Abstract
Background
Screening for Fabry disease (FD) in high risk populations yields a significant number of individuals with novel, ultra rare genetic variants in the GLA gene, largely without classic manifestations of FD. These variants often have significant residual α-galactosidase A activity. The establishment of the pathogenic character of previously unknown or rare variants is challenging but necessary to guide therapeutic decisions.
Objectives
To present 2 cases of non-classical presentations of FD with renal involvement as well as to discuss the importance of high risk population screenings for FD.
Results
Our patients with non-classical variants were diagnosed through FD screenings in dialysis units. However, organ damage was not limited to kidneys, since LVH, vertebrobasilar dolichoectasia and cornea verticillata were also present. Lyso-Gb3 concentrations in plasma were in the pathologic range, compatible with late onset FD. Structural studies and in silico analysis of p.(Cys174Gly) and p.(Arg363His), employing different tools, suggest that enzyme destabilization and possibly aggregation could play a role in organ damage.
Conclusions
Screening programs for FD in high risk populations are important as FD is a treatable multisystemic disease which is frequently overlooked in patients who present without classical manifestations.
Keywords: Fabry disease, GLA variants, Late onset variants, Lyso-Gb3
1. Introduction
Fabry disease (FD, MIM #301500) is an inherited metabolic disorder that results from the deficient activity of the lysosomal enzyme α-galactosidase A (α-Gal A, EC 3.2.1.22) [1]. This lysosomal hydrolase, encoded by the GLA gene (locus Xq22.1), catalyzes the removal of terminal α-linked galactosyl residues from neutral glycosphingolipids, the most prominent being globotriaosylceramide (Gb3) [2]. Patients with FD show progressive accumulation of Gb3 and related glycosphingolipids in neurons, podocytes, cardiomyocytes, endothelial, perithelial and vascular smooth muscle cells. Recently, the deacylated soluble derivative globotriaosylsphingosine (lyso-Gb3) and analogs, were found to be increased in plasma and urine of FD patients [3], [4]. FD has an estimated birth incidence of 1:40.000 newborns, although recent systematic screenings in different low and high risk cohorts indicate a higher prevalence [5], [6], [7]. The phenotypic spectrum of the disease comprises the classic or early onset form and the non-classical form, diagnosed later in life time, formerly referred as heart or renal variants [1]. In the classic form, the first disease manifestations may become evident during childhood or adolescence as neuropathic distal pain in limbs, hypohydrosis, angiokeratoma, gastrointestinal symptoms, cornea verticillata, dysautonomia, fatigue and auditive impairment [2]. During adulthood, most males develop left ventricular hypertrophy (LVH) and/or arrhythmia, renal insufficiency and/or stroke [1], [2]. The concept of late onset or non-classical variants arose in 1995 after enzymatic screening in patients with idiopathic LVH [8], [9]. Recent increase of screenings in high risk populations, due to treatment availability, allowed the identification of variants causative of non-classical disease. A third category of genetic alterations in GLA, genetic variants of unclear significance (GVUS), also called fringe alleles due to their unclear pathological consequences, further adds complexity to FD diagnosis and management [10], [11].
2. Patients materials and methods
Patients
Individuals carrying the GLA variants p.(Cys174Gly) and p.(Arg363His) alleles were identified on systematic screenings at renal units, using dried blood spots (DBS) and leukocyte α-Gal A levels as screening methods.
Structural analysis
To evaluate the location of an amino acid residue involved in substitution, the solvent-accessible surface area (ASA) value of an amino acid in the wild type GLA was calculated using Stride (http://webclu.bio.wzw.tum.de/stride/). Structural models of mutant AGAL_HUMAN proteins with p.(Cys174Gly) and p.(Arg363His) were built using TINKER (http://dasher.wustl.edu/tinker/). As a template, the crystal structure of human AGAL_HUMAN (PDB: 1R46) was used and energy minimization was performed. The root-mean-square gradient value was set at 0.05 kcal/mol · Å. Then, each mutant model was superimposed on the wild type GLA structure based on the Cα atoms by the least-square-mean fitting algorithm, in which the optimal rotations and translations are found by minimizing the sum of the squared distances among all structures in the superposition. We defined that the atom was affected by an amino acid substitution when the position of the atom in a mutant differed from that in the wild type structure by more than 0.15 Å. For evaluation of the structural changes caused by amino acid substitutions, we calculated the root-mean-square distance (RMSD) values, as described previously [12]. Furthermore, coloring of the influenced atoms in the three-dimensional structure of the enzyme was performed to determine the influence of the amino acid substitutions geographically and semi-quantitatively. The colors of affected atoms were shown on the basis of the distance between the wild type and mutant one.
In silico mutations evaluation
The mutations in the GLA coding region c.520C > G and c.1088G > A, that changes the translation of codons 174 and 363 to p.(Cys174Gly) and p.(Arg363His), were analyzed using several web-based tools. MutationTaster [13], SIFT [14] PolyPhen-2 [15], PhD-SNP [16], V7 and V8 Methods [17], SNPeffect 4.0 [18] I-Mutant Suite 3.0 [19] and SDM [20], in order to assess their potential pathogenicity. Biochemical studies: Lyso-Gb3 quantitation in plasma was performed as previously described [21].
3. Results
3.1. Clinical data
Case 1
A 46 year old male, with no family history of renal disease, sought care for lower limb edema. The patient was normotensive and physical exam was unremarkable. Laboratory evaluation showed 1.3 g/24hs of proteinuria (with normal urinary sediment). Work up for his proteinuria ruled out diabetes and autoimmune nephropathies. Renal ultrasound was also normal. He initiated treatment with an angiotensin converting enzyme inhibitors (ACEi). Two years later, proteinuria had increased to 2.8 g/24 h, lower limb edema worsened and serum creatinine was 1.07 mg/dl (95.5 μmol/l). Three years after his initial presentation, he was found to have increased proteinuria at 4 g/24h and a serum creatinine of 1.29 mg/dl (114 μmol/l). Renal biopsy was performed (electron microscopy was not done) but did not yield a definitive diagnosis. At age 50, serum creatinine continued to rise to 2.35 mg/dl (207 μmol/l) and proteinuria reached 8.5 g/24 h. A subsequent renal biopsy, employing light microscopy, revealed segmental sclerosis in two glomeruli and cytoplasmic vacuolization in podocytes, tubulo-interstitial fibrosis and moderate tubular atrophy. Electron microscopy (EM) showed zebra bodies and cytoplasmic vacuolization in podocytes, with prominent foot process effacement [22], specific findings of FD. Zebra bodies were notably absent in endothelial, mesangial and tubular cells. Leukocyte α-Gal A activity was 2.8 nmol/h/mg (Normal Values: 30.5–57.7 nmol/h/mg) and GLA sequencing identified the hemizygous missense variant c.520C > G; p.(Cys174Gly) in exon 3. Lyso-Gb3 measurement in plasma was 2.9 ng/ml or 3.6 nM (Normal Range: ≤ 0.9 ng/ml or 1.1 nM). Brain magnetic resonance images (MRI) showed severe vertebrobasilar dolichoectasia (VBD), without evidence of white matter involvement. Echocardiographic studies revealed mild LVH. Upon further review, the patient did not manifest hypohidrosis, neuropathic pain, or angiokeratomas. The patient was started on enzyme replacement therapy (ERT), however, despite this he progressed to end stage renal disease (ESRD), requiring renal