Highlights
-
•
MR using hepatocyte specific contrast may potentially assess liver function.
-
•
Covariance between contrast uptake and histo-pathological scoring of liver fibrosis.
-
•
No relationship between visually assessed biliary contrast excretion and fibrosis scoring.
-
•
No relationship between visually assessed biliary excretion and contrast uptake parameters.
Abbreviations: Gd-EOB-DTPA, gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid; CLD, chronic liver disease; DCE-MRI, Dynamic Contrast Enhanced Magnetic Resonance Imaging; LSC_N, normalised liver-to-spleen contrast ratio; KHep, contrast uptake rate; ALT, alanine aminotransferase; ALP, alkaline phosphatase; NAFLD, non-alcoholic fatty liver disease; PSC, primary sclerosing cholangitis; HCV, hepatitis C; AIH, autoimmune hepatitis; AAT deficiency, α1-antitrypsin deficiency; DILI, drug induced liver injury; MANA, multi scale adaptive normalizing averaging; AUROC, area under the receiver operating characteristic curve; OATP, organic anion transporting polypeptides; MRP, multidrug resistance protein; RE, relative enhancement; FA, flip angle; SNR, signal to noise ratio
Keywords: Gd-EOB-DTPA, Dynamic contrast enhanced MRI, Liver, Bile, Excretion
Abstract
Objectives
To qualitatively evaluate late dynamic contrast phases, 10, 20 and 30 min, after administration of Gd-EOB-DTPA with regard to biliary excretion in patients presenting with elevated liver enzymes without clinical signs of cirrhosis or hepatic decompensation and to compare the visual assessment of contrast agent excretion with histo-pathological fibrosis stage, contrast uptake parameters and blood tests.
Methods
29 patients were prospectively examined using 1.5 T MRI. The visually assessed presence or absence of contrast agent for each of five anatomical regions in randomly reviewed time-series was summarized on a four grade scale for each patient. The scores, including a total visual score, were related to the histo-pathological findings, the quantitative contrast agent uptake parameters, expressed as KHep or LSC_N, and blood tests.
Results
No relationship between the fibrosis grade or contrast uptake parameters could be established. A negative correlation between the visual assessment and alkaline phosphatase (ALP) was found. Comparing a sub-group of cholestatic patients with fibrosis score and Gd-EOB-DTPA dynamic parameters did not add any additional significant correlation.
Conclusions
No correlation between visually assessed biliary excretion of Gd-EOB-DTPA and histo-pathological or contrast uptake parameters was found. A negative correlation between the visual assessment and alkaline phosphatase (ALP) was found.
1. Introduction
With the introduction in 2004 of the hepatocyte specific contrast agent, Gd-EOB-DTPA (Primovist® Bayer Schering Pharma, Berlin, Germany) a possible agent for demonstrating alterations in signal intensity (SI) related to histo-pathological changes in patients with chronic liver disease (CLD) and also evaluating liver function was present. Approximately 50% of the administered dose will be transported through the hepatocytes and excreted into the bile and the remaining 50% through the kidneys. Focus has been on the uptake of Gd-EOB-DTPA in the hepatocytes and pathophysiological conditions linked to that and a number of reports, including results from both animal and human studies, have been published. A correlation between contrast uptake and histo-pathological scoring of fibrosis has been described [1], [2], [3], [4], [5], [6], [7], and since the prognosis of CLD largely depends on the extent and progression of liver fibroisis the results may indicate the potential of dynamic contrast enhanced (DCE) MRI as a non invasive tool in the assessment of fibrosis.
There are also reports evaluating alterations in biliary and renal excretion in patients with diffuse liver disease. Tamada et al. made a qualitative analysis to visually assess the biliary excretion and found a significantly delayed biliary excretion in cirrhotic patients compared to a control group. They also found that the enhancement effects measured as the relative enhancement before and after contrast administration were significantly higher in the cirrhotic liver than those of normal liver when assessed in the renal medulla and portal vein 20 min after the contrast infusion [8].
Tschirch et al. also made a qualitative assessment of the visualization of different parts of the biliary ducts in cirrhotic patients. Only 40% of the patients showed an overall sufficient image quality for anatomical diagnosis 30 min after contrast administration. They found a cut-off value of 30 μmol/L for total bilirubin for insufficient visualization of the biliary tree for anatomical diagnosis 20 min after contrast administration [9].
