Summary
In this work, we aimed to evaluate the levels of ferritin enriched in H subunits (H‐ferritin) and ferritin enriched in L subunits (L‐ferritin) and the cells expressing these two molecules in the lymph node (LN) biopsies obtained from adult‐onset Still's disease (AOSD) patients, and the possible correlation among these data and the severity of the disease. Ten patients with AOSD underwent LN biopsy. All the samples were stained by immunofluorescence. A statistical analysis was performed to estimate the possible correlation among both H‐ferritin and L‐ferritin tissue expression and the clinical picture of the disease. Furthermore, the same analysis was performed to evaluate the possible correlation among the number of CD68+/H‐ferritin+ or CD68+/L‐ferritin+ cells and the clinical picture. Immunofluorescence analysis demonstrated an increased tissue H‐ferritin expression in the LNs of AOSD patients. This increased expression correlated with the severity of the disease. An increased number of CD68 macrophages expressing H‐ferritin was observed in the LN samples of our patients. Furthermore, we observed that the number of CD68+/H‐ferritin+ cells correlated significantly with the severity of the clinical picture. Our data showed an imbalance between the levels of H‐ and L‐ferritin in LNs of AOSD patients and the evidence of an increased number of CD68+/H‐ferritin+ cells in the same organs. Furthermore, a correlation among both the tissue H‐ferritin levels and the CD68+/H‐ferritin+ cells and the clinical picture was observed.
Keywords: adult‐onset Still's disease, H‐ferritin, hyperferritinaemic syndrome, macrophage
Introduction
Adult‐onset Still's disease (AOSD) is a rare systemic inflammatory disorder of unknown aetiology, characterized by quotidian high spiking fevers, arthritis and multi‐organ involvement, requiring immunosuppressive therapies 1, 2, 3. A large percentage of AOSD patients showed an evanescent salmon‐pink or erythematous maculopapular eruption which appears frequently during febrile attacks, and is found predominantly on the proximal limbs and trunk 3, 4. Furthermore, both splenomegaly and mild–severe enlargement of cervical lymph nodes (LNs) are observed frequently in AOSD patients and lymphoma should be always considered in the differential diagnosis of this clinical picture 1, 2, 3, 4.
Recently, it has been suggested that AOSD and other uncommon medical conditions such as macrophage activation syndrome (MAS), catastrophic anti‐phospholipid syndrome and septic shock, which share similar clinical and laboratory features, may be considered an intermediate phenotype of the same inflammatory process, affecting target cells killed by cytotoxic T cells and natural killer (NK) cells 5. In this context the cytokine storm observed in these conditions, associated with hyperferritinaemia, may further activate both NK and cytotoxic T cells with consequent release of uncontrolled granzyme system and perforin release, thus amplifying the cytokine storm and the production of proinflammatory cytokines 6, 7.
Ferritin is an intracellular iron storage protein including 24 subunits: heavy (H) subunits and light (L) subunits on the bases of their molecular weight 8, 9. The H‐/L‐subunits ratio may change, depending on the specific tissue and the physiological status of the cell. In normal conditions, ferritin enriched in L subunits (L‐ferritin) has been found in the liver and in the spleen; on the contrary, the ferritin enriched in H subunits (H‐ferritin), may be observed mainly in the heart and kidneys 8, 9. Although the secretory pathway of serum ferritin has not been clarified fully, hepatocytes, macrophages and Küpffer cells may be involved in its secretion 8, 9, 10, 11.
For many years, ferritin has been considered as a potential immunosuppressant, inducing suppression of delayed‐type hypersensitivity 12, suppression of antibody production by B lymphocytes 13, decreasing the phagocytosis by granulocytes 14 and regulating granulo‐mono‐cytopoiesis. More recently, it has been suggested that H‐ferritin induces production of the anti‐inflammatory cytokine in lymphocytes 15 and may act as a negative regulator of the CXC chemokine receptor 4 (CXCR4), impairing the signalling leading to the activation of mitogen‐activated protein kinase (MAPK), a kinase that is known to play an important role in cell proliferation, differentiation and migration 16.
