cultured as described previously 8. MRL/lpr mouse blood samples were obtained from the heart. The serum was obtained by centrifugation of blood at 1000 g for 10 min at 4°C. The concentration of mouse anti‐dsDNA antibody was detected by enzyme‐linked immunosorbent assay (ELISA) (BioVendor, Brno, Czech Republic). Mouse serum was added into podocyte medium according to the concentration of mouse anti‐dsDNA antibody (10 μg/ml) 9. Podocytes were collected after stimulation with serum for 24 and 48 h. Anti‐DC‐SIGN antibody was added into the medium of podocytes in the intervention group when mouse serum was added.
Analysis of major histocompatibility complex (MHC) class II, DC‐SIGN and CD80 expressed on podocytes
Briefly, 1 × 106 podocytes were stained with FITC‐ and PE‐labelled mAbs specific for MHC‐II and CD80 (eBioscience, Santiago, CA, USA). In addition, 1 × 106 podocytes were stained indirectly for DC‐SIGN using a goat anti‐mouse DC‐SIGN polyclonal antibody (Santa Cruz Biotechnology) and a PE‐conjugated donkey anti‐goat IgG mAb (Santa Cruz Biotechnology). Phenotypical analysis was performed by flow cytometry using fluorescence activated cell sorter (FACS)Calibur (BD FACSAriaTM Cell Sorter; BD Biosciences, San Jose, CA, USA) and analysed using FlowJo (TreeStar Inc., Ashland, OR, USA.
Western blot analysis
Mouse podocytes were collected and total protein was obtained by cell lysis and protease inhibitor. Protein concentration was detected by bicinchonininc acid. Podocyte proteins were run on a 10% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE), transferred to a polyvinylidene fluoride membrane, and blocked by immersing in 10% bovine serum albumin in PBS for 2 h at room temperature. After rewashing, the membranes were cut into strips and each strip incubated in a 1 : 500 dilution of anti‐DC‐SIGN antibody as primary antibody at 4°C overnight. Each strip was then incubated after diluting (1 : 1000) with donkey anti‐goat IgG conjugated with horseradish peroxidase for 1 h at room temperature. After washing with PBS‐T, the antigen–antibody complex was developed using ECL Plus (Pierce, Appleton, WI, USA) chemiluminescence reagent method and imaged using X‐ray. GS‐800 calibrated absorbance spectrometer was used to scan the film. The images were analysed using Image J (National Institutes of Health, Bethesda, Maryland, USA).
Immunoprecipitation
Renal tissues of LN mice at 28 weeks of age and mouse podocytes stimulated by mouse serum with LN for 48 h were lysed, respectively, in lysis buffer (Cell Signaling, Danvers, MA, USA) containing protease inhibitors (Roche, Indianapolis, IN, USA). Cell lysates were immunoprecipitated with DC‐SIGN‐coated, nephrin‐coated or mouse IgG‐coated agarose A/G beads (Pierce, Appleton, WI, USA), following the manufacturer's instructions. Briefly, cell lysates were incubated with DC‐SIGN‐coated, nephrin‐coated or mouse IgG‐coated beads overnight at 4°C on an orbital shaker. The beads were then collected and washed three times with 800 μl of ice‐cold PBS followed by pulse centrifugation. After washing, beads were resuspended in 60 μl of ×2 sample buffer and gently mixed. DC‐SIGN or nephrin was detected by Western blotting. Cell lysates without immunoprecipitation were used as positive controls.
Mixed lymphocyte reaction (MLR)
The ability of podocytes to stimulate CD4+ T cells was assayed in a mixed lymphocyte reaction. Allogeneic CD4+ T cells, isolated from peripheral blood mononuclear cells using magnetic bead‐labelled anti‐mouse CD4 mAb (Miltenyi Biotec, Bergisch Gladbach, Germany), were incubated with irradiated (30 Gy) podocytes at a ratio of 10 : 1 in a 96‐cell U‐bottomed plate at 37°C for 5 days. After 5 days, [3H]‐TdR (1 μCi/well) was added for 12–16 h before the end of culture. Wells were cultured in triplicate for each group.
