Summary
Anti‐DNA antibodies play a pivotal role in the pathogenesis of lupus nephritis by cross‐reacting with renal antigens. Previously, we demonstrated that the binding affinity of anti‐DNA antibodies to self‐antigens is isotype‐dependent. Furthermore, significant variability in renal pathogenicity was seen among a panel of anti‐DNA isotypes [derived from a single murine immunoglobulin (Ig)G3 monoclonal antibody, PL9‐11] that share identical variable regions. In this study, we sought to select peptide mimics that effectively inhibit the binding of all murine and human anti‐DNA IgG isotypes to glomerular antigens. The PL9‐11 panel of IgG anti‐DNA antibodies (IgG1, IgG2a, IgG2b and IgG3) was used for screening a 12‐mer phage display library. Binding affinity was determined by surface plasmon resonance. Enzyme‐linked immunosorbent assay (ELISA), flow cytometry and glomerular binding assays were used for the assessment of peptide inhibition of antibody binding to nuclear and kidney antigens. We identified a 12 amino acid peptide (ALWPPNLHAWVP, or ‘ALW’) which binds to all PL9‐11 IgG isotypes. Preincubation with the ALW peptide reduced the binding of the PL9‐11 anti‐DNA antibodies to DNA, laminin, mesangial cells and isolated glomeruli significantly. Furthermore, we confirmed the specificity of the amino acid sequence in the binding of ALW to anti‐DNA antibodies by alanine scanning. Finally, ALW inhibited the binding of murine and human lupus sera to dsDNA and glomeruli significantly. In conclusion, by inhibiting the binding of polyclonal anti‐DNA antibodies to autoantigens in vivo, the ALW peptide (or its derivatives) may potentially be a useful approach to block anti‐DNA antibody binding to renal tissue.
Keywords: anti‐DNA antibodies, lupus nephritis, phage display library, SLE
Introduction
Among the pathogenic autoantibodies found commonly in the sera of patients with systemic lupus erythematosus (SLE), immunoglobulin (Ig)G anti‐DNA antibodies attract particular attention for their key role in the pathogenesis of lupus nephritis 1. Anti‐DNA antibodies contribute to renal injury by indirect binding to the glomerular basement membrane mediated by DNA/nucleosomes or by direct binding to glomerular antigens, the latter of which is mediated by cross‐reactivity 2. The cross‐reaction between anti‐DNA IgGs and particular self‐antigens may explain the specificity of tissue damage in lupus nephritis. Besides complement activation, binding of anti‐DNA antibodies to live cells can modulate gene expression and cell metabolism, enhance cellular proliferation and induce phenotypical changes which are found in glomerular resident cells in lupus kidneys 3, 4, 5, 6, 7. Therefore, blocking the cross‐reaction of anti‐DNA IgGs with glomerular antigens may be a valuable approach to the management of lupus nephritis initiated and/or perpetuated by circulating autoantibodies.
Previously, using class‐switching in vitro, we generated IgG1, IgG2a and IgG2b versions of the murine monoclonal PL9‐11 IgG3 anti‐DNA antibody 8, which all share an identical light chain and heavy chain variable region, but differ from each other in the heavy chain constant region (i.e. isotype) 9. Surprisingly, we identified significant differences in both antigenic specificity and renal pathogenicity between these PL9‐11 isotypes, due to the effects of the different constant regions on antigen–antibody interactions 10. These findings may also explain why certain subclasses of lupus anti‐DNA antibodies are associated more closely with pathogenic potential 11, 12. Thus, the blocking of multiple anti‐DNA antibody subsets, especially high‐affinity or more pathogenic isotypes, may be a worthy therapeutic goal.
Screening phage display libraries with antibodies is a useful method for selecting peptides that mimic the antigenic partners with which these antibodies interact. Using pathogenic anti‐DNA antibodies as the ‘bait’ and isolating peptide mimics by a phage display approach has been suggested as a promising approach for identifying novel therapies that can protect target organs from antibody‐mediated injury in SLE 13, 14, 15, 16, 17, 18, 19, 20, 21. Here, we screened a phage peptide display library with each of the four isotypes of the pathogenic PL9‐11 anti‐DNA antibody. Our goals were to discover both common and isotype specific dsDNA mimics, and identify peptides that would inhibit the cross‐reaction of lupus autoantibodies with DNA and glomerular antigens.
Materials and methods
Scanning of a phage display library
Monoclonal antibodies derived from the PL9‐11 parent clone, having differential affinity to dsDNA (IgG3 > IgG2a > IgG1 > IgG2b), were purified from culture supernatants, as described 9. The Ph.D.™‐12‐phage display library (New England Biolabs, Ipswich, MA, USA) was used for peptide selection 22. Bound phages selected by the members of the PL9‐11 antibody panel were isolated (11 distinct phages by IgG1, 19 phages by IgG2a, 18 phages by IgG2b and 12 phages by IgG3; Supporting information, Figure S1). From these phage clones, a peptide with the sequence of ALWPPNLHAWVP (abbreviated as ALW) was chosen for further study as it was the sequence isolated most frequently by all four isotypes. Biotin‐labelled or unlabelled ALW peptides and two size‐identical peptide controls (P1 peptide, SPNQHTPPWMLK; ‘PLP’ peptide, PLPHNPWVLAAW, scrambled randomly from ALW) were synthesized at the Proteomics Resource Center of Rockefeller University (NY, USA). PLP was used as the primary control peptide, as this peptide is identical in amino acid composition (but not in sequence) and length to ALW. P1 is the same length as ALW, and also binds to an irrelevant IgG3 monoclonal antibody (clone 3E5) that exhibits no binding to DNA 9, 23.
Surface plasmon resonance
The affinity of the PL9‐11 monoclonal antibodies for the ALW peptide was determined by surface plasmon resonance (SPR) analysis using a Biacore 3000 instrument (Biacore, Piscataway, NJ, USA) 9. In brief, the antibodies were immobilized on a CM sensor chip (GE Healthcare, Port Washington, NY, USA) at a concentration of 10 nM. The ALW peptide (0–250 nM) in HEPES buffer (pH 7·4, with 0.05% Tween 20) was injected over the chip. The simple Langmuir model (A + B ↔ AB) was used for calculating the binding kinetics, including association (Ka) and dissociation (Kd) values.