Intriguingly, in recent years, a proinflammatory role of extracellular ferritin has been suggested for some specific cells, such as hepatic stellate cells 17. Cells treated with ferritin activate PI3 kinase phosphorylation, protein kinase C zeta activation and MAPK activation, culminating in nuclear factor‐kappa B (NF‐κB) activation. This activation leads to the production of proinflammatory molecules, inducible nitric oxide synthase and others. Of note, this function is independent of the iron content of ferritin, suggesting that exogenous ferritin may play active roles independently of its main function 17. In fact, ferritin synthesis may be regulated not only in response to iron availability, but also by different inflammatory cytokines such as interleukin (IL)‐1β and IL‐6, and by different biological stimuli such as oxidative stress, hypoxia–ischaemia, hyperoxia and lipopolysaccharide (LPS) toxicity 7, 9, 10, 11. H‐ferritin specifically binds a member of the T cell immunoglobulin and mucin‐domain (TIM) gene family, TIM‐2 7, which is considered a defined marker of T helper type 2 (Th2) cells 17, 18, 19. During the inflammatory response other cells, including macrophages, may express TIM‐2 on their surface 18, 19, 20, 21, 22, 23. In this setting, instead of modulating down‐regulation of the immune response, as observed in normal conditions, the H‐ferritin/TIM‐2 binding up‐regulates strongly the expression of inflammatory cytokines, playing a pivotal role in the development of an extremely severe condition known as a ‘cytokine storm’ 7, 8, 9, 10, 11.
Although a growing body of evidence suggests that macrophages are associated with the immunopathogenic mechanism of this rare condition, at present their exact role is not fully understood 1, 7. It is well known that, together with other myeloid and lymphoid cells, macrophages in the subcapsular sinus and medulla of secondary LNs contribute to the innate and adaptive responses of the host, shaping the nature and quality of inflammation 24.
In this paper, we show both an increased tissue expression of H‐ferritin and an increased number of CD68+/H‐ferritin+ macrophages in the LNs of AOSD patients, and these results correlate with the disease activity and the serum ferritin levels of the same patients, suggesting a pathogenic role of these macrophages in this inflammatory disease.
Patients and methods
In the last 10 years, 10 consecutive patients with AOSD referred to the Rheumatology Clinic of L'Aquila University and to the Rheumatology Clinic of Palermo University were enrolled into this study. All patients fulfilled the criteria proposed by Yamaguchi et al. 25 for AOSD and showed a flare of the disease in the context of a polycyclic or a chronic form of the disease, according with the criteria proposed by Cush et al. 26. Serum levels of ferritin, erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP) were obtained from the patients. Pouchot's score was used to evaluate the disease activity 27. This score ranges from 0 to 12 and is calculated by assigning and adding 1 point for each of the following manifestations occurring during a disease flare: fever, evanescent rash, pleuritis, pneumonia, pericarditis, hepatomegaly or abnormal liver function tests, splenomegaly, lymphadenopathy, white blood cells > 15 000/mm3, sore throat, myalgias and abdominal pain. The demographic data of our patients are reported in Table 1.
Table 1.
Demographic data of adult‐onset Still's disease (AOSD) patients.
| Women (men) | 5 (5) |
| Disease duration, median (range) months | 19 (15, 30) |
| Polycyclic form (chronic form) | 5 (5) |
| Serum ferritin, median (range) ng/ml | 1476·5 (734, 3021) |
| Erythrocyte sedimentation rate mm/h | 82 (54, 120) |
| C‐reactive protein mg/dl | 31 (12, 150) |
| White blood count 10/ml | 15·5 (10·1, 18·9) |
| AST U/l | 82 (10, 101) |
| ALT U/l | 110 (41, 154) |
| Pouchot's score | 6 (4, 10) |
ALT = alanine transaminase; AST = aspartate transaminase.
Ten LN samples were collected from patients who had undergone biopsies for differential diagnosis with a lymphoproliferative disease. These samples were matched with LN samples obtained from subjects without any symptoms or history for autoimmune or autoinflammatory diseases who had undergone biopsies to exclude lymphoproliferative or granulomatous diseases.
The San Salvatore University Hospital ethics committee approved this study. It was performed according to Good Clinical Practice guidelines, and written informed consent was obtained from all patients according to the Declaration of Helsinki.
Histological analysis of biopsies
Sequential sections (thickness 3 µm) were obtained from LN biopsies, fixed by formaldehyde and paraffin‐embedded. For conventional smear preparations, glass smear slides were fixed with 95% ethanol for at least 15 min, and then treated with water for 1 min, haematoxylin for 1 min, running water for 15 min, eosin for 30 s, 95% ethanol for 10 min and 100% ethanol for 10 min. Stained slides were coverslipped with Permount. Haematoxylin and eosin images were acquired using an Olympus BX53 fluorescence microscope with CellSens software (Olympus America Inc., Center Valley, PA, USA).