Quantitation of interferon (IFN)‐γ and interleukin (IL)‐4 levels by ELISA
The supernatants from the MLR were collected. The concentrations of IFN‐γ and IL‐4 were determined using ELISA kits (Biosource, Camarillo, CA, USA) according to the manufacturer's instructions.
Statistical analysis
spss software version 13.0 (SPSS Inc., Chicago, IL, USA), was used for statistical analysis. Data are presented as mean ± standard deviation (s.d.) and evaluated by analysis of variance and Student–Newman–Keuls test (SNK).
Results
DC‐SIGN and IgG1 expression in renal tissues of LN patients
Expression of DC‐SIGN and IgG1 in renal tissues of 15 LN patients with different LN classes and normal renal tissues was detected. DC‐SIGN and IgG1 were almost undetectable in the normal renal tissues. However, compared with normal renal tissues, DC‐SIGN and IgG1 expression was observed in renal tissues of LN patients including classes III, IV and V, especially in the glomeruli (Fig. 1a,b). Furthermore, it displayed that DC‐SIGN was expressed along with nephrin in renal tissues of LN patients by confocal laser scanning microscope (Fig. 1c). However, immunoprecipitation showed no interaction between DC‐SIGN and nephrin (Supporting information, Fig. S1).
Figure 1.
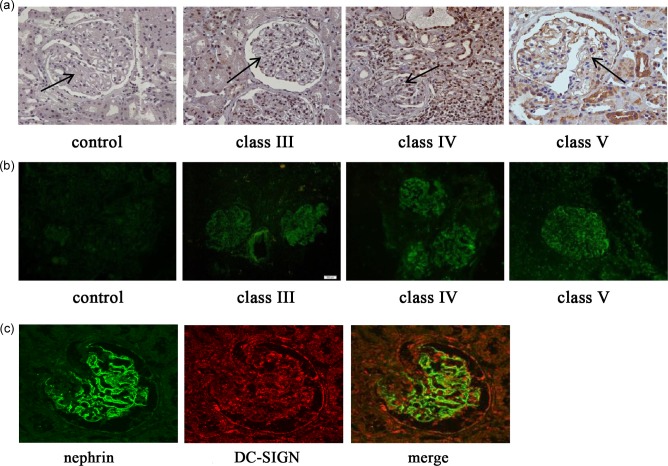
Dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN) and IgG1 expression in renal tissues of different classes of lupus nephritis (LN) patients. (a) Immunohistochemistry of DC‐SIGN in renal tissues of LN patients (final magnification ×400). (b) Immunofluorescence of immunoglobulin (Ig)G1 in renal tissues of LN patients (final magnification ×200). (c) Double‐labelling with nephrin and DC‐SIGN in renal tissues of LN patients (final magnification ×400).
Changes of renal function and renal histopathology in LN mice
MRL/lpr mice were used to develop LN. Changes of renal function and renal histopathology were observed first in LN mice. Compared with the control group, 24‐h urine protein of LN mice in the experimental group increased from 16 weeks (P < 0·01), while serum creatinine increased significantly from 24 weeks (P < 0·01). After treatment with anti‐DC‐SIGN antibody, 24‐h urine protein of LN mice decreased (P < 0·01) and renal function improved (Tables 1 and 2). In addition, from 20 weeks, mouse renal tissue showed proliferation of mesangial cells, mesangial matrix and epithelial cells on renal capsule, and the fall and atrophy of part of the renal tubules. After treatment with anti‐DC‐SIGN antibody, the number of cells in the glomerulus decreased, proliferation of renal capsule wall layers of epithelial cells reduced and the opening of glomerular capillary increased (Fig. 2).
Table 1.
24‐h urine protein of mice.
| Age | Experimental group (mg/24 h) | Anti‐DC‐SIGN antibody group (mg/24 h) | Control group (mg/24 h) |
|---|---|---|---|
| 16 weeks | 2·08 ± 1·02* | 2·01 ± 0·98 | 0·20 ± 0·10 |
| 20 weeks | 2·63 ± 0·39* | 2·43 ± 0·12† | 0·21 ± 0·09 |
| 24 weeks | 3·66 ± 0·98* | 3·01 ± 0·55† | 0·22 ± 0·13 |
| 28 weeks | 4·01 ± 1·87* | 3·24 ± 1·09† | 0·22 ± 0·11 |
*P < 0·01, compared with control group.