Recently Dahlqvist Leinhardt et al. have proposed a procedure for quantifying the hepatocyte-specific uptake of Gd-EOB-DTPA. Based on the normalized signal intensity (SI), normalized liver-to-spleen contrast ratios (LSC_N) were calculated at 10 min (LSC_N10) and 20 min (LSC_N20) and the contrast uptake rate (KHep) was calculated by using a pharmacokinetic two-compartment model of the liver and spleen [10].
Applying this novel quantification procedure prospectively on a group of patients referred for evaluation of elevated serum alanine aminotransferase (ALT) and/or alkaline phosphatase (ALP), but without signs of liver cirrhosis in laboratory tests or at physical examination, the main finding was that liver fibrosis stage strongly influences the hepatocyte-specific uptake of Gd-EOB-DTPA [11].
The purpose of this prospective study was to compare the visual assessment of the contrast elimination via the bile ducts with (1) the histo-pathological fibrosis scoring, (2) the quantified uptake of Gd-EOB-DTPA defined as KHep and LSC_N (10 and 20 min), and (3) results from the liver and renal blood tests in order to determine the extent of possible co-variation.
2. Materials and methods
2.1. Subjects
In this prospective study, 29 patients were studied. The group consisted of 16 males (median age: 37, range: 20–57 years) and 13 females (median age: 60, range: 28–78 years). They were referred for evaluation of elevated serum ALT and/or ALP levels. Physical examination and laboratory tests revealed no signs of liver cirrhosis. Three patients were symptomatic (one with episodes of cholangitis and two with jaundice) while the remaining 26 were asymptomatic. Two male patients had a median weekly alcohol consumption exceeding 140 g (192 g and 148 g respectively).
The diagnostic work-up resulted in the following definite or plausible causes of elevated liver enzymes: non-alcoholic fatty liver disease (NAFLD) in 8 subjects, primary sclerosing cholangitis (PSC) in 7 subjects, hepatitis C (HCV) in 4 subjects, autoimmune hepatitis (AIH) in 6 subjects, primary biliary cirrhosis (PBC) in 1 subject, α1-antitrypsin deficiency (AAT deficiency) in 1 subject and drug induced liver injury (DILI) due to thioguanine in 1 subject. In the remaining one case, no diagnosis could be determined by liver biopsy.
Demographic variables, clinical diagnosis, results from liver and renal function tests are presented in Table 1.
Table 1.
Demographic variables, clinical diagnosis and results from liver and renal function tests.
| Plausible diagnosis prior to liver biopsy | Age Mean ± SD |
Sex |
BMI Mean ± SD |
Bilirubina | ASTb | ALTc | ALPd | GGTe | Kreatininf | |
|---|---|---|---|---|---|---|---|---|---|---|
| Female No of subjects | Male No of subjects | |||||||||
| NAFLD | 49.5 ± 14.9 | 4 | 4 | 28.7 ± 3.4 | 10.1 ± 3.1 | 0.7 ± 0.2 | 1.4 ± 1.1 | 1.4 ± 0.8 | 1.4 ± 1.3 | 74.6 ± 17.3 |
| PSC | 42.0 ± 20.0 | 2 | 5 | 24.6 ± 3.2 | 10.4 ± 2.9 | 1.1 ± 0.6 | 2.1 ± 1.4 | 4.1 ± 3.0 | 8.6 ± 5.8 | 81.0 ± 16.0 |
| HCV | 49.0 ± 9.1 | 2 | 2 | 25.7 ± 1.1 | 10.8 ± 3.3 | 0.9 ± 0.3 | 1.1 ± 0.4 | 1.1 ± 0.3 | 2.7 ± 2.7 | 70.5 ± 8.7 |
| AIH | 47.0 ± 16.8 | 3 | 3 | 25.0 ± 4.2 | 21.0 ± 14.7 | 1.4 ± 0.8 | 2.5 ± 1.7 | 3.4 ± 2.7 | 5.0 ± 5.3 | 66 ± 10.1 |
| PBC | 48 | 1 | – | 24.2 | 8 | 0.8 | 0.9 | 1.4 | 1.3 | 76 |
| AAT-deficiency | 66 | 1 | – | 20.2 | 12 | 0.6 | 0.3 | 0.4 | 0.6 | 89 |
| DILI (thioguanine) | 48 | – | 1 | 28.4 | 15 | 0.5 | 0.6 | 0.8 | 0.4 | 82 |
| Unclear | 35 | – | 1 | 27.4 | 10 | 1.5 | 2.0 | 1.5 | 7.4 | 95 |
| 13 | 16 | |||||||||
NAFLD: non-alcoholic fatty liver disease; PSC: primary sclerosing cholangitis; HCV: hepatitis C virus; AIH: autoimmune hepatitis; PBC: primary biliary cholangitis; AAT-deficiency: α-1-anti-trypsine deficiency; DILI, drug-induced liver injury.