Enzyme‐linked immunosorbent assay (ELISA)
dsDNA, laminin and matrigel ELISAs were performed as described previously 9. For inhibition with ALW, the PL9‐11 IgGs (at 2 µg/ml) were preincubated with serially diluted peptide (from 0·16 to 80 µg/ml) at 37°C for 2 h, followed by transfer to antigen‐coated 96‐well plates. Alkaline phosphatase (AP)‐conjugated goat IgG anti‐mouse κ chain was the secondary antibody. The percentage of inhibition was calculated from the optical density (OD) values of peptide‐preincubated wells (inhibited) and non‐preincubated wells (non‐inhibited, as baseline). Similarly, peptide inhibition was performed with sera (diluted 1 : 200) from female Murphy Roths Large lymphoproliferation (MRL/lpr) mice (38–54 weeks old), male Sle1·3 mice (30 weeks old) or human SLE patients with active disease. Studies were approved by the Institutional Animal Care and Use Committee and the Institutional Review Board of the Albert Einstein College of Medicine/Montefiore Medical Center, respectively.
Assessing the direct binding of PL9‐11 antibodies to the ALW peptide was carried out by coating plates with streptavidin (5 µg/ml) and then adding biotin‐labelled peptide (5 µg/ml). Next, serial dilutions of each antibody (starting at 10 µg/ml) were added before colour development using alkaline phosphatase (AP)‐conjugated goat IgG anti‐mouse κ chain (2 µg/ml) and substrate. The selected phages were also used for ELISA by direct coating onto 96‐well plates.
Peptide inhibition of antibody binding to mesangial cells was assessed by a cell surface ELISA 9. Mesangial cells were treated with or without DNase I, before addition of the individual antibodies (at 2 µg/ml) that were preincubated or not with ALW at 37°C for 2 h.
Flow cytometry
Mesangial cells were removed from tissue culture plates using a trypsin‐ethylenediamine tetraacetic acid (EDTA) solution. After blocking, cells were incubated with the individual antibodies (at 2 µg/ml) which were ALW preincubated (80 µg/ml, 37°C for 1 h) or not. Following repeated washing, phycoerythrin‐conjugated goat anti‐mouse κ chain was added (Southern Biotech, Birmingham, AL, USA). The stained cells were analysed by using a LSRII instrument (BD Biosciences, San Jose, CA, USA). In other experiments, mesangial cells were first treated with DNase I before antibody incubation 9.
Glomerular binding assay
The binding of PL9‐11 antibodies (or lupus sera) to isolated rat glomeruli was analysed by the glomerular binding assay (GBA) 9, 24. Briefly, glomeruli adherent to glass slides were treated with DNase I, followed by the addition of the individual antibodies (at 2 µg/ml) or sera (diluted 1 : 200) that were preincubated with the ALW peptide (80 µg/ml) at 37°C for 2 h. Fluorescein isothiocyanate‐conjugated goat IgG anti‐mouse κ chain was used as the secondary antibody. The fluorescence intensity was quantitated by ImageJ (National Institutes of Health, Bethesda, MD, USA). A murine IgG3 monoclonal antibody (mAb) (3E5), which does not bind dsDNA, was used as an isotype control 9, 25.
Alanine scanning
To evaluate the role of the amino acid sequence in the specific binding of ALW to anti‐DNA antibodies, alanine scanning was performed in which single amino acid residues in the ALW sequence were replaced with alanine. Alanine is non‐bulky and chemically inert, and its substitution for individual amino acids can determine whether each specific residue plays a significant role in the bioactivity of the original peptide 26. In a direct binding assay, the alanine‐replaced ALW peptides were coated onto plates at 140 µM in carbonate buffer (pH 9·6) overnight at 4°C. After blocking, the serially diluted antibodies (starting at 10 µg/ml) were added, and AP‐conjugated goat IgG anti‐mouse κ chain (2 µg/ml) and substrate were used for colour development.
Statistical analysis
All results were shown as mean ± the standard error. Analysis of variance (anova) was used for the comparison of more than two groups. A two‐tailed t‐test was used when two groups were compared for statistical differences, with P < 0·05 considered significant.
Results
ALW exhibits isotype‐dependent binding to anti‐DNA antibodies
Using SPR to determine the binding kinetics of the antibody/antigen interaction, we observed binding of the PL9‐11 IgG3 antibody to the ALW peptide in a concentration as low as 5 nM (Fig. 1a). Furthermore, we found that the ALW peptide possesses differential binding affinity to anti‐DNA antibodies in the order of IgG2b > IgG2a > IgG3 > IgG1 (Fig. 1b; Table 1). An irrelevant IgG3 isotype control, 3E5, repeatedly showed no specific binding to the ALW peptide in these assays (data not shown), demonstrating that the binding of ALW to PL9‐11 IgG3 was variable region‐specific, rather than being a function of the isotype. This result is highly consistent with our previous report that DNA, which is mimicked by ALW (as this peptide prevents the binding of the PL9‐11 antibodies to DNA) is also bound only by members of the PL9‐11 panel but not isotype control antibodies 9. Taken together, the conclusion from these results would be that binding of ALW and DNA to PL‐11 antibodies is mediated by the antibody binding site, rather than being a non‐specific Fc‐dependent effect. Similarly, no significant binding to PL9‐11 IgG2a and IgG3 was observed using PLP (scrambled ALW peptide), with maximal RU values of 8 versus 370 units (IgG2a) and 20 versus 761 units (IgG3) for PLP and ALW, respectively (Table 1 and data not shown). ELISA data using biotin‐labelled ALW revealed a similar pattern ro the SPR analysis (Fig. 1c).
Figure 1.