† P < 0·01, compared with the experimental group. DC‐SIGN = dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin.
Table 2.
Serum creatinine of mice.
| Age | Experimental group (μmol/l) | Anti‐DC‐SIGN antibody group (μmol/l) | Control group (μmol/l) |
|---|---|---|---|
| 16 weeks | 18·84 ± 1·97 | 18·42 ± 2·06 | 18·91 ± 1·90 |
| 20 weeks | 18·83 ± 2·77 | 18·35 ± 3·01 | 19·01 ± 2·57 |
| 24 weeks | 23·76 ± 1·23* | 19·86 ± 2·87† | 19·23 ± 2·49 |
| 28 weeks | 26·01 ± 3·12* | 21·76 ± 1·87† | 19·43 ± 1·76 |
*P < 0·01, compared with control group.
† P < 0·01, compared with the experimental group. DC‐SIGN = dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin.
Figure 2.
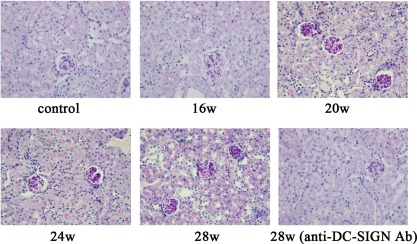
Pathology of renal tissues in mice. Periodic acid‐Schiff (PAS) staining of renal tissues and corresponding quantification (final magnification ×400). Renal tissues of Murphy Roths Large/lymphoproliferation (MRL/lpr) mice in the experimental group were harvested at 16, 20, 24 and 28 weeks of age. Renal tissues of mice in intervention group were harvested at 28 weeks.
DC‐SIGN and IgG1 expressions in LN mouse renal tissues
The expression of DC‐SIGN and IgG1 was detected in the renal tissues of LN mice. DC‐SIGN are usually scattered throughout the normal renal tissues. However, the expression of DC‐SIGN in renal tissues increased from 20 weeks in the experimental group, observed mainly in the glomerulus and tubulointerstitium and also present in the tubules, increased with the progression of LN. After treatment with anti‐DC‐SIGN antibody, DC‐SIGN expression in renal tissues decreased (Fig. 3a,b). The expression of IgG1 was observed rarely in the renal tissues of normal mice. In the experimental group, from 20 weeks, with the progression of LN, IgG1 was seen in the glomerulus and tubulointerstitium. After the intervention of anti‐DC‐SIGN antibody, IgG1 expression in renal tissues showed no obvious change (Fig. 4a,b).
Figure 3.
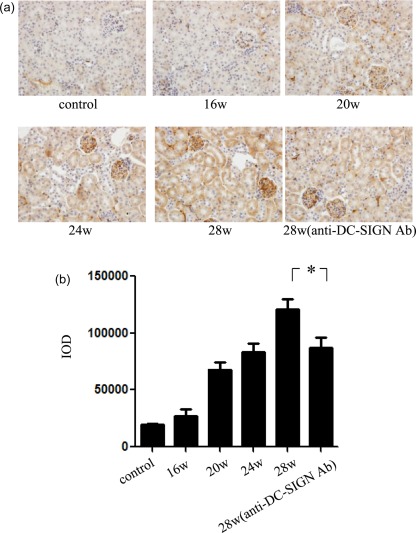
Dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN) expression in mice renal tissues. (a) Immunohistochemistry of DC‐SIGN in mice and corresponding quantification (final magnification ×400). Renal tissues of Murphy Roths Large/lymphoproliferation (MRL/lpr) mice in experimental group were harvested at 16, 20, 24, and 28 weeks of age. Renal tissues of mice in intervention group were harvested at 28 weeks. (b) Integrated optical density (IOD) analysis of DC‐SIGN expression in renal tissues. *P < 0·05.
Figure 4.