For immunofluorescence, antigen retrieval was carried out using target retrieval solution (Dako, Glostrup, Denmark). Sections were treated with Dako protein block to block non‐specific binding and successively with H‐ferritin, L‐ferritin and CD68 antibodies. Ferritin L chain (H‐45, sc‐25616; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) is a rabbit polyclonal antibody [immunoglobulin (Ig)G] raised against amino acids 131–175 of the human‐origin ferritin L chain. The ferritin H chain (B‐12, sc‐376594; Santa Cruz Biotechnology Inc.) is a mouse monoclonal antibody (IgG2a) raised against amino acids 131–183 of the human‐origin ferritin H chain. The immunoreaction was revealed by using a fluorescence secondary antibody (Alexa Fluor 488‐conjugated and Alexa Fluor 555‐conjugated; Invitrogen, Carlsbad, CA, USA) and negative controls were obtained by omitting the primary antibody. Cell nuclei were visualized using 4′,6‐diamidino‐2‐phenylindole (DAPI). Sections were examined and photographed under a light microscope (Olympus BX53). Immunofluorescence images were acquired using an Olympus BX53 fluorescence microscope with CellSens software (Olympus America Inc.).
Fluorescence was analysed using an Olympus BX53 fluorescence microscope and the immunofluorescence optical density was performed using ImageJ software (NIH, Bethesda, MD, USA). The number of CD68+/H‐ferritin+ and CD68+/L‐ferritin+ cells was determined as follows: 10 random high‐power microscopic fields for each area (10 000 μm2) were selected and the numbers of CD68+/H‐ferritin+ and CD68+/L‐ferritin+ cells were counted using NIH ImageJ version 1·43 (http://rsbweb.nih.gov/ij/) freeware.
Statistical analysis
GraphPad Prism version 5·0 software was used for statistical analyses. Results are expressed as median (range). Due to the non‐parametric distribution of our data, the Mann–Whitney U‐test was used as appropriate for analyses. Spearman's correlation analysis and linear regression were performed to evaluate possible correlations between the tissue expression of H‐ and L‐ferritin and the number of CD68+/H‐ferritin+ cells or CD68+/L‐ferritin+ cells and clinical and laboratory data. Statistical significance was expressed by a P‐value < 0·05.
Results
Increased expression of H‐ferritin
Immunofluorescence analysis shown in Fig. 1a shows an increased extracellular H‐ferritin expression in the LN samples of AOSD patients when compared with healthy controls (HC). Conversely, the results in Fig. 1b concerning L‐ferritin do not show increased immunofluorescence expression in AOSD patients when compared to HC. The quantitative analyses of the optical density for both H‐ and L‐ferritin expression showed a significant increase of H‐ferritin when compared with both L‐ferritin in AOSD patients and with HC (P < 0·001; P < 0·001, respectively) (Fig. 1g).
Figure 1.

Increased expression of H‐ferritin in lymph node (LN) samples of adult‐onset Still's disease (AOSD) patients. Immunofluorescence analyses in LN sample of subcapsular sinus shows: (a) increased H‐ferritin expression in AOSD patients when compared with healthy controls (HC) (d). No increase of L‐ferritin (b) has been detected in patients when compared with HC (e). (g) H‐ferritin expression levels are significantly higher when compared with the levels of L‐ferritin (***P < 0·001) (c) (f) no co‐localization between H‐ and L‐ferritin has been shown in both AOSD patients and HC.
Correlation of H‐ferritin with clinical features
Our analyses showed a positive correlation between the increased levels of extracellular H‐ferritin in the LN tissues and disease activity. H‐ferritin tissue levels, showing the classical granular deposition in the tissue, correlated strongly with both Pouchot's score and serum ferritin (P < 0·0001; P < 0·0001, respectively). Furthermore, there was a positive correlation between these levels of H‐ferritin and ESR (P < 0·05; P < 0·05, respectively). No correlation was found between CRP and H‐ferritin. Figure 2 shows the linear regression performed among these parameters. Of note, correlation analyses concerning L‐ferritin tissues and the same parameters did not show significant results.
Figure 2.