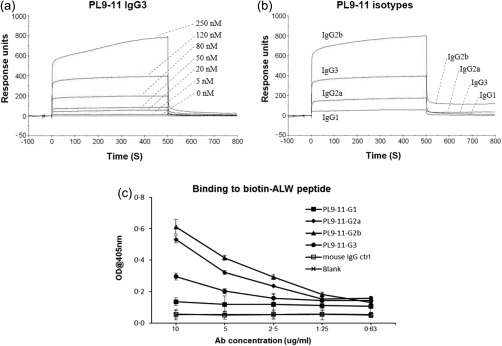
Anti‐DNA antibodies bind to the ALWPPNLHAWVP (ALW) peptide. (a) Representative graph of surface plasmon resonance (SPR) analysis for ALW peptide (0–250 nM) binding to PL9‐11 immunoglobulin (Ig)G3. (b) Representative graph of SPR analysis for PL9‐11 isotypes binding to the ALW peptide (120 nM). (c) PL9‐11 antibodies bind to biotin‐labelled ALW in order of IgG2b > IgG2a > IgG3 > IgG1. Polyclonal mouse IgG was used as control. The difference in binding affinity observed between the panels in this figure reflects methodological variations in both the peptide antigen preparation and binding assay, with the peptide immobilized on the chip and the binding measured by SPR in (a) and (b), versus biotin labelling and enzyme‐linked immunosorbent assay (ELISA) in (c), respectively. Data were from three independent experiments.
Table 1.
Kinetics of PL9‐11 binding to ALWPPNLHAWVP (ALW).
| Isotype | ka (M−1s−2) | kd (μs−1) | KD (μM)† | R max |
|---|---|---|---|---|
| IgG2b | 109·0 | 0·80 | 0·01* | 792·34 |
| IgG2a | 0·8 | 0·52 | 0·64* | 369·50 |
| IgG3 | 3·84 | 44·90 | 11·69 | 760·61 |
| IgG1 | 5·68 | 1830·00 | 322·18* | 107·48 |
*P < 0.05, compared to immunoglobulin (Ig)G3.
†Lower KD value (=kd/ka) means higher binding affinity (n = 3). Δ Maximum value of response unit at the range of 0 to 250 nM of peptide.
ALW inhibits the binding of anti‐DNA antibodies to multiple antigens
Inhibition ELISA showed that preincubation with ALW reduced significantly the binding of PL9‐11 IgGs to dsDNA (Fig. 2a,b) and laminin (Fig. 2c). The magnitude of inhibition of dsDNA binding was similar for the different isotypes at high ALW concentrations, but was isotype‐dependent at lower ALW concentrations (1·25–20 µg/ml) (Fig. 2a). A similar finding was seen with dsDNA inhibition of PL9‐11 panel antibodies binding to ALW (data not shown). The data were fitted to a one‐site binding model to gain isotype‐dependent Kd values of 4·6 × 10−6 (IgG1), 4·1 × 10−6 (IgG2a), 4·2 × 10−6 (IgG2b) and 6·2 × 10−6 (IgG3), respectively, for ALW inhibition of PL9‐11 binding to dsDNA (Fig. 2b). Although ALW exhibits a relatively higher inhibition of the binding of the PL9‐11 IgG2a and IgG2b isotypes to dsDNA, IgG2b and IgG3 were the isotypes inhibited most effectively by ALW in binding to laminin (Fig. 2c), indicating that the degree and isotype dependence of peptide inhibition can vary depending on the nature of the antigen.
Figure 2.
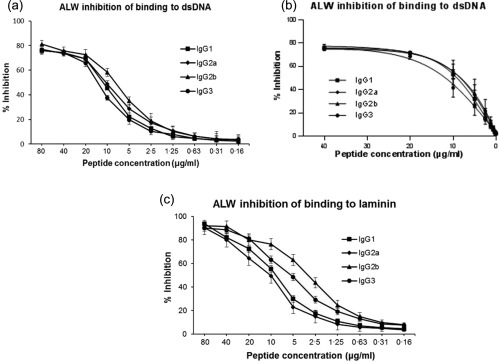
The ALWPPNLHAWVP (ALW) peptide inhibits the binding of PL9‐11 antibodies to dsDNA and laminin. (a) The ALW peptide (0·16–80 µg/ml) inhibits the binding of PL9‐11 isotypes (2 µg/ml) to dsDNA in the order of immunoglobulin (Ig)G2b > IgG2a > IgG1 > IgG3. (b) The graph for one site binding curve analysis is shown. (c) ALW inhibits the binding of PL9‐11 antibodies (2 µg/ml) to laminin in the order of IgG2b > IgG3 > IgG1 > IgG2a. The inhibitory capacity of the unmodified ALW in PL9‐11 binding to dsDNA and laminin was higher than that of the scrambled peptide (data not shown). (a,c) Representative graphs from three independent experiments are shown.
Because binding to glomerular antigens in vitro has been shown to be associated with pathogenicity in vivo, we next performed quantitative analysis of PL9‐11 antibody binding to murine mesangial cell surface antigens. Similar to other lupus‐associated autoantibodies, PL‐9‐11 antibodies not only bind to DNA but also cross‐react directly with renal self‐antigens, an important determinant of the pathogenicity of this type of autoantibody 2, 3, 9. Therefore, binding to kidney cell antigens was assessed with and without DNase I pretreatment to exclude the possibility that ALW blocks the binding only of PL9‐11 IgGs to DNA, but not other self‐antigens. Cell surface ELISA showed effective inhibition by ALW of PL9‐11 IgG2a, IgG2b and IgG3 binding to DNase I‐treated mesangial cells (Fig. 3a). Similarly, strong inhibition by the ALW peptide was seen in IgG2a and IgG2b binding to non‐DNase I‐treated mesangial cells (data not shown). In a confirmatory study using flow cytometry, ALW similarly exhibited strong inhibition of the binding of the PL9‐11 isotypes to mesangial cells treated (Fig. 3b,c) or not (data not shown) with DNase I, consistent with the results from the cell surface ELISA.
Figure 3.