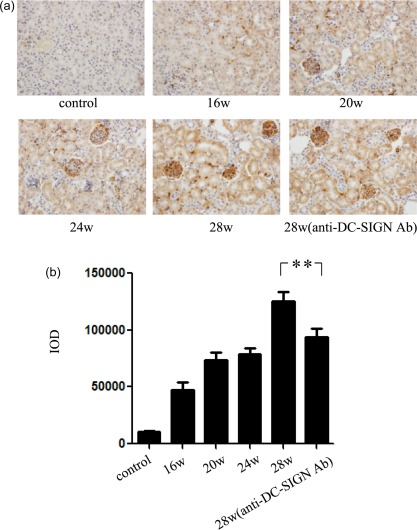
Immunoglobulin (Ig)G1 expression in mouse renal tissues. (a) Immunohistochemistry of IgG1 in mice and corresponding quantification (final magnification ×400). Renal tissues of Murphy Roths Large/lymphoproliferation (MRL/lpr) mice in experimental group were harvested at 16, 20, 24 and 28 weeks of age. Renal tissues of mice in intervention group were harvested at 28 weeks. (b) Integrated optical density (IOD) analysis of IgG1 expression in renal tissues. **P < 0·01.
Changes of phenotype and function of mouse podocytes with serum from LN mice
Podocytes are important resident cells in the kidney, which participate actively in the development of glomerular injury caused by immune cells. To investigate further the function of podocytes in LN, mouse podocytes were used. In the control group, podocytes expressed low levels of DC‐SIGN, MHC‐II and CD80 (Fig. 5a,b). The ability to stimulate T cell proliferation is weak (Fig. 6a). The level of IFN‐γ and IL‐4 secreted in MLR supernatants is low (Fig. 6b). After stimulation with LN mouse serum for 24 h, the expression of DC‐SIGN, MHC‐II and CD80 on mouse podocytes increased (Fig. 5a,b). The ability of podocytes to stimulate T cell proliferation strengthened (P < 0·01) (Fig. 6a). The level of IFN‐γ and IL‐4 and the ratio of IFN‐γ/IL‐4 in MLR supernatant increased (P < 0·01) (Fig. 6b). After stimulation with LN mouse serum for 48 h, the expression of DC‐SIGN decreased and the expression of MHC‐II and CD80 on podocytes showed mild down‐regulation (Fig. 5a,b). The ability of podocytes to stimulate T cell proliferation weakened (Fig. 6a). The ratio of IFN‐γ/IL‐4 also decreased significantly (P < 0·01) (Fig. 6b). In addition, anti‐DC‐SIGN antibody obviously suppressed the level of IFN‐γ (P < 0·01), while it had no effect on the level of IL‐4 (Fig. 6b).
Figure 5.
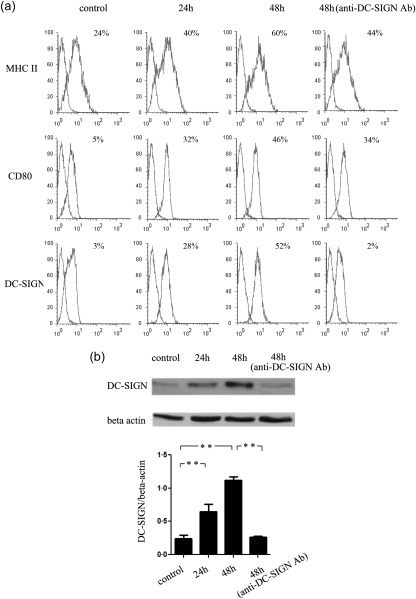
Dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN), major histocompatibility complex (MHC) class II and CD80 expression on mouse podocytes. (a) Mouse podocytes were harvested after serum stimulation for 24 and 48 h. DC‐SIGN, MHC class II and CD80 expression of podocytes were detected by flow cytometry. (b) DC‐SIGN expression of mouse podocytes was detected by Western blotting. **P < 0·01.
Figure 6.