Reference value:
3–20 mol/L.
<0.76 kat/L (in men), <0.61 kat/L (in women).
<1.1 kat/L (in men), <0.75 kat/L (in women).
0.6–1.8 kat/L.
0.1–1.3 kat/L.
50–90 μmol/L (women), 60–100 μmol/L (men).
The study was approved by the regional ethics committee in Linköping, Sweden, registration number M72-07 T5-08. Patients participated in this study after their informed consent was obtained.
2.2. Liver biopsy and histo-pathology
Liver biopsy was performed on the same day immediately after the MR-examination on an outpatient basis using a 1.6 mm Biopince needle (Medical Device Technologies Inc., FL, USA) in order to assess the histological severity of the underlying liver disease, to confirm the plausible diagnosis or, in the case with the single patient in whom prior diagnostic work-up was negative, to elucidate the reason for the elevated liver enzymes. Biopsies were graded and classified according to the Batts and Ludwig system, i.e. fibrosis was staged as: no fibrosis (F0), portal fibrosis (F1), periportal fibrosis (F2), septal fibrosis (F3) and probable or obvious cirrhosis (F4). [12]. All biopsies were read by the same liver pathologist, who was blinded to patient details and the MR examination results.
2.3. Data acquisition
The data were acquired as previously described [10], [11]. A 1.5 T Achieva MR-scanner (Philips Healthcare, Best, The Netherlands) was used together with a phase-array body coil with 16 coil elements. Single breath-hold symmetrically sampled two-point Dixon 3D images [11] were acquired with sensitivity encoding (SENSE).
All subjects received a bolus injection of Gd-EOB-DTPA (0.025 mmol/kg) administered intravenously at a rate of 1 mL/s using a power injector (Medrad Spectris Solaris, Pittsburgh, PA, USA) followed by a 30 mL saline flush. Image time-series were acquired pre- and post-contrast injection (native, arterial and venous portal phase, 3, 10, 20 and 30 min post-injection). The delayed and non-enhanced images were captured using the following parameters: repetition time = 6.5 ms, echo time = 2.3 and 4.6 ms, flip angle = 13°, typical acquisition matrix = 168/168, typical FOV = 261 mm × 200 mm × 342 mm, slice thickness = 4 mm, slice interval 2 mm, typical imaging time = 20.2 s.
The acquired in- and opposed-phase images were reconstructed into water and fat images using the inverse gradient method [13], [14], [15]. In order to correct for intensity heterogeneity in water/fat Dixon images, and to gain reference scaling throughout the time-series, the intensity of voxels identified as containing pure adipose tissue was used as an internal reference. This correction was performed using the multi scale adaptive normalizing averaging (MANA) method [16], [17], [18].
Quantitative measurements of the uptake of Gd-EOB-DTPA, defined as KHep and LSC_N (10 and 20 min), were performed as previously described, [10], [11].
2.4. Image analysis
The qualitative analysis of the bile duct excretion of Gd-EOB-DTPA was based on visual consensus reading of the water – only images performed by two experienced radiologists (B.N. and N.D.) on axial reformats. The readers were blinded to patient and time point data as well as histo-pathological findings. Axials of the 10, 20 and 30 min post-contrast image time-series for all 29 patients were reviewed randomly.
Five anatomical regions (peripheral bile ducts in the left and right liver lobe, right and left intra hepatic main branch and the extra hepatic bile ducts) for each time-series were assessed and the presence of contrast agent was graded as 1 (‘yes‘) or 0 (‘no’).
The presence of Gd-EOB-DTPA in each anatomical region was after the reviewing process summarized on a four graded scale; 3 = contrast visible at 10 min, 2 = contrast visible at 20 min but not at 10 min, 1 = contrast visible at 30 min but not at 10–20 min, 0 = no contrast visible at 10–30 min.
Finally the five scores, one for each anatomical region, as well as a total visual score, obtained by adding the five separate scores, were related to the histo-pathological findings, the quantitative contrast agent uptake parameters and blood tests.
2.5. Statistics
Statistical analysis was performed using Stata 12.0 (StataCorp, College Station, TX, USA). Results are reported as means and standard deviation.