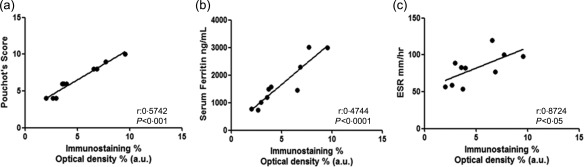
Correlation of H‐ferritin in lymph nodes (LNs) and different markers of severity of adult‐onset Still's disease (AOSD) patients. The non‐parametric Spearman's r correlation coefficient is calculated.
CD68+/H‐ferritin+ cells in LNs from AOSD patients
In the histological analyses of LN samples, performed according to Jeon et al. 28, two patterns were observed. The atypical paracortical hyperplasia pattern was characterized by paracortical hyperplasia with vascular proliferation and mixed cell infiltration in five patients (Fig. 3a), and the burnt‐out histiocytic pattern was characterized by exuberant paracortical hyperplasia with vascular proliferation and extensive sinus macrophage infiltration in the other five patients (Fig. 3b). The immunofluorescence analysis showed that CD68+/H‐ferritin+ cells, with enlarged cytoplasm, were distributed widely in the LN tissue of AOSD patients (Fig. 4b), without specific localization in both the subcapsular and medullary sini, which represent the areas colonized normally by resident macrophages in the LNs. With regard to L‐ferritin, no co‐localization with CD68 molecule was observed in our patients.
Figure 3.
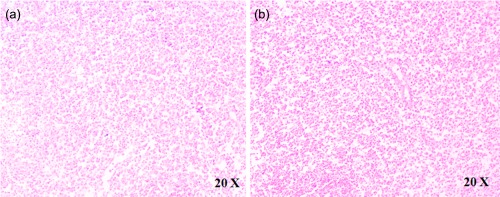
The histological analyses of lymph node (LN) samples. The haematoxylin and eosin analysis of LN samples shows: (a) the atypical paracortical hyperplasia pattern and (b) the burnt‐out histiocytic pattern.
Figure 4.
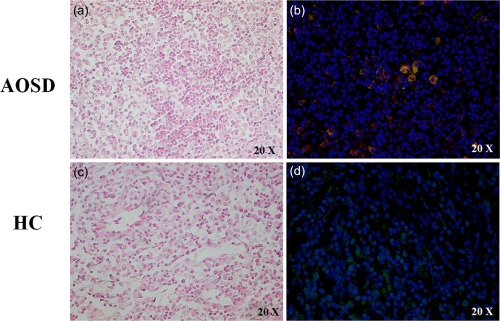
CD68 macrophages expressing H‐ferritin in lymph node (LN) samples of adult‐onset Still's disease (AOSD) patients. The hematoxylin eosin analysis of LNs samples of medullary sinus in AOSD patients (a) and HC (c). The immunofluorescence analysis in LN sample of medullary sinus shows: (b) CD68 macrophages expressing H‐ferritin.
Correlation of number of CD68+/H‐ferritin+ cells in LN with disease activity
A significantly increased number of CD68+/H‐ferritin+ cells was observed in LNs of AOSD patients when compared with HC [AOSD: 4.5 (3·5, 5·8) versus HC: 0·6 (0·2, 1·2); P < 0·0001]. In addition, a significant correlation between the number of H‐ferritin+/CD68+cells and Pouchot's score was observed (P < 0·01). Furthermore, we demonstrated a strong positive correlation between the number of CD68+/H‐ferritin+ cells and serum ferritin (P < 0·0001). Conversely, as shown in Fig. 5, no significant correlations were observed among the number of CD68+/H‐ferritin+ cells and ESR and CRP.
Figure 5.
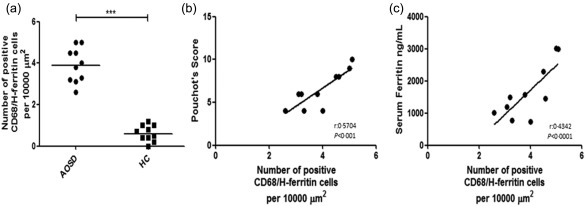
Increased number of CD68+/H‐ferritin+ cells and correlation with severity of adult‐onset Still's disease (AOSD). (a) An increased number of CD68+/H‐ferritin+ cells in lymph node (LN) samples of AOSD patients when compared to healthy controls (HC) (***P < 0·001). (b,c) The non‐parametric Spearman's r correlation coefficient is calculated.