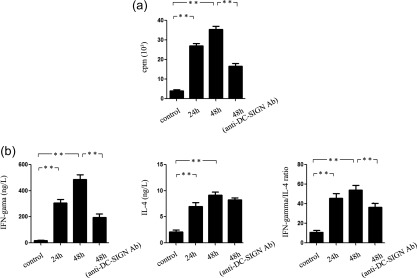
Immunological function of mouse podocytes after stimulation with serum of Murphy Roths Large/lymphoproliferation (MRL/lpr) mice. (a) Ability of podocytes to stimulate T cell proliferation. (b) Ability of podocytes to stimulate T cells secreting T helper type 1 (Th1) and Th2 cytokines. Interferon (IFN)‐γ and interleukin (IL)‐4 expression in supernatant of co‐culture of podocytes and T cells were detected by enzyme‐linked immunosorbent assay (ELISA). **P < 0·01.
Discussion
Renal biopsies of SLE patients suggest renal injuries of varying levels. The degree of renal injury directly affects the prognosis of SLE 10, 11. As an important complication of SLE, the pathogenesis of LN is unclear. It is considered that LN is characterized by IC, which comprises antigens such as dsDNA and antibody that binds onto the glomerulus and causes inflammatory cell infiltration and local immunoinflammation. Podocytes are the major target of IC.
Podocytes are the most vulnerable cells in the glomeruli. They are not only victims in renal injury, but also active participants in the development of all kinds of glomerular diseases 12. It is known that nearly all glomerular diseases, primary or secondary, are immune response‐mediated inflammatory diseases. The injury on renal tissues by IC deposited under the epithelial cells of glomeruli is related closely to podocytes. Recently, it was found that podocytes can express different immune molecules that participate actively and play an important role in local immune response after IC attack. Banas et al. found that podocytes in nephritis could express Toll‐like receptor, which can secrete chemokines stimulated by ligands 13. An alternative pathway of complement activation could be activated abnormally, as podocytes express different complementary regulatory proteins abnormally, which enhances the susceptibility of the injury of podocytes against the complement 14. Podocytes, stimulated by inflammatory factors, can express MHC‐I, MHC‐ΙΙ and co‐stimulatory molecules B7 and they have the ability to process and present antigen, and thus function as antigen‐presenting cells 15, 16, 17. Podocytes can also express adhesion molecules intercellular adhesion molecule 1 (ICAM‐1), chemokine CXCL16 and CD2AP, the important molecules that participate in the formation of immune synapse 3, 4, 15. Our results showed that podocytes up‐regulate MHC‐II and CD80 after being stimulated by serum of LN mice. All the above suggest further that podocytes have the function of immune cells, can participate actively in renal immune response and have an important regulatory role in renal diseases.
As mentioned above, podocytes can participate in HIV internalization into CD4+ T cells in HIV‐related nephritis through DC‐SIGN 1. As an innate immune molecule, DC‐SIGN links innate immune and adaptive immune responses. It is a pattern recognition receptor (PRR) and an adhesion receptor that interacts with molecular pattern groups such as Lewis X antigen in pathogens and cells, which mediates dendritic cells (DCs) participating in the immune escape of pathogens and tumor molecules 2, 3, 4. It can also mediate DC interaction with vascular endothelial cells, neutrophils and initial T cells, which regulates DC migration and immune response 2, 3, 4, 5, 6, 18. Therefore, DC‐SIGN plays an important role in the positive and negative modulation of immune response 2, 3, 4. It is speculated that in LN characterized by local immune reaction, DC‐SIGN expressed on podocytes may play a prominent function in local immune response. The present study showed that DC‐SIGN is present in podocytes, as well as tubules and interstitium, of LN patients and mice. The expression of DC‐SIGN strengthened with the progression of LN, related closely to the pathological changes and renal function. After intervention of the anti‐DC‐SIGN antibody, the pathological changes and renal function improved. In terms of expression of DC‐SIGN in the tubules and interstitium, the results were consistent with our previous study, which suggested that DC‐SIGN is present in tubular epithelial cells in primary and secondary glomerular diseases 19. It also corresponds to the reports that DC‐expressing DC‐SIGN infiltrates into renal tubulointerstium and participate in the local immune response 20. Therefore, DC‐SIGN could mediate podocytes by playing an important regulatory role in glomerular immune response in LN.