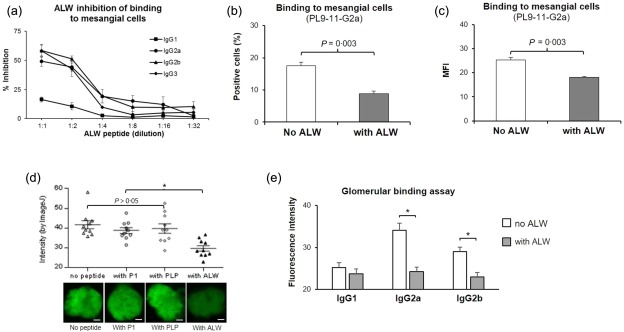
The ALWPPNLHAWVP (ALW) peptide inhibits PL9‐11 antibody binding to DNase I‐treated mesangial cells and glomeruli. (a) ALW inhibits the binding of PL9‐11 immunoglobulins (Ig)Gs to mesangial cells. Serial dilutions from an initial peptide concentration of 80 µg/ml were tested in this experiment. (b,c) Flow cytometry shows a significant decrease in both the percentage of positive cells (b) and mean fluorescence intensity (MFI) (c) after PL9‐11 IgG2a was preincubated with the ALW peptide before binding to the mesangial cells. The other PL9‐11 isotypes showed similar results (not shown). (a–c) Data are representative of three independent experiments. (d) ALW, but not the control peptides (P1, PLP), inhibit the binding of PL9‐11 IgG3 to isolated glomeruli. There were no significant differences in the inhibition between the three control groups. The bottom part of the panel depicts immunoglobulin binding to a single glomerulus fixed to the glass slide. (e) Binding of PL9‐11 IgG2a and IgG2b to glomeruli was inhibited similarly by ALW. (d,e) Ten glomeruli in each group were stained, and representative images are shown. Scale bar = 25 µm. *P < 0·05, compared to the no ALW (or control peptide) group accordingly.
The GBA is a useful technique for modelling glomerular binding of anti‐DNA antibodies ex vivo, with the degree of GBA binding correlating well with in‐vivo pathogenicity 24. We found that the ALW peptide decreased the binding of PL9‐11 isotypes to DNase I‐treated rat glomeruli significantly (Fig. 3d,e). Once again, the inhibition by ALW was isotype‐dependent (data not shown). In contrast, an irrelevant IgG3 monoclonal antibody (3E5)‐selected peptide P1 and the scrambled ALW peptide PLP did not inhibit the binding of the PL9‐11 IgG3 anti‐DNA antibody to glomeruli ex vivo (Fig. 3d). The inhibition by ALW of anti‐DNA antibody binding to non‐DNase I‐treated glomeruli was similarly significant (data not shown).
ALW binding is sequence‐specific
Alanine scanning was performed to determine whether specific amino acid residues contribute to the binding of ALW to PL9‐11‐derived anti‐DNA antibodies. We found that the replacement of a single amino acid, especially at the third or eighth residue in the sequence, decreased peptide binding (Fig. 4a). The magnitude of the decrease in binding following particular substitutions varied with the individual PL9‐11 IgG isotypes, although the strongest effects were generally seen with these two specific sites of alanine replacement (Fig. 4b).
Figure 4.
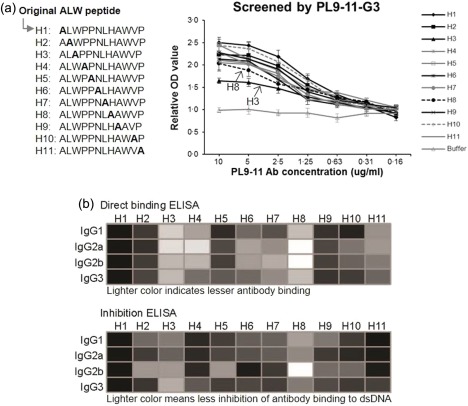
Alanine scanning of the ALWPPNLHAWVP (ALW) peptide. (a) Alanine replacement at the third or eighth amino acid residue decreases immunoglobulin (Ig)G3 binding to ALW. The other PL9‐11 IgG isotypes exhibited similar results (not shown). (b) In both direct and inhibition enzyme‐linked immunosorbent assays (ELISAs), reduction of peptide binding was most seen frequently when either the third (W) or eighth (H) amino acid residue was replaced by alanine. The optical density (OD) values were normalized to the original ALW peptide, and the degree of binding or inhibition is indicated by the shade of colour.
ALW was selected for its binding to all PL9‐11 isotypes. To establish the specificity of the amino acid sequence in interaction between PL9‐11 isotypes and the selected peptides, ELISA analysis with size‐identical phages (selected by PL9‐11 antibodies) was performed. We found that PL9‐11 antibody binding is both peptide‐ and isotype‐dependent (Fig. 5). Phages selected exclusively by any of the individual PL9‐11 isotypes during panning of the peptide display library subsequently exhibited no specific binding to the other members of the antibody panel (Fig. 5a). The differences in binding to specific peptide‐containing phage (Fig. 5a), despite the negligible length of the different peptide inserts (12 amino acids) compared to the phage coat protein and linker (408 amino acids), supports a specific binding interaction of the different PL9‐11 isotypes only to the indicated peptides. Indeed, the isotype‐selected peptides inhibited only the binding of the selecting isotype to its cognate antigen; shown, for example, in Fig. 5b,c is the selective inhibition by the VHLQAGELMNPK (VHL)‐containing phage of the binding of PL9‐11 IgG3 to dsDNA and laminin, respectively. In contrast, the VHL‐containing phage did not inhibit the binding of the other PL9‐11 isotypes (Fig. 5b,c). The converse was also true; only the phage containing the isotype‐specific peptide, but not the peptide selected by the other isotypes, inhibited the binding of the selecting isotype to autoantigens (Fig. 5d,e). Although the exact concentration of peptide needed for inhibition is not obvious from these particular experiments, nevertheless the small relative molecular weight of the peptide relative to the phage as well as the lack of cross‐inhibition that was observed are supportive of the specificity of the binding interactions between individual peptides and members of the PL9‐11 panel, and the highly sensitive detection of small structural differences between different isotypes sharing a variable region using the peptide library approach. Finally, the lack of inhibition by the control peptides (P1 and PLP; Fig. 3d) is also supportive of a conclusion that the specificity of amino acid sequence, rather than the size, is critical in determining the binding to anti‐DNA antibodies.