Discussion
In this work, we have shown that both the H‐ferritin tissue expression and the number of CD68+/H‐ferritin+ cells were increased in the LNs of AOSD patients, and these results correlated significantly with disease severity. To our knowledge, this is the first work that has aimed to investigate the evidence of these cells in secondary lymphoid organs during AOSD.
It has been proposed recently that AOSD, MAS, catastrophic anti‐phospholipid syndrome and septic shock should be included in ‘hyperferritinaemic syndrome’, in which the higher levels of ferritin may be considered to be not only a consequence of the inflammation, but are probably involved in a vicious loop leading to the cytokine storm 5. The release during erythrophagocytosis, the impaired tissue clearance by macrophages and the secretion of ferritin by these cells have been considered as possible causes of hyperferritinaemia in these patients 29, 30.
As mentioned previously, we reported an increased number of CD68+/H‐ferritin+. In this context, it is well known that macrophages are distributed widely throughout the body, performing vital homeostatic and defence functions after local and systemic perturbation within tissues. Macrophages are central players in innate and adaptive defence reactions, directly neutralizing pathogens by phagocytosis and orchestrating the responses of other immune cells 31. In response to local adaptation, tissue macrophages display different functional specializations which is reflected in their phenotype, as well as gene expression profiles 31. Basically, subcapsular sinus macrophages (SSMs) and medullary sinus macrophages (MSMs) in the LNs have been recognized 24, 31. The role of SSMs and MSMs in immune response has been studied extensively 32, 33. SSMs may capture lymph‐borne substances, including virus particles, through different mechanisms, including lectins, scavenger receptors and CR3, but are poorly endocytic. Antigens and immunocomplexes bind to SSM heads, are translocated without internalization to their tails, and are finally presented to follicular B cells, migrating randomly through the dense net of tails. Cognate B cells are activated directly from SSMs via the B cell receptor 24, 31. In contrast, MSMs recognize and transfer antigens into conspicuous phagolysosomes. Furthermore, MSMs play an important role in determining survival and clearance of short‐lived early immune cells 24, 31, 32, 33.
In our study, we observed that the CD68+/H‐ferritin+ cells were distributed widely in the tissue, without specific colonization of the subcapsular and/or medullary sini, which are the specific resident macrophage areas in the LNs 24. The majority of these resident macrophages are established prenatally, and these cellular compartments self‐maintain locally independently from each other within their tissue of residence and are self‐sufficient and independent of further haematopoietic input 31, 32, 33. In contrast, our data suggest that the CD68+/H‐ferritin+ cells are part of the additional CD68+ macrophage pool of LN macrophages, including interfollicular cells, which migrate into the LNs after immune activation, under the influence of inflammatory stimuli, and interact with various innate and adaptive cells 24, 31, 32, 33. Although our study does not address the origin of H‐ferritin in LN tissue, our results might allow us to speculate that during AOSD, migratory macrophages colonizing the LNs may produce and secrete ferritin, thus leading to the release of inflammatory mediators 7. In fact, it has been shown that macrophages may produce ferritin in an animal model, and after inflammatory stimuli macrophages may release H‐ferritin 34, 35, 36, 37. Furthermore, it must be pointed out that IL‐1, IL‐6, interferon (IFN)‐γ and tumour necrosis factor (TNF)‐α, largely over‐expressed in AOSD patients 1, 5, 38, may induce the expression of H‐ferritin 34, 35 via Fer2 activation, which is able to stimulate the synthesis of H‐ferritin and further production of many proinflammatory cytokines, thus perpetuating the inflammatory state 11, 36, 37, 39, 40. The experimental evidence reported in previous studies strongly supports our data of increased production of H‐ferritin during AOSD, which is characterized by elevated levels of proinflammatory cytokines 1, 5, 34, 35, 36, 37, 38, 39, 40.
Recently, ferritin distribution was shown in the B area of germinal centres in LN samples from one patient with AOSD 41, and the authors suggested that ferritin may act as an antigen in AOSD enhancing the inflammatory response via the activation of B cells and ferritin autoantibody production 41, although their presence in AOSD has still not been confirmed as observed in other diseases 42, 43.