In the present study, we found that after treatment with the serum of LN mice, podocytes expressed DC‐SIGN with the up‐regulation of MHC‐II and CD80, stimulated T cell proliferation and promoted T cells to secrete T helper type 1 (Th1) and Th2 cytokines by increasing the ratio of IFN‐γ and IL‐4. Anti‐DC‐SIGN antibody weakened the ability of the podocytes to stimulate T cell proliferation and obviously suppressed T cell secretion of IFN‐γ by down‐regulating Th1/Th2 bias. Our study showed that podocytes expressed DC‐SIGN in LN and thus have the ability of DC‐like functions such as stimulating T cell proliferation and promotion of Th1 immune reaction. Therefore, podocytes may take part actively in the regulation of renal immune reaction and are related to the progression of LN. In addition, inflammatory cells such as neutrophils are seen in the glomeruli of LN renal tissues. A previous study reported that pattern group Mac‐1 on neutrophils could interact with DC‐SIGN, participating in the proliferation and activation of T cells stimulated by DC, and thus link innate and adaptive immunity 21. Therefore, it is speculated that podocytes in LN may interact with neutrophils infiltrating into the renal tissues through DC‐SIGN and take part in the local immune reaction, which will be explored in a future study.
Our study indicated further that DC‐like functions of podocytes in LN may be mediated by DC‐SIGN. However, the molecular pattern group that could interact with DC‐SIGN expressed on podocytes to trigger the DC‐like functions is unknown. Recent studies showed that DC‐SIGN could interact with glycan groups which include the oligosaccharides in IgA and agalactosylated bi‐antennary N‐glycans in IgG1 22, 23. Therefore, it is speculated that glycoproteins in IgA and IgG1 in LN glomeruli may interact with DC‐SIGN expressed on podocytes, and then participates in the local immune reaction. As a receptor, DC‐SIGN may also transport some phlogogenic molecules which, however, lack relevant researches. Further studies are warranted to explore the relevant mechanisms.
Disclosure
All the authors declare no disclosures.
Author contributions
W. J Y. and T. Z. conceived the study design, participated in its design and in the acquisition of data. M. C. C. and X. W. carried out the experiments, participated in the acquisition of data, analysis and interpretation, and drafting of the manuscript. M. H. S. helped to analyse the pathology of renal tissues. Y. Y. Z. analysed the data. M. C. L. was involved in revision of figures. C. D. X. helped to draft and revise the manuscript. All authors read and approved the final submitted version of the manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Figure S1. Dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN) have no interaction with nephrin. (a) Renal tissues of LN mice at 28 weeks of age were lysed by lysis buffer. Lysates were immunoprecipitated with anti‐DC‐SIGN antibody or anti‐nephrin antibody, followed by immunoblotting with anti‐nephrin antibody or anti‐DC‐SIGN antibody. (b) Mouse podocytes were stimulated by serum of mouse with lupus nephritis (LN) for 48 h. Podocytes were lysed by lysis buffer. Lysates were immunoprecipitated with anti‐DC‐SIGN antibody or anti‐nephrin antibody, followed by immunoblotting with anti‐nephrin antibody or anti‐DC‐SIGN antibody.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81200508, 81270801, 81470941 and 81170363).
References
- 1. Mikulak J, Teichberg S, Arora S et al DC‐specific ICAM‐3‐grabbing nonintegrin mediates internalization of HIV‐1 into human pdocytes. Am J Physiol Renal Physiol 2010; 299:F664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Vliet SJ, García‐Vallejo JJ, van Kooyk Y. Dendritic cells and C‐type lectin receptors: coupling innate to adaptive immune responses. Immunol Cell Biol 2008; 86:580–7. [DOI] [PubMed] [Google Scholar]
- 3. den Dunnen J, Gringhuis SI, Geijtenbeek TB. Innate signaling by the C‐type lectin DC‐SIGN dictates immune responses. Cancer Immunol Immunother 2009; 58:1149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou T, Chen Y, Hao L, Zhang Y. DC‐SIGN and immunoregulation. Cell Mol Immunol 2006; 3:279–83. [PubMed] [Google Scholar]
- 5. Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C‐type lectin DC‐SIGN modulates Toll‐like receptor signaling via Raf‐1 kinase‐dependent acetylation of transcription factor NF‐kappaB. Immunity 2007; 26:605–16. [DOI] [PubMed] [Google Scholar]
- 6. Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J. The role of Toll‐like receptors in renal diseases. Nat Rev Nephrol 2010; 6:224–35. [DOI] [PubMed] [Google Scholar]
- 7. Cai M, Zhou T, Li X et al DC‐SIGN modulates DC maturation and function in rat renal tubulointerstitial lesions. Front Biosci 2012; 17:1795–803. [DOI] [PubMed] [Google Scholar]
- 8. Mundel P, Reiser J, Zúñiga Mejía Borja A et al Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997; 236:248–58. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Yang J, Jiang S et al The lupus‐derived anti‐double‐stranded DNA IgG contributes to myofibroblast‐like phenotype in mesangial cells. J Clin Immunol 2012; 32:1270–8. [DOI] [PubMed] [Google Scholar]
- 10. Ortega LM, Schultz DR, Lenz O, Pardo V, Contreras GN. Review: lupus nephritis: pathologic features, epidemiology and a guide to therapeutic decisions. Lupus 2010; 19:557–74. [DOI] [PubMed] [Google Scholar]
- 11. Contreras G, Roth D, Pardo V, Striker LG, Schultz DR. Lupus nephritis: a clinical review for practicing nephrologists. Clin Nephrol 2002; 57:95–107. [DOI] [PubMed] [Google Scholar]
- 12. Liu Y. New insights into epithelial–mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 2010; 21:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Banas MC, Banas B, Hudkins KL et al TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 2008; 19:704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Wu J, Wu H et al UCH‐L1 expression of podocytes in diseased glomeruli and in vitro. J Pathol 2009; 217:642–53. [DOI] [PubMed] [Google Scholar]
- 15. Coers W, Brouwer E, Vos JT et al Podocyte expression of MHC class I and II and intercellular adhesion molecule‐1 (ICAM‐1) in experimental pauci‐immune crescentic glomerulonephritis. Clin Exp Immunol 1994; 98:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reiser J, von Gersdorff G, Loos M et al Induction of B7‐1 in podocytes is associated with nephrotic syndrome. J Clin Invest 2004; 113:1390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reiser J, Mundel P. Danger signaling by glomerular podocytes defines a novel function of inducible B7‐1 in the pathogenesis of nephrotic syndrome. J Am Soc Nephrol 2004; 15:2246–8. [DOI] [PubMed] [Google Scholar]
- 18. Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol 2006; 17:1253–63. [DOI] [PubMed] [Google Scholar]
- 19. Zhou T, Li X, Zou J et al Effects of DC‐SIGN expression on renal tubulointerstitial fibrosis in nephritis. Front Biosci 2009; 14:3814–24. [DOI] [PubMed] [Google Scholar]
- 20. Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol 2013; 13:738–53. [DOI] [PubMed] [Google Scholar]
- 21. van Gisbergen KP, Sanchez‐Hernandez M, Geijtenbeek TB, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation‐dependent interactions between Mac‐1 and DC‐SIGN. J Exp Med 2005; 201:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yabe R, Tateno H, Hirabayashi J. Frontal affinity chromatography analysis of constructs of DC‐SIGN, DC‐SIGNR and LSECtin extend evidence for affinity to agalactosylated N‐glycans. FEBS J 2010; 277:4010–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC‐SIGN: implications for immune surveillance in the intestine. Immunol Lett 2010; 131:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Figure S1. Dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN) have no interaction with nephrin. (a) Renal tissues of LN mice at 28 weeks of age were lysed by lysis buffer. Lysates were immunoprecipitated with anti‐DC‐SIGN antibody or anti‐nephrin antibody, followed by immunoblotting with anti‐nephrin antibody or anti‐DC‐SIGN antibody. (b) Mouse podocytes were stimulated by serum of mouse with lupus nephritis (LN) for 48 h. Podocytes were lysed by lysis buffer. Lysates were immunoprecipitated with anti‐DC‐SIGN antibody or anti‐nephrin antibody, followed by immunoblotting with anti‐nephrin antibody or anti‐DC‐SIGN antibody.