Figure 5.
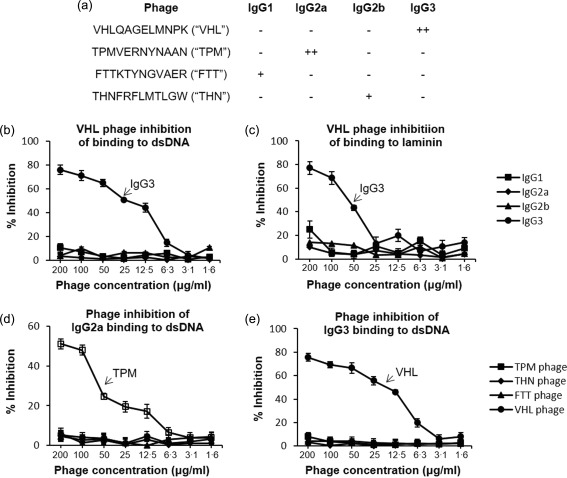
Identification of isotype specific peptide sequences. (a) Four phage clones were used for phage inhibition enzyme‐linked immunosorbent assays (ELISAs), each of them selected exclusively by a single PL9‐11 isotype during library screening. ++ = Strong binding; + = moderate binding; – = weak or no binding. (b,c) The immunoglobulin (Ig)G3‐selected VHLQAGELMNPK (VHL) phage only inhibited PL9‐11 IgG3 but not the binding of the other isotypes to dsDNA (b) or laminin (c). (d) The binding of PL9‐11 IgG2a to dsDNA was inhibited exclusively by the phage (TPM) selected singularly by that particular isotype. (e) Similar inhibition specificity was also seen with PL9‐11 IgG3 selected phage. Three independent experiments were performed, and representative graphs are shown in panels (b) to (e).
ALW abrogates lupus serum binding to renal antigens
To determine whether the ALW peptide represents an epitope recognized broadly by polyclonal anti‐DNA antibodies, we analysed the effect of ALW on the binding of lupus sera in vitro. Marked inhibition of the binding of lupus sera to self‐antigens was seen after preincubation with ALW peptide (Fig. 6), but not the PLP control peptide (scrambled ALW) (data not shown). Specifically, the binding of sera from MRL/lpr mice to dsDNA (Fig. 6a), laminin (Fig. 6b) and matrigel (data not shown) was largely inhibited. Similar results were seen with sera from Sle1.Sle3 mice (Fig. 6c,d, and data not shown). Analysing serum samples from human lupus patients, significant inhibition of binding to dsDNA (∼ 50%) and laminin (>70%) was seen following sera pretreatment with the ALW peptide (Fig. 6e,f), but not the PLP peptide (data not shown). In the GBA, ALW inhibited significantly the binding of both MRL/lpr and human lupus sera to isolated rat glomeruli (Fig. 6g,h). Despite some differences in the degree of binding affinity/extent of inhibition in individual samples and between the MRL/lpr, Sle1.Sle3 and human lupus groups, the fact that significant inhibition was often seen suggests strongly that the PL9‐11 peptide contains motifs shared by anti‐DNA antibodies from different individuals, lupus mouse strains and species.
Figure 6.
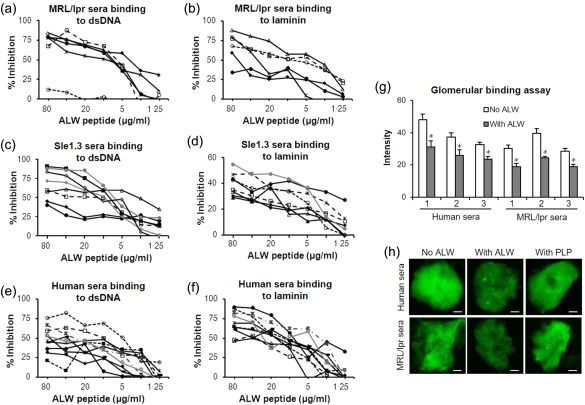
ALWPPNLHAWVP (ALW) inhibits lupus sera binding to self‐antigens. (a,b) Sera from Murphy Roths Large lymphoproliferation (MRL)lpr) mice (n = 6) binding to dsDNA (a) or laminin (b) were inhibited by ALW. (c,d) ALW inhibition of the binding of Sle1.3 mice sera (n = 8) to dsDNA (c) and laminin (d). (e,f) ALW inhibition of the binding to dsDNA (e) or laminin (f) by sera from lupus patients (n = 10). Each individual line in the graphs from (a) to (f) represents the binding of an individual serum sample. (g) Preincubation with ALW decreased the binding of human and mouse lupus sera (n = 3 of each group) to isolated glomeruli (n = 4 for each serum sample). (h) Representative images of the glomerular binding assay results are shown. Scale bar = 25 µm. *P < 0·05, comparing binding intensity with and without ALW.
Discussion
We demonstrated in this study that the ALW peptide mimics the antigenicity of dsDNA, and exhibits differential binding affinity to the variable region‐identical PL9‐11 anti‐DNA isotype family. The interaction of the ALW peptide with these antibodies was sequence‐specific. Furthermore, ALW inhibits significantly the binding of anti‐DNA IgGs to glomerular antigens in an isotype‐ and antigen‐dependent manner. Finally, the interaction of polyreactive lupus sera from either lupus mice or human patients with dsDNA, laminin and matrigel was blocked significantly by the ALW peptide. Our results demonstrate clearly the potential promise of the ALW peptide (or its derivatives) in inhibiting the cross‐reaction between anti‐DNA antibodies and glomerular antigens.
The anti‐DNA antibodies we used for identifying peptide dsDNA mimics originated from the same parent PL9‐11 clone (IgG3) and therefore share identical variable regions in both heavy and light chains, thus providing the rationale for direct comparison of their binding affinity 9. As we had found significant diversity in the antigenic specificity and renal pathogenicity of the PL9‐11 antibody panel, we surmised that screening the peptide library with these different antibodies may help to identify particular epitopes that effectively block autoantibodies with specific renal effects or that display higher nephritogenicity. Moreover, using both circular dichroism and tryptophan fluorescence, we had found previously that differences in the binding affinity among PL9‐11 isotypes to autoantigens are due to differential alterations in secondary structures arising during antigen–antibody interactions; that is, the binding of identical variable regions to the same antigen can lead to different structural changes in the antigen‐binding pocket because of the contribution from constant regions 10. In addition, changes of antibody secondary structures depended partially on the molecular properties and structure of the interacting protein antigens, which are determined by their amino acid sequence. Therefore, the differences we found among PL9‐11 isotypes in the nature of their selected peptides strengthen our previous findings significantly, indicating a role for the constant region in determining the structure of the antigen binding end of the antibody molecule, because otherwise all selected peptides, which are mirror images of the antigen‐binding pocket, would be identical among the four isotypes. Our results further support the importance of specific linear, secondary or tertiary structural motifs in the binding of autoantigens to anti‐DNA antibodies. We also found that individual PL9‐11 isotypes can bind to several peptides with different amino acid residues (e.g. PL9‐11 IgG3 binding to ALW and VHL). Cross‐reactivity with multiple antigens is a feature of pathogenic lupus associated autoantibodies 2, 9, and may occur due to similarities between these peptides in spatial (linear or conformational) structure or charge. In our future studies we plan to solve the crystal structure of one or more members of the PL9‐11 antibody panel (alone and complexed with cognate antigen) to understand more clearly the structural basis for this cross‐reactivity, and help to identify more accurately the optimal epitope recognized by these antibodies.
The ALW peptide was the sequence most often selected during library screening by all PL9‐11 isotypes. Interestingly, using an identical phage display library, this same ALW sequence was selected by antibodies that bind to the RNA polymerase of hepatitis C virus 27 or influenza virus M2 protein 28. Nevertheless, our ELISA and SPR results clearly demonstrated a strong affinity of ALW for the PL9‐11 antibodies. Furthermore, the significant differences in affinity for the peptide between the PL9‐11 isotypes indicate further that it is their intrinsic attributes, rather than non‐specific stickiness, that underlie the interaction between members of the PL9‐11 panel and the ALW peptide. Future structural studies may be useful in clarifying the mechanism by which ALW binds to antibodies with seemingly disparate specificities.
Previously, we found that immunization with a peptide (DWEYSVWLSN) selected by the murine IgG2b anti‐DNA antibody R4A induces autoantibody production and renal immunoglobulin deposition in non‐autoimmune BALB/c mice 13, 14, accelerates autoimmune manifestations and nephritis in lupus‐prone mice 15 and, when given therapeutically (in a truncated D‐form), can protect mice from renal deposition of the anti‐DNA antibody 16. A peptidomimetic designed to mimic the molecular structure of DWEYSVWLSN showed much higher affinity for anti‐DNA antibodies and enhanced blocking of binding to renal and brain tissue 21. In this paper we did not compare directly the binding affinity and inhibitory capacity of DWEYSVWLSN to ALWPPNLHAWVP, due to their disparate lengths. Furthermore, the former was selected by a single isotype, while the latter peptide described in this paper bound to all four IgG isotypes and thus, potentially, would more broadly inhibit the pathogenic anti‐DNA response, which is polyclonal. It is interesting, however, that both these peptides have tryptophan residues at similar positions. In future studies the relative importance of this particular amino acid at these positions will be determined. Similarly, other DNA mimicking peptides (that is, peptides with specific affinity for antibodies that also bind DNA) selected by the murine F14.6, F4.1 and J20.8 IgG2a anti‐DNA monoclonal antibodies not only bound to serum antibodies from human patients with SLE, but also elicited the production of IgG3 anti‐dsDNA antibodies by immunization of BALB/c mice 19. Polyclonal human anti‐DNA autoantibodies (from lupus sera) were used in the selection of the DNA mimicking peptides ASPVTARVLWKASHV and RLTSSLRYNP, the former of which inhibited serum binding to DNA 17, and the latter which bound to anti‐nucleosome antibodies and induced IgG anti‐DNA antibodies in rabbits 20. Therefore, DNA mimicking peptides can augment autoantibody production or block antibody–antigen interactions, depending on the mode of peptide administration 29. Nevertheless, although ALW is not the first peptide to be described as a dsDNA mimic, previous peptides were identified by screening a L100 (gene VIII) phage library 13, 14, 15, 16, 17, 18, 19, 20. In contrast, in the present study we utilized a M13KE pIII genome phage library, which provides only a single copy rather than both fused and unfused copies of gene III. The reduced valency of pIII compared to pVIII libraries renders the M13KE library more suitable for the discovery of higher‐affinity ligands 30, 31. Indeed, although direct comparison is not possible and the antibodies are different, the molar ratio of peptide to antibody concentration required to inhibit 50% of dsDNA binding for ALW was similar or lower than what had been described previously 14, 17. Moreover, peptides described previously were selected by a single anti‐DNA clone 13, 14, 15, 16, 19 or by polyclonal anti‐DNA antibodies isolated from human lupus sera 17, 18, 20. Such peptides might then display biased binding potential, with potentially lower affinity for specific isotypes or antibody subsets of therapeutic importance. Finally, considering the low potential toxicity of purified peptides and the highly variable and cross‐reactive autoantibody repertoire present in vivo, using multiple peptides identified by different routes in combination may result in increased inhibition and greater clinical efficacy, without increasing side effects. Interestingly, while the sequence of the ALW peptide is quite different from that of other dsDNA mimics discussed above, it still exhibits strong affinity for all anti‐DNA isotypes, including isotypes with particularly high affinity to renal antigens 9. Therefore, the ALW peptide displays useful properties that can be helpful in inhibiting the binding of pathogenic anti‐DNA antibodies to kidney antigens.
One notable finding is the significant blocking of lupus sera binding to glomerular antigens by the ALW peptide. The MRL/lpr and Sle1.3 lupus‐prone strains have high serum titres of polyclonal anti‐DNA antibodies 32, 33, which are reactive with multiple antigens 34. Similarly, during active disease, lupus patients often exhibit elevated serum levels of anti‐DNA antibodies 35, while in a proof‐of‐concept study the removal of serum anti‐DNA antibodies by immunoadsorption reduced proteinuria and stabilized global disease activity 36. Therefore, if and when down‐regulation of pathogenic autoantibodies cannot be achieved quickly, easily or selectively by other therapeutic interventions, effective blocking of the cross‐reaction of serum anti‐DNA antibodies with self‐antigens may be efficacious in controlling organ involvement, especially renal damage. Because ALW inhibits self‐antigen binding of sera efficiently from both murine lupus models and lupus patients, at the appropriate circulating dose this peptide may reduce the nephritogenic potential of serum anti‐DNA antibodies effectively and/or down‐regulate the production of pathogenic antibodies via induction of anergy.
The primary purpose of the inhibition experiments with human lupus sera performed in this study was to serve as a proof‐of‐concept that a peptide mimic identified by mouse autoantibodies could also inhibit the binding of human lupus autoantibodies to renal antigens. Indeed, consistent with the known structural similarities between human and mouse lupus autoantibodies, we were able to conclude that murine antibody‐selected peptides are also relevant to human disease. Closer investigation of any correlation between the degree of inhibition of human lupus sera and disease features may therefore be of translational interest. Nevertheless, considering the highly variable nature of disease between individual human lupus patients (which was also reflected in their differential response to inhibition by ALW peptide), carefully examining any possible association with lupus disease severity or clinical symptoms will require a separate, and much larger, dedicated study.
In conclusion, we found that the ALW peptide markedly inhibits the binding of nephritogenic anti‐DNA antibodies and human and mouse lupus sera to multiple self‐antigens, presumably by mimicking their antigenic properties. The ALW peptide is a small molecule (MW = 1·4 kDa), which dissolves readily in either water or phosphate‐buffered saline, and which can be administered easily by intravenous injection. Furthermore, ALW may possess enhanced physiological stability because of the absence of methionine, cysteine and glutamine, which are related to the propensity for peptide oxidation, cyclization and degradation 37. Finally, the ALW peptide has a relatively neutral net charge (pI = 7·38), that would probably reduce unwanted, non‐specific protein–protein interactions. Thus, ALW or its peptide analogues are then potential candidates for a novel therapeutic approach in lupus.
Disclosure
The authors declare no financial or commercial disclosures.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Peptides selected by members of the PL9‐11 antibody panel. Using the Ph.D.™ 12‐phage display library, all peptides selected by PL9‐11 immunoglobulin (Ig)G1 (a), IgG2a (b), IgG2b (c) and IgG3 (d) are shown. The number (n) of sequence‐identical phage clones isolated by each isotype is shown on the right side of each panel. Based on their relative frequency of selection, amino acid residues at each position were selected to derive a consensus 12 amino acid peptide (ALWPPNLHAWVP).
Acknowledgements
This work was supported by grants from the NIH (AR048692 and DK090319) to C. P.). We thank Dr Huiyong Cheng (Department of Biochemistry, Albert Einstein College of Medicine) for help with the Biacore analysis.
References
- 1. Yung S, Chan TM. Anti‐DNA antibodies in the pathogenesis of lupus nephritis – the emerging mechanisms. Autoimmun Rev 2008; 7:317–21. [DOI] [PubMed] [Google Scholar]
- 2. Putterman C. New approaches to the renal pathogenicity of anti‐DNA antibodies in systemic lupus erythematosus. Autoimmun Rev 2004; 3:7–11. [DOI] [PubMed] [Google Scholar]
- 3. Qing X, Zavadil J, Crosby MB et al Nephritogenic anti‐DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum 2006; 54:2198–210. [DOI] [PubMed] [Google Scholar]
- 4. Qing X, Pitashny M, Thomas DB, Barrat FJ, Hogarth MP, Putterman C. Pathogenic anti‐DNA antibodies modulate gene expression in mesangial cells: involvement of HMGB1 in anti‐DNA antibody‐induced renal injury. Immunol Lett 2008; 121:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yung S, Tsang RC, Sun Y, Leung JK, Chan TM. Effect of human anti‐DNA antibodies on proximal renal tubular epithelial cell cytokine expression: implications on tubulointerstitial inflammation in lupus nephritis. J Am Soc Nephrol 2005; 16:3281–94. [DOI] [PubMed] [Google Scholar]
- 6. Yung S, Zhang Q, Zhang CZ, Chan KW, Lui SL, Chan TM. Anti‐DNA antibody induction of protein kinase C phosphorylation and fibronectin synthesis in human and murine lupus and the effect of mycophenolic acid. Arthritis Rheum 2009; 60:2071–82. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y, Yang J, Jiang S et al The lupus‐derived anti‐double‐stranded DNA IgG contributes to myofibroblast‐like phenotype in mesangial cells. J Clin Immunol 2012; 32:1270–8. [DOI] [PubMed] [Google Scholar]
- 8. Losman MJ, Fasy TM, Novick KE, Monestier M. Relationships among antinuclear antibodies from autoimmune MRL mice reacting with histone H2A‐H2B dimers and DNA. Int Immunol 1993; 5:513–23. [DOI] [PubMed] [Google Scholar]
- 9. Xia Y, Pawar RD, Nakouzi AS et al The constant region contributes to the antigenic specificity and renal pathogenicity of murine anti‐DNA antibodies. J Autoimmun 2012; 39:398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xia Y, Janda A, Eryilmaz E, Casadevall A, Putterman C. The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol Immunol 2013; 56:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bijl M, Dijstelbloem HM, Oost WW et al IgG subclass distribution of autoantibodies differs between renal and extra‐renal relapses in patients with systemic lupus erythematosus. Rheumatology (Oxf) 2002; 41:62–7. [DOI] [PubMed] [Google Scholar]
- 12. Krishnan MR, Wang C, Marion TN. Anti‐DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice. Kidney Int 2012; 82:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Putterman C, Diamond B. Immunization with a peptide surrogate for double‐stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med 1998; 188:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Putterman C, Deocharan B, Diamond B. Molecular analysis of the autoantibody response in peptide‐induced autoimmunity. J Immunol 2000; 164:2542–9. [DOI] [PubMed] [Google Scholar]
- 15. Beger E, Deocharan B, Edelman M, Erblich B, Gu Y, Putterman C. A peptide DNA surrogate accelerates autoimmune manifestations and nephritis in lupus‐prone mice. J Immunol 2002; 168:3617–26. [DOI] [PubMed] [Google Scholar]
- 16. Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti‐DNA antibody. Proc Natl Acad Sci USA 1997; 94:1955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang W, Reichlin M. A peptide DNA surrogate that binds and inhibits anti‐dsDNA antibodies. Clin Immunol 2005; 117:214–20. [DOI] [PubMed] [Google Scholar]
- 18. Sun Y, Fong KY, Chung MC, Yao ZJ. Peptide mimicking antigenic and immunogenic epitope of double‐stranded DNA in systemic lupus erythematosus. Int Immunol 2001; 13:223–32. [DOI] [PubMed] [Google Scholar]
- 19. Sibille P, Ternynck T, Nato F, Buttin G, Strosberg D, Avrameas A. Mimotopes of polyreactive anti‐DNA antibodies identified using phage‐display peptide libraries. Eur J Immunol 1997; 27:1221–8. [DOI] [PubMed] [Google Scholar]
- 20. Dieker JW, Sun YJ, Jacobs CW et al Mimotopes for lupus‐derived anti‐DNA and nucleosome‐specific autoantibodies selected from random peptide phage display libraries: facts and follies. J Immunol Methods 2005; 296:83–93. [DOI] [PubMed] [Google Scholar]
- 21. Bloom O, Cheng KF, He M et al Generation of a unique small molecule peptidomimetic that neutralizes lupus autoantibody activity. Proc Natl Acad Sci USA 2011; 108:10255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma A, Saha A, Bhattacharjee S, Majumdar S, Das Gupta SK. Specific and randomly derived immunoactive peptide mimotopes of mycobacterial antigens. Clin Vaccine Immunol 2006; 13:1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janda A, Eryilmaz E, Nakouzi A, Cowburn D, Casadevall A. Variable region identical immunoglobulins differing in isotype express different paratopes. J Biol Chem 2012; 287:35409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Budhai L, Oh K, Davidson A. An in vitro assay for detection of glomerular binding IgG autoantibodies in patients with systemic lupus erythematosus. J Clin Invest 1996; 98:1585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torres M, Fernández‐Fuentes N, Fiser A, Casadevall A. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J Biol Chem 2007; 282:13917–27. [DOI] [PubMed] [Google Scholar]
- 26. Faussner A, Wennerberg G, Schüssler S et al Alanine scanning of the intracellular loops of the human bradykinin B2 receptor – effects on receptor maintenance, G protein activation and internalization. FEBS J 2009; 276:3491–3503. [DOI] [PubMed] [Google Scholar]
- 27. Thueng‐in K, Thanongsaksrikul J, Srimanote P et al Cell penetrable humanized‐VH/VHH that inhibit RNA dependent RNA polymerase (NS5B) of HCV. PLoS One 2012; 7:e49254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pissawong T, Maneewatch S, Thueng‐In K et al Human monoclonal ScFv that bind to different functional domains of M2 and inhibit H5N1 influenza virus replication. Virol J 2013; 10:148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schall N, Page N, Macri C, Chaloin O, Briand JP, Muller S. Peptide‐based approaches to treat lupus and other autoimmune diseases. J Autoimmun 2012; 39:143–53. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Wang H, Zhang Q, Kim HJ, Gee SJ, Hammock BD. Phage‐displayed peptide that mimics aflatoxins and its application in immunoassay. J Agric Food Chem 2013; 61:2426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bazan J, Całkosiński I, Gamian A. Phage display–a powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum Vaccin Immunother 2012; 8:1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu J, Mohan C. SLE 1, 2, 3… genetic dissection of lupus. Adv Exp Med Biol 2007; 601:85–95. [DOI] [PubMed] [Google Scholar]
- 33. Furukawa F, Yoshimasu T. Animal models of spontaneous and drug‐induced cutaneous lupus erythematosus. Autoimmun Rev 2005; 4:345–50. [DOI] [PubMed] [Google Scholar]
- 34. Yadav P, Tran H, Ebegbe R et al Antibodies elicited in response to EBNA‐1 may cross‐react with dsDNA. PLOS ONE 2011; 6:e14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanaoka H, Okazaki Y, Satoh T et al Circulating anti‐double‐stranded DNA antibody‐secreting cells in patients with systemic lupus erythematosus: a novel biomarker for disease activity. Lupus 2012; 21:1284–93. [DOI] [PubMed] [Google Scholar]
- 36. Stummvoll GH, Schmaldienst S, Smolen JS, Derfler K, Biesenbach P. Lupus nephritis: prolonged immunoadsorption (IAS) reduces proteinuria and stabilizes global disease activity. Nephrol Dial Transplant 2012; 27:618–26. [DOI] [PubMed] [Google Scholar]
- 37. Hoeppe S, Schreiber TD, Planatscher H et al Targeting peptide termini, a novel immunoaffinity approach to reduce complexity in mass spectrometric protein identification. Mol Cell Proteomics 2011; 10:M110.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Peptides selected by members of the PL9‐11 antibody panel. Using the Ph.D.™ 12‐phage display library, all peptides selected by PL9‐11 immunoglobulin (Ig)G1 (a), IgG2a (b), IgG2b (c) and IgG3 (d) are shown. The number (n) of sequence‐identical phage clones isolated by each isotype is shown on the right side of each panel. Based on their relative frequency of selection, amino acid residues at each position were selected to derive a consensus 12 amino acid peptide (ALWPPNLHAWVP).