In our work, both the increased H‐ferritin levels in LNs and the number of CD68+/H‐ferritin+ cells colonizing the lymphatic tissue correlated significantly with disease activity and serum ferritin, and these data mirror what has been observed with other biomarkers of macrophage activation, such as macrophage–colony‐stimulating factor, IFN‐γ, sCD163 and macrophage inhibitory factor, which correlates significantly with the AOSD activity 41, 44, 45, 46. It should be pointed out that LNs are highly dynamic structures: during immune response there is a continuous quick entry of lymphocytes and macrophages from the bloodstream via the afferent lymphatics and a slow egress of recirculating lymphocytes and effector cells via the efferent lymphatics, determining LN enlargement 47, 48, as reported frequently during AOSD 5, 6, 49. In this context, the finding of increased CD68+/H‐ferritin+ cells in the affected LNs and the strong correlation between the number of cells and the severity of disease may allow us to suppose that these cells may be involved in the pathogenic mechanism of AOSD and confirm our previous results, in which CD68+/H‐ferritin+ cells, infiltrating the bone marrow, correlated strongly with the mortality of MAS‐associated AOSD patients 50.
It has been suggested recently that AOSD may be categorized as a multi‐genic inflammatory disorder at the crossroads of autoinflammatory and autoimmune diseases 51. In fact, it has been suggested that IL‐18 and IL‐1β, which are processed through the inflammasome machinery, may play an important role in AOSD pathogenesis, modulating both IL‐6 and Th1 cytokine secretion as well as NK cell dysregulation and macrophage activation 51. According to our results we might speculate that the activated CD68+/H‐ferritin+ cells, via the production and secretion of large amount of H‐ferritin, which display a proinflammatory function, may amplify the up‐regulation and production of inflammatory mediators.
In conclusion, our observational study shows an increased level of extracellular H‐ferritin in the lymphatic tissue of AOSD patients, associated with a CD68+/H‐ferritin+ macrophage colonization of the same tissue. Although our study does not address the origin of H‐ferritin in these affected tissues, the strong correlation of these data with the severity of disease suggests a pathogenic role of H‐ferritin and H‐ferritin‐expressing macrophages during this disease. Further studies are ongoing in our laboratory in order to elucidate more thoroughly the role of these cells and H‐ferritin in AOSD.
Acknowledgement
The authors thank Mrs Federica Sensini for her technical assistance.
Disclosure
The authors have no disclosures.
Author contributions
P. R.: study conception and design, data interpretation, literature search, figure creation, writing, paper revision and acceptance; F. C. I.: study conception and design, data interpretation, literature search, writing, paper revision and acceptance; P. C.: data collection, data interpretation, literature search, paper revision and acceptance; G. G.: data collection, data interpretation, literature search, paper revision and acceptance; P. D. B.: data collection, literature search, paper revision and acceptance; A. R.: data collection, data interpretation, literature search, paper revision and acceptance; V. L.: data collection, literature search, paper revision and acceptance; O. B.: data collection, literature search, paper revision and acceptance; F. C. A.: data collection, data interpretation, literature search, paper revision and acceptance; G. T.: data collection, data interpretation, literature search, paper revision and acceptance; and R. G.: study design, data interpretation, writing, paper revision and acceptance. All authors gave final approval for submitting the manuscript for review and agree to be accountable for all aspects of the work.
References
- 1. Maria AT, Le Quellec A, Jorgensen C et al Adult onset Still's disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun Rev 2014; 13:1149–59. [DOI] [PubMed] [Google Scholar]
- 2. Cipriani P, Ruscitti P, Carubbi F et al Tocilizumab for the treatment of adult‐onset Still's disease: results from a case series. Clin Rheumatol 2014; 33:49–55. [DOI] [PubMed] [Google Scholar]
- 3. Kim YJ, Koo BS, Kim YG et al Clinical features and prognosis in 82 patients with adult‐onset Still's disease. Clin Exp Rheumatol 2014; 32:28–33. [PubMed] [Google Scholar]
- 4. Kim HA, Kwon JE, Yim H et al The pathologic findings of skin, lymph node, liver, and bone marrow in patients with adult‐onset still disease: a comprehensive analysis of 40 cases. Medicine (Balt) 2015; 94:e787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosário C, Zandman‐Goddard G, Meyron‐Holtz EG et al The hyperferritinemic syndrome: macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med 2013; 11:185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med 2009; 10:387–92. [DOI] [PubMed] [Google Scholar]
- 7. Zandman‐Goddard G, Shoenfeld Y. Hemophagocytic syndrome with hyperferritinemia: a stormy immunological response. Isr Med Assoc J 2013; 15:187–8. [PubMed] [Google Scholar]
- 8. Wang W, Knovich MA, Coffman LG et al Serum ferritin: past, present and future. Biochim Biophys Acta 2010; 1800:760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood 2002; 99:3505–16. [DOI] [PubMed] [Google Scholar]
- 10. Cohen LA, Gutierrez L, Weiss A et al Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 2010; 116:1574–84. [DOI] [PubMed] [Google Scholar]
- 11. Recalcati S, Invernizzi P, Arosio P et al New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun 2008; 30:84–9. [DOI] [PubMed] [Google Scholar]
- 12. Hann HW, Stahlhut MW, Lee S et al Effects of isoferritins on human granulocytes. Cancer 1989; 63:2492–6. [DOI] [PubMed] [Google Scholar]
- 13. Morikawa K, Oseko F, Morikawa S. H and L‐rich ferritins suppress antibody production, but not proliferation, of human B lymphocytes in vitro . Blood 1994; 83:737–43. [PubMed] [Google Scholar]
- 14. Broxmeyer HE, Bognacki J, Dorner MH et al Identification of leukemia‐associated inhibitory activity as acidic isoferritins. A regulatory role for acidic isoferritins in the production of granulocytes and macrophages. J Exp Med 1981; 153:1426–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gray CP, Franco AV, Arosio P et al Immunosuppressive effects of melanoma‐derived heavy‐chain ferritin are dependent on stimulation of IL‐10 production. Int J Cancer 2001; 92:843–50. [DOI] [PubMed] [Google Scholar]
- 16. Li R, Luo C, Mines M et al Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem 2006; 281:37616–27. [DOI] [PubMed] [Google Scholar]
- 17. Ruddell RG, Hoang‐Le D, Barwood JM et al Ferritin functions as a proinflammatory cytokine via iron‐independent protein kinase C zeta/nuclear factor kappaB‐regulated signaling in rat hepatic stellate cells. Hepatology 2009; 49:887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen TT, Li L, Chung DH et al TIM‐2 is expressed on B cells and in liver and kidney and is a receptor for H‐ferritin endocytosis. J Exp Med 2005; 202:955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuchroo VK, Umetsu DT, DeKruyff RH et al The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol 2003; 3:454–62. [DOI] [PubMed] [Google Scholar]
- 20. Chakravarti S, Sabatos CA, Xiao S et al Tim‐2 regulates T helper type 2 responses and autoimmunity. J Exp Med 2005; 202:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knickelbein JE, de Souza AJ, Tosti R et al Cutting edge: inhibition of T cell activation by TIM‐2. J Immunol 2006; 177:4966–70. [DOI] [PubMed] [Google Scholar]
- 22. Smith EP, Shanks K, Lipsky MM et al Expression of neuroimmune semaphorins 4A and 4D and their receptors in the lung is enhanced by allergen and vascular endothelial growth factor. BMC Immunol 2011; 12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez‐Manzanet R, DeKruyff R, Kuchroo VK et al The costimulatory role of TIM molecules. Immunol Rev 2009; 229:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gordon S, Plüddemann A, Mukhopadhyay S. Sinusoidal immunity: macrophages at the lymphohematopoietic interface. Cold Spring Harb Perspect Biol 2014; 7:a016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamaguchi M, Ohta A, Tsunematsu T et al Preliminary criteria for classification of adult Still's disease. J Rheumatol 1992; 19:424–30. [PubMed] [Google Scholar]
- 26. Cush JJ, Medsger TA Jr, Christy WC et al Adult‐onset Still's disease. Clinical course and outcome. Arthritis Rheum 1987; 30:186–94. [DOI] [PubMed] [Google Scholar]
- 27. Pouchot J, Sampalis JS, Beaudet F et al Adult Still's disease: manifestations, disease course, and outcome in 62 patients. Medicine (Balt) 1991; 70:118–36. [PubMed] [Google Scholar]
- 28. Jeon YK, Paik JH, Park SS et al Spectrum of lymph node pathology in adult onset Still's disease; analysis of 12 patients with one follow up biopsy. J Clin Pathol 2004; 57:1052–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramos‐Casals M, Brito‐Zerón P, López‐Guillermo A et al Adult haemophagocytic syndrome. Lancet 2014; 383:1503–16. [DOI] [PubMed] [Google Scholar]
- 30. Knovich MA, Storey JA, Coffman LG et al Ferritin for the clinician. Blood Rev 2009; 23:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol 2015; 33:643–75. [DOI] [PubMed] [Google Scholar]
- 32. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014; 41:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smirnov IM, Bailey K, Flowers CH et al Effects of TNF‐alpha and IL‐1beta on iron metabolism by A549 cells and influence on cytotoxicity. Am J Physiol 1999; 277:L257–63. [DOI] [PubMed] [Google Scholar]
- 35. Wei Y, Miller SC, Tsuji Y et al Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun 1990; 169:289–96. [DOI] [PubMed] [Google Scholar]
- 36. Piñero DJ, Hu J, Cook BM et al Interleukin‐1beta increases binding of the iron regulatory protein and the synthesis of ferritin by increasing the labile iron pool. Biochim Biophys Acta 2000; 1497:279–88. [DOI] [PubMed] [Google Scholar]
- 37. Ben‐Neriah Y, Karin M. Inflammation meets cancer, with NF‐κB as the matchmaker. Nat Immunol 2011; 12:715–23. [DOI] [PubMed] [Google Scholar]
- 38. Chen DY, Lan JL, Lin FJ et al Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset Still's disease. J Rheumatol 2004; 31:2189–98. [PubMed] [Google Scholar]
- 39. Silva‐Gomes S, Bouton C, Silva T et al Mycobacterium avium infection induces H‐ferritin expression in mouse primary macrophages by activating Toll‐like receptor 2. PLOS ONE 2013; 8:e82874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zandman‐Goddard G, Shoenfeld Y. Ferritin in autoimmune diseases. Autoimmun Rev 2007; 6:457–63. [DOI] [PubMed] [Google Scholar]
- 41. Colafrancesco S, Priori R, Alessandri C et al sCD163 in AOSD: a biomarker for macrophage activation related to hyperferritinemia. Immunol Res 2014; 60:177–83. [DOI] [PubMed] [Google Scholar]
- 42. Régent A, Ly KH, Blet A et al Contribution of antiferritin antibodies to diagnosis of giant cell arteritis. Ann Rheum Dis 2013; 72:1269–70. [DOI] [PubMed] [Google Scholar]
- 43. Baerlecken NT, Linnemann A, Gross WL et al Association of ferritin autoantibodies with giant cell arteritis/polymyalgia rheumatica. Ann Rheum Dis 2012; 71:943–7. [DOI] [PubMed] [Google Scholar]
- 44. Matsui K, Tsuchida T, Hiroishi K et al High serum level of macrophage‐colony stimulating factor (M‐CSF) in adult‐onset Still's disease. Rheumatology (Oxf) 1999; 38:477–8. [DOI] [PubMed] [Google Scholar]
- 45. Jung S‐Y, Park Y‐B, Ha Y‐J et al Serum calprotectin as a marker for disease activity and severity in adult‐onset Still's disease. J Rheumatol 2010; 37:1029–34. [DOI] [PubMed] [Google Scholar]
- 46. Zou Y‐Q, Lu L‐J, Li S‐J et al The levels of macrophage migration inhibitory factor as an indicator of disease activity and severity in adult‐onset Still's disease. Clin Biochem 2008; 41:519–24. [DOI] [PubMed] [Google Scholar]
- 47. Benahmed F, Ely S, Lu TT. Lymph node vascular‐stromal growth and function as a potential target for controlling immunity. Clin Immunol 2012; 144:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev 2013; 251:160–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gerfaud‐Valentin M, Maucort‐Boulch D, Hot A et al Adult onset Still's disease: manifestations, treatments, outcome, and prognostic factors in 57 patients. Medicine (Balt) 2014; 93:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruscitti P, Cipriani P, Di Benedetto P et al Increased level of H‐ferritin and its imbalance with L‐ferritin, in bone marrow and liver of patients with adult onset Still's disease, developing macrophage activation syndrome, correlate with the severity of the disease. Autoimmun Rev 2015; 14:429–37. [DOI] [PubMed] [Google Scholar]
- 51. Gerfaud‐Valentin M, Jamilloux Y, Iwaz J et al Adult‐onset Still's disease. Autoimmun Rev 2014; 13:708–22. [DOI] [PubMed] [Google Scholar]