Spearman rank correlation was used to compare visual assessment of bile duct excretion of Gd-EOB-DTPA vs. histo-pathological grading of fibrosis, described by the fibrosis score (F0–F4) as well as by a binary variable indicating the presence or absence of advanced fibrosis or cirrhosis (F3–4), dynamic contrast enhancement parameters and liver and renal blood tests. Diagnostic ability of visually assessed bile duct excretion of Gd-EOB-DTPA with respect to the presence or absence of advanced fibrosis (F3–4) was performed by calculating the area under the receiver operating characteristic curve (AUROC) with a 95% confidence interval.
A p value < 0.05 was considered statistically significant.
3. Results
3.1. Final diagnosis and fibrosis scoring
The subject in whom no plausible diagnosis could be established prior to liver biopsy had minor histo-pathological changes in accordance with autoimmune hepatitis. The patient who took thioguanine was diagnosed with NAFLD and had no histo-pathological evidence of drug-induced liver injury. In three patients with suspected autoimmune hepatitis histo-pathological examination confirmed this diagnosis. They also showed findings in accordance with overlap syndrome (one with PBC and two with PSC). In the remaining 24 patients liver biopsy confirmed the diagnosis. The final diagnosis and fibrosis scoring are shown in Table 2.
Table 2.
Final diagnosis and fibrosis scoring.
| Final diagnosis | F0 | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| NAFLD | 5 | – | 3 | – | 1 |
| PSC | – | 2 | 2 | 3 | – |
| HCV | – | 2 | 2 | – | – |
| AIH | 1 | 2 | 2 | 1 | 1 |
| PBC | – | – | 1 | – | – |
| AAT-deficiency | – | – | – | 1 | – |
| Total no of subjects | 6 | 6 | 10 | 5 | 2 |
Data are presented as number of subjects. NAFLD: non-alcoholic fatty liver disease; PSC: primary sclerosing cholangitis; HCV: hepatitis C virus; AIH: autoimmune hepatitis; PBC: primary biliary cholangitis; AAT-deficiency: α-1-anti-trypsine deficiency.
3.2. Analysis of Gd-EOB-DTPA excretion
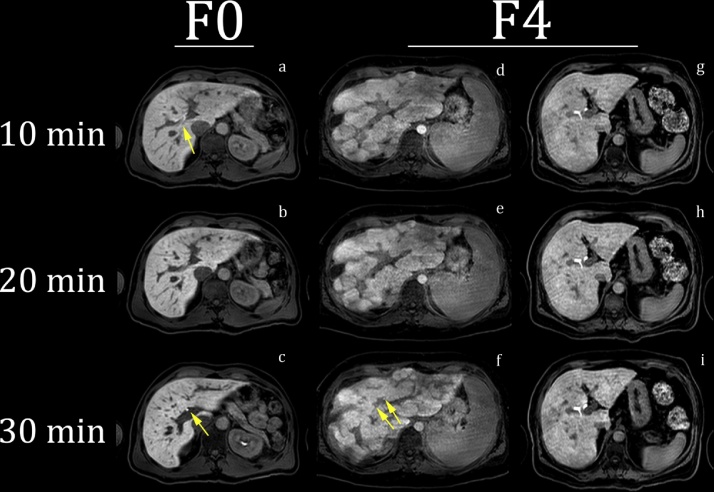
The Gd-EOB-DTPA excretion was visually assessed vs. histopathology, quantitative contrast uptake parameters and blood tests. Fig. 1 illustrates the evaluation of the three time series.
Fig. 1.
Example of Gd-EOB-DTPA excretion in the bile ducts in the tree time series in one F0 patient with the diagnosis of AIH, panels a–c, and two F4 patients with the diagnosis of AIH and NAFLD, panels d, f and g, i, respectively. In the F0 patient intrahepatic contrast agent is observed at 10 min, indicated with an arrow in image a. A reduced amount of contrast in central intra hepatic bile ducts was observed between 10 and 20 min. Contrast in the CBD, as indicated with an arrow at 30 min in image c, was observed for all time series (not shown). The difference in contrast excretion between the two F4 patient may be noted – only poorly visible contrast at 30 min in central intra hepatic bile ducts in patient d–f indicated with arrows in image f.
3.2.1. Gd-EOB-DTPA excretion vs. histopathology
No significant correlation between visual assessment of bile duct excretion of Gd-EOB-DTPA and histo-pathological grading of fibrosis was found, Table 3. The diagnostic ability of visually assessed bile duct excretion of contrast is shown in Table 4.
Table 3.
Visual assessment of bile duct excretion of Gd-EOB-DTPA vs. histopathological grading of fibrosis.
| Extra hepatic bile duct | Main duct right liver lobe | Segmental branches right liver lobe | Main duct left liver lobe | Segmental branches left liver lobe | Total visual score | |
|---|---|---|---|---|---|---|
| Fibrosis grade (F0–4) | −0.34 | −0.18 | −0.13 | −0.20 | −0.12 | −0.17 |
| Advanced fibrosis (F3–4) vs. F0–2. | −0.30 | −0.10 | −0.03 | −0.07 | −0.08 | −0.06 |
Spearman correlation (ρ). No correlations are significant (all p > 0.05).
Table 4.
Diagnostic ability of visually assessed bile duct excretion of Gd-EOB-DTPA with respect to the presence or absence of advanced fibrosis (F3–4).
| AUROC | 95% confidence interval | |
|---|---|---|
| Extra hepatic bile duct | 0.67 | 0.44–0.90 |
| Main duct right liver lobe | 0.56 | 0.32–0.80 |
| Segmental branches right liver lobe | 0.51 | 0.30–0.74 |
| Main duct left liver | 0.54 | 0.29–0.79 |
| Segmental branches left liver lobe | 0.55 | 0.31–0.79 |
| Total visual score | 0.54 | 0.23–0.86 |
Area under the receiver operating characteristic curve (AUROC) with 95% confidence interval.
3.2.2. Gd-EOB-DTPA excretion vs. quantitative contrast uptake parameters
No significant correlation between visual assessment, one score for each anatomical region as well as a total visual score, and the quantified uptake of Gd-EOB-DTPA defined as KHep and LSC_N was found, see Table 5.
Table 5.
Visual assessment of bile duct excretion of Gd-EOB-DTPA vs. dynamic contrast enhancement parameters.
| Extra hepatic bile duct | Main duct right liver lobe | Segmental branches right liver lobe | Main duct left liver lobe | Segmental branches left liver lobe | Total visual score | |
|---|---|---|---|---|---|---|
| kHep | 0.01 | 0.04 | −0.03 | 0.13 | −0.01 | −0.03 |
| LSC10 | 0.07 | 0.21 | 0.17 | 0.30 | 0.04 | 0.20 |
| LSC20 | 0.05 | 0.21 | 0.21 | 0.30 | 0.07 | 0.22 |
| LSC_N10 | 0.25 | 0.24 | −0.30 | 0.30 | −0.14 | −0.08 |
| LSC_N20 | 0.21 | 0.23 | −0.17 | 0.29 | −0.09 | −0.03 |
Spearman correlation (ρ). No correlations are significant (all p > 0.05).
3.2.3. Gd-EOB-DTPA excretion vs. blood tests
Concerning correlation between visual assessment and liver and renal blood tests significant negative correlations were found between ALP and the visual assessment of bile duct excretion in several anatomic regions see Table 6.
Table 6.
Visual assessment of bile duct excretion of Gd-EOB-DTPA vs. liver and renal blood tests.
| Extra hepatic bile duct | Main duct right liver lobe | Segmental branches right liver lobe | Main duct left liver lobe | Segmental branches left liver lobe | Total visual score | |
|---|---|---|---|---|---|---|
| Bilirubin | 0.19 | 0.21 | 0.18 | 0.20 | −0.20 | 0.13 |
| Albumin | 0.07 | 0.09 | 0.04 | 0.06 | 0.31 | 0.14 |
| AST | −0.06 | −0.02 | −0.16 | −0.08 | −0.20 | −0.12 |
| ALT | 0.05 | 0.12 | −0.10 | 0.13 | −0.24 | −0.06 |
| ALP | −0.42* | −0.47* | −0.36 | −0.42* | −0.20 | −0.43* |
| GGT | −0.18 | −0.20 | −0.24 | −0.22 | −0.08 | −0.19 |
| Kreatinin | 0.18 | 0.21 | 0.03 | 0.22 | 0.01 | 0.14 |
Spearman correlation (ρ).
Significance level, p < 0.05.
4. Discussion
In this prospective study, with patients without any signs of cirrhosis at the time of inclusion and all patients corresponding to grade “A” according to the Child–Pugh scoring system, the qualitative assessment of contrast excretion could not establish a relationship between the fibrosis grade or contrast uptake parameters expressed as KHep or LSC_N. In a recent prospective study however, where physical examination and laboratory tests likewise could not reveal any signs of liver cirrhosis, we found that liver fibrosis stage strongly influences the hepatocyte-specific uptake of Gd-EOB-DTPA [11].
The hepatobiliary molecular transport system is complex and includes multiple substrate specific uptake and export proteins located at the hepatocyte sinusoidal and canalicular membranes, which differ in their biochemical and functional characteristics. Bile formation is dependent on these mechanisms and dysfunction at the level of the hepotocyte may result in e.g. cholestasis. Other reasons for cholestasis are malfunction of the bile secretion and impaired flow in the bile ducts. In order to protect the hepatocytes from intracellular retention of toxic bile acids alterations in the transport mechanism may occur [19], [20], [21].
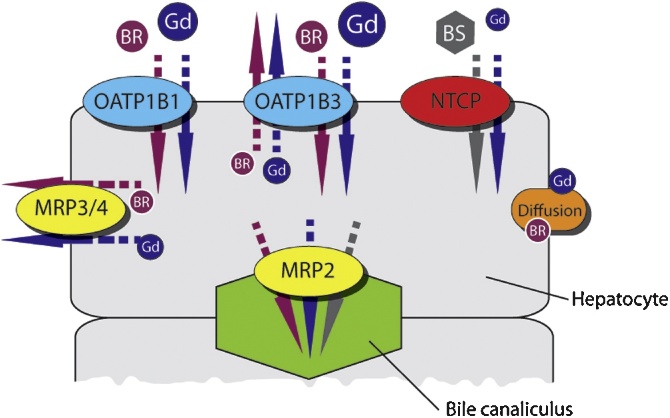
Gd-EOB-DTPA is transported over the sinusoidal membrane via the organic anion transporting polypeptides OATP1B1 and OATP1B3. Bidirectional transport is seen within the OATP's. Gd-EOB-DTPA is not metabolized within the hepatocytes and excreted into the bile through the multidrug resistance protein 2, MRP2. The MRP2 excretion is a unidirectional ATP-dependant transporter; moreover MRP2 has a limited rate which in turn yields retention of Gd-EOB-DTPA in the hepatocytes. Located at the sinusoidal membrane there are two other members of the MRP family, the MRP3 and MRP4, which may be expressed and up-regulated under cholestatic conditions returning bile salt to portal circulation. [21], [22]. See Fig. 2.
Fig. 2.
Uptake of liver specific contrast agent, Gd-EOB-DTPA (‘Gd’), bilirubin (‘BR’) and bile salts (‘BS’) into hepatocytes. The colored arrows correspond to direction of transport as the transported molecules and MRP3/4 and MRP2 represent transporting proteins other than diffusion; MR2 into the bile canaliculus. The sodium/taurocholate co-transporting polypeptide (NCTP) is also to some extent involved in the uptake of Gd-EOB-DTPA. The size of the symbols reflects their relative importance. There is a very strong competition between the contrast agent and naturally occurring molecules. Appreciate the many different possible routes for flux contrast agent into and out of the hepactocytes. Omitted in this picture is the transporting protein OATP2 which facilitates bile salts and bilirubin transfer into the hepatocyte [18].
In advanced liver disease such as cirrhosis the uptake system is likely to be hampered via a number of processes such as a lower OATP1 activity as described by van Beers et al., a competitively reduced Gd-EOB-DTPA uptake due to high concentrations of other metabolites such as bilirubin and a reduced amount of hepatocytes. The findings by Tamada et al. of a prolonged relative enhancement (RE) of the portal vein and a higher RE of the renal medulla suggest a compensatory increase in the renal excretion of Gd-EOB-DTPA could support this as well as the finding by Tschirch et al. of a cut of value for total bilirubin of ≥30 μmol/L for insufficient visualization of the biliary tree for anatomical diagnosis 20 min after contrast administration [8], [9], [22]. But to what extent is the balance between uptake and excretion of the contrast agent affected with regard to the fibrosis stage?
Is the dominating feature in healthier patients a disturbance in uptake mechanism and in clinically severe cases a shift toward a gradually more impaired excretion? Primarily cholestatic diseases such as PSC and PBC will have to be excluded from this assumption since they primarily affect the excretion mechanism and decreased expression of MRP2 is seen in PBC and PSC patients [20], [23].
Interestingly enough, we found a negative correlation between the visual assessment and ALP, elevated levels indicating intra-hepatic cholestasis. Additional statistical analysis, comparing the sub-group of cholestatic patients (n = 8), assuming a connection between PSC and PBC and elevated ALP, with fibrosis score and Gd-EOB-DTPA dynamic parameters did not add additional significant correlation.
In order to further improve the evaluation of the hepatocyte excretion pharmaco-dynamics of Gd-EOB-DTPA the following measures may be considered: (1) to acquire time series with shorter intervals, thereby achieving more detailed information concerning contrast timing and characteristics (e.g. 2 min), (2) to include coronal reformats in the evaluation process and (3) to increase the flip angle (FA) thereby gaining a stronger T1 weighting and an improved (signal to noise ratio) SNR.
5. Conclusions
Visually assessed biliary excretion of Gd-EOB-DTPA could not establish a relationship between the fibrosis grade or contrast uptake parameters expressed as KHep or LSC_N. However, a negative correlation between the visual assessment and ALP was found. The complexity in uptake/excretion mechanism is highlighted by the finding in a previous study where liver fibrosis stage strongly influences the hepatocyte-specific uptake of Gd-EOB-DTPA. Further methodological improvements and larger prospective studies are desirable.
Acknowledgements
Financial support from the Swedish Research Council (VR/M 2007-2884), the Medical Research Council of Southeast Sweden (FORSS 12621), the Linköping University, Linköping University Hospital Research Foundations and the County Council of Östergötland are gratefully acknowledged.
Contributor Information
Bengt Norén, Email: bengt.noren@gmail.com.
Nils Dahlström, Email: nils.dahlstrom@liu.se.
Mikael Fredrik Forsgren, Email: mikaelf@wolfram.com.
Olof Dahlqvist Leinhard, Email: olof.dahlqvist.leinhard@liu.se.
Stergios Kechagias, Email: stergios.kechagias@liu.se.
Sven Almer, Email: sven.almer@ki.se.
Staffan Wirell, Email: staffan.wirell@liu.se.
Örjan Smedby, Email: orjan.smedby@liu.se.
Peter Lundberg, Email: peter.lundberg@liu.se.
References
- 1.Tsuda N., Okada M., Murakami T. New proposal for the staging of nonalcoholic steatohepatitis: evaluation of liver fibrosis on Gd-EOB-DTPA-enhanced MRI. Eur J Radiol. 2010;73:137–142. doi: 10.1016/j.ejrad.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 2.Ryeom H.K., Kim S.H., Kim J.Y., Kim H.J., Lee J.M., Chang Y.M. Quantitative evaluation of liver function with MRI using Gd-EOB-DTPA. Korean J Radiol. 2014;5:231–239. doi: 10.3348/kjr.2004.5.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motosugi U., Ichikawa T., Sou H., Sano K., Tominaga L., Kitamura T. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement. J Magn Reson Imaging. 2009;30:1042–1046. doi: 10.1002/jmri.21956. [DOI] [PubMed] [Google Scholar]
- 4.Yamada A., Hara T., Li F., Fujinaga Y., Ueda K., Kadoya M. Quantitative evaluation of liver function with use of gadoxetate disodium-enhanced MR imaging. Radiology. 2011;260:727–733. doi: 10.1148/radiol.11100586. [DOI] [PubMed] [Google Scholar]
- 5.Lee W.J., Cha S.H., Kim M.Y., Chung H.H., Lee S.W., Yi A. Quantitative evaluation of the hepatic parenchymal change in patients with chronic liver disease using Gd-EOB-DTPA-enhanced MRI: comparison with normal liver. J Korean Soc Radiol. 2011;64:49–55. [Google Scholar]
- 6.Watanabe H., Kanematsu M., Goshima S., Kondo H., Onozuka M., Moriyama N. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging – preliminary observations. Radiology. 2011;259:142–150. doi: 10.1148/radiol.10100621. [DOI] [PubMed] [Google Scholar]
- 7.Chen B.B., Hsu C.Y., Yu C.W., Wei S.Y., Kao J.H., Lee H.S. Dynamic contrast-enhanced magnetic resonance imaging with Gd-EOB-DTPA for the evaluation of liver fibrosis in chronic hepatitis patients. Eur Radiol. 2012;22:171–180. doi: 10.1007/s00330-011-2249-5. [DOI] [PubMed] [Google Scholar]
- 8.Tamada T., Ito K., Sone T., Kanki A., Sato T., Higashi H. Gd-EOB-DTPA enhanced MR imaging: evaluation of biliary and renal excretion in normal and cirrhotic livers. Eur J Radiol. 2011;8:e207–e211. doi: 10.1016/j.ejrad.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 9.Tshirch F., Struwe A., Petrowsky H., Kakales I., Marinec B., Weishaupt D. Contras-enhanced MR cholangiography with Gd-EOB-DTPA in patients with liver cirrhosis: visualization of the biliary ducts in comparison with patients with normal liver parenchyma. Eur Radiol. 2008;18:1577–1586. doi: 10.1007/s00330-008-0929-6. [DOI] [PubMed] [Google Scholar]
- 10.Dahlqvist Leinhard O., Dahlstrom N., Kihlberg J., Sandstrom P., Brismar T.B., Smedby O. Quantifying differences in hepatic uptake of the liver specific contrast agents Gd-EOB-DTPA and Gd-BOPTA: a pilot study. Eur Radiol. 2012;22:642–653. doi: 10.1007/s00330-011-2302-4. [DOI] [PubMed] [Google Scholar]
- 11.Noren B., Forsgren M.F., Dahlqvist Leinhard O., Dahlström N., Kihlberg J., Romu T. Separation of Advanced from mild fibrosis by quantification of the hepatobiliary uptake of Gd-EOB-DTPA. Eur Radiol. 2013;23:174–181. doi: 10.1007/s00330-012-2583-2. [DOI] [PubMed] [Google Scholar]
- 12.Batts K.P., Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Rydell J., Knutsson H., Pettersson J., Johansson A., Farnebäck G., Dahlqvist O. Phase sensitive reconstruction for water/fat separation in MR imaging using inverse gradient. Med Image Comput Comput Assist Interv. 2007;10:210–218. doi: 10.1007/978-3-540-75757-3_26. [DOI] [PubMed] [Google Scholar]
- 14.Rydell J., Johansson A., Leinhard O.D., Knutsson H., Farnebäck G., Lundberg P. Proc of the int soc mag reson in med (ISMRM’08), vol. 16. 2008. Three dimensional phase sensitive reconstruction for water/fat separation in MR imaging using inverse gradient; p. 1519. [Google Scholar]
- 15.Romu T., Dahlqvist Leinhard O., Forsgren M.F., Almer S., Dahlström N., Kechagias S. Proc of the int soc mag reson in med (ISMRM’11), vol. 19. 2011. Fat water classification of symmetrically sampled two-point dixon images using biased partial volume effects; p. 2711. [Google Scholar]
- 16.Dahlqvist Leinhard O., Johansson A., Rydell J., Smedby O., Nystrom F., Lundebrg P. Proceedings of the 19th international conference of pattern recognition (ICPR’08) 2008. Quantitative abdominal fat estimation using MRI. [Google Scholar]
- 17.Romu T., Borga M., Dahlqvist O. Proceedings in the IEEE international symposium on biomedical imaging; from nano to macro (ISBI’11) 2011. MANA – multi scale adaptive normalized averaging; pp. 361–364. [Google Scholar]
- 18.Andersson T., Romu T., Norén B., Forsgren M., Smedby Ö., Almer S. Proc of the int soc mag reson in med (ISMRM’12), vol. 20. 2012. Selfcalibrated DCE-MRI using Scale Adaptive Normalized Averaging (MANA) p. 2445. [Google Scholar]
- 19.Müller M., Jansen P.L. Molecular aspects of hepatobiliary transport. Am J Physiol. 1997;272(June (6 Pt 1)):G1285–G1303. doi: 10.1152/ajpgi.1997.272.6.G1285. [DOI] [PubMed] [Google Scholar]
- 20.Zollner G., Trauner M. Mechanisms of cholestasis. Clin Liver Dis. 2008;12:1–26. doi: 10.1016/j.cld.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Trauner M., Boyer J.L. Bile salt transporters: molecular characterization, function and regulation. Physiol Rev. 2003;83(2):633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 22.Van Beers B.E., Pastor C.M., Hussain H.K. Primovist, eovist: what to expect? J Hepatol. 2012;57(August (2)):421–429. doi: 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Kullak-Ublick G.A., Baretton G.B., Oswald M., Renner E.L., Paumgartner G., Beuers U. Expression of the hepatocyte canalicular multidrug resistance protein (MRP2) in primary biliary cirrhosis. Hepatol Res. 2002;23(1):78–82. doi: 10.1016/s1386-6346(01)00159-0. [DOI] [PubMed] [Google Scholar]