Abstract
Complex III deficiency due to a MT-CYB mutation has been reported in patients with myopathy. Here, we describe a 15-year-old boy who presented with metabolic acidosis, ketotic hypoglycemia and carnitine deficiency. Electron transport chain analysis and mitochondrial DNA sequencing on muscle tissue lead to the eventual diagnosis of complex III deficiency. This case demonstrates the critical role of muscle biopsies in a myopathy work-up, and the clinical efficacy of supplement therapy.
Abbreviations: GSD, glycogen storage disease; CK, creatine phosphokinase; MRS, magnetic resonance spectroscopy; MCT, medium chain triglyceride; G-tube, gastric feeding tube; CSF, cerebrospinal fluid; ETC, electron transport chain; mtDNA, mitochondrial DNA
Keywords: Mitochondrial myopathy, Cytochrome b, Complex III deficiency, Ketotic hypoglycemia, Carnitine deficiency
1. Introduction
Mitochondrial complex III in humans consists of 11 subunits, all of which are encoded by nuclear genes except for cytochrome b (EC 1.10.2.2) which is encoded by MT-CYB in the mitochondrial genome [1]. Complex III deficiency is most commonly caused by mutations in MT-CYB or the nuclear gene BCS1L. Mutations in MT-CYB cause isolated complex III deficiency leading to a variety of symptoms including failure to thrive, exercise intolerance, cardiomyopathy, encephalomyopathy, and Leber hereditary optic neuropathy [2], [3], [4], [5]. Some phenotypic variability is thought to be related to the mutant load or types of mutation. In-frame deletions in the gene have been reported in patients with exercise intolerance [6], [7], or with additional complications of hearing loss, visual impairment, brain atrophy, cardiomyopathy and gastroparesis [3]. MT-CYB mutations are often undetectable in the blood or skin fibroblast and require testing of muscle mtDNA. We report a fifteen-year-old boy who was initially treated as having a glycogen storage disease (GSD); however a muscle biopsy led to an eventual diagnosis of complex III deficiency.
2. Case report
The patient presented at age seven years with intermittent vomiting, diarrhea, constipation, weight loss and fatigue that has persisted for over 18 months. Routine work-up showed significant metabolic acidosis with low serum bicarbonate concentrations. He was started on oral sodium citrate and admitted for evaluation, which revealed low serum glucose of 3.05 mmol/L (55 mg/dL) and very low plasma l-carnitine (total carnitine:7 μmol/L, reference range: 25–69). Serum creatine phosphokinase (CK) and transaminases were elevated and continued to increase as he clinically deteriorated, with CK peaking at 202 μkat/L (12,086 U/L), AST at 341 U/L and ALT at 190 U/L. Ophthalmologic and audiologic exams were normal. Differential diagnoses included fatty acid oxidation defects, GSDs, and mitochondrial oxidative phosphorylation disorders. The patient had started on carnitine and cornstarch but continued to deteriorate, with multiple admissions for ketotic hypoglycemia and severe lactic acidosis. Brain magnetic resonance imaging was normal, while brain magnetic resonance spectroscopy (MRS) showed lactate peaks in the left basal ganglion and lateral ventricle. As this finding was suggestive of a mitochondrial disorder, treatment with coenzyme Q10, riboflavin, creatine monohydrate, alpha-lipoic acid [8], and medium chain triglyceride (MCT) oil was initiated, while continuing carnitine supplementation. Leucovorin was later added due to a low cerebrospinal fluid (CSF) 5-methyltetrahydrofolate level. At age eight, he required a gastric feeding tube (G-tube) placement for malnutrition and continued weight loss.
The urine organic acid profile was notable for elevations of lactate, ethylmalonate, 3-methylglutaconate, and branched-chain ketoacids, suggestive of mitochondrial dysfunction. Cultured skin fibroblast testing was performed for suspected defects in fatty acid oxidation, pyruvate carboxylase (EC 6.4.1.1), and pyruvate dehydrogenase complex (EC 1.2.4.1, EC 2.3.1.12, EC 1.8.1.4) with normal results [9]. Analysis of acylcarnitines in cultured skin fibroblasts incubated with palmitic acid and l-carnitine (in vitro probe assay) showed marked elevations of long-chain species, suggestive of carnitine palmitoyltransferase II or carnitine–acylcarnitine translocase deficiency. CSF analysis showed mild elevation of lactate of 3.1 mmol/L (< 2.8), and a low normal level of 5-methyltetrahydrofolate. Transaminitis was initially attributed to a possible GSD; glycogen content was elevated in both liver (10%; control range 3.3 +/− 1.7%) and muscle (3.0%; control range 0.94 +/− 0.55). However, sequencing of PHKA1 and PHKG2 genes (GSD IX) and AGL gene (GSD III) was normal. Activities of debranching enzyme (EC 2.4.1.25 GSD III) and hepatic phosphorylase kinase (GSD IX, EC 2.7.11.19) in the liver and muscle, and glucose-6-phosphatase (GSD Ia, EC 3.1.3.9) in the liver were also normal.
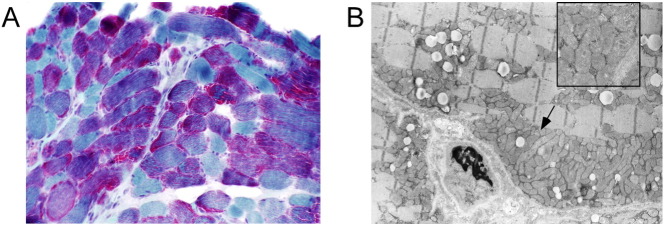
Further evaluation for a suspected mitochondrial disorder revealed normal electron transporter chain (ETC) activity in cultured skin fibroblasts. Muscle histopathology demonstrated prominent granular red staining of the fibers with a trichrome stain and numerous mitochondria aggregates by electron microscopy, characteristic of a mitochondrial myopathy (Fig. 1). ETC testing on frozen muscle revealed deficient complex III activity of 455 (mean 1461, standard deviation 473); mtDNA sequencing of the muscle sample revealed a 9 basepair deletion with loss of 3 amino acids Leu-Ala-Thr (m.15319_15327delCCTAGCAAC; p.Leu192_Thr194del, Fig. 2) in the MT-CYB gene encoding cytochrome b subunit of complex III, with > 90% mutant load, confirming complex III deficiency. MtDNA sequencing in the liver and blood did not reveal the mutation, although low heteroplasmy for the mutation was detected in uncultured skin fibroblasts.
Fig. 1.
A. Trichrome stain. Marked increase in red-staining indicates excess of mitochondria. 10 × objective. B. Electron micrograph. Confirmation of excess mitochondria. Original magnification × 4400; inset × 16900.
Fig. 2.
Cross-species sequence comparison of m.15319_15327.
Since the diagnosis, ongoing cardiac evaluations to monitor for cardiomyopathy or rhythm disturbance have been normal. The child dramatically improved with the start of G-tube feeding and dietary supplements, with increased energy level, improved exercise tolerance and progress in academic performance. Previously confined to a wheel chair, he started to walk independently and actively. Lactic acid, transaminases and CK levels have stabilized, although lactate showed occasional elevations as high as 11 mmol/L without symptoms of metabolic decompensation. His G-tube was removed at age 12 years and he stopped taking supplements, and he continues to do remarkably well physically.
3. Discussion
This case demonstrates mitochondrial complex III deficiency due to an in-frame deletion mutation in MT-CYB encoding cytochrome b. This deletion occurs near the iron binding site for heme b566 at amino acid 196. The two cytochrome b iron containing heme groups are critical to electron transport through complex III. Low heteroplasmy for the mutation in the skin tissue and elevated lactate and pyruvate in CSF suggest that the mutation is present across different tissues. The patient was characterized by failure-to-thrive, recurrent emesis, ketotic hypoglycemia, lactic acidosis, and urine organic acid abnormalities, mimicking GSDs. Additionally, CK was markedly elevated, a common finding in patients with a cytochrome b mutation. Difficulty gaining weight, hypoglycemia, profound deconditioning and CK elevation were not observed in the prior cases with isolated exercise intolerance [6], [7]. He would need a surveillance for heart, hearing and vision impairment as reported in a case with 18 basepair in-frame deletion [3]. A definitive diagnosis required histology, ETC analysis and mtDNA sequencing in a muscle specimen. Effective treatment included proper nutrition with dietary supplements and resulted in a dramatic clinical improvement.
While mutant load or inheritance was not confirmed in our case, the absence or extremely low levels of the mutation in tissues with low replicative potential suggests that segregation varied significantly during embryogenesis [7]. The absence of MT-CYB mutations in blood complicates clinical diagnosis and testing of at risk relatives for genetic counseling.
In general, mitochondrial disorders can present with non-specific symptoms as described. Considerable phenotypic overlap exists among mitochondrial disorders. Diagnosis of mtDNA disorders is also complicated by heteroplasmy [1]. MtDNA sequencing in leukocytes may not necessarily detect mutations present in affected tissues due to different heteroplasmic levels across tissues. Thus a muscle biopsy may be required to establish a diagnosis. As abnormalities of mitochondrial oxidative phosphorylation in muscle may occur secondary to other disorders, mtDNA analysis in muscle is also important for confirmation [8].
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Research grants to reference: John Shoffner, M.D. was supported by the Department of Defense under award numbers W81XWH-09-1-0367 and W81XWH-10-1-0547. Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
References
- 1.Bénit P., Lebon S., Rustin P. Respiratory-chain diseases related to complex III deficiency. Biochim. Biophys. Acta BBA - Mol. Cell Res. 2009;1793:181–185. doi: 10.1016/j.bbamcr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Keightley J.A., Anitori R., Burton M.D., Quan F., Buist N.R.M., Kennaway N.G. Mitochondrial encephalomyopathy and complex III deficiency associated with a stop-codon mutation in the cytochrome b gene. Am. J. Hum. Genet. 2000;67:1400–1410. doi: 10.1086/316900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carossa V., Ghelli A., Tropeano C.V., Valentino M.L., Iommarini L., Maresca A. A novel in-frame 18-bp microdeletion in MT-CYB causes a multisystem disorder with prominent exercise intolerance. Hum. Mutat. 2014;35:954–958. doi: 10.1002/humu.22596. [DOI] [PubMed] [Google Scholar]
- 4.Emmanuele V., Sotiriou E., Rios P.G., Ganesh J., Ichord R., Foley A.R. A novel mutation in the mitochondrial DNA cytochrome b gene (MTCYB) in a patient with mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes syndrome. J. Child Neurol. 2013;28:236–242. doi: 10.1177/0883073812445787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaral-Fernandes M.S., Marcondes A.M., Miranda P.M.d.A.D., Maciel-Guerra A.T., Sartorato E.L. Mutations for Leber hereditary optic neuropathy in patients with alcohol and tobacco optic neuropathy. Mol. Vis. 2011;17:3175–3179. [PMC free article] [PubMed] [Google Scholar]
- 6.Taivassalo T., Shoubridge E.A., Chen J., Kennaway N.G., DiMauro S., Arnold D.L. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann. Neurol. 2001;50:133–141. doi: 10.1002/ana.1050. [DOI] [PubMed] [Google Scholar]
- 7.Andreu A.L., Hanna M.G., Reichmann H., Bruno C., Penn A.S., Tanji K. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N. Engl. J. Med. 1999;341:1037–1044. doi: 10.1056/NEJM199909303411404. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez M.C., MacDonald J.R., Mahoney D.J., Parise G., Beal M.F., Tarnopolsky M.A. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 9.Shen J.J., Matern D., Millington D.S., Hillman S., Feezor M.D., Bennett M.J. Acylcarnitines in fibroblasts of patients with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency and other fatty acid oxidation disorders. J. Inherit. Metab. Dis. 2000;23:27–44. doi: 10.1023/a:1005694712583. [DOI] [PubMed] [Google Scholar]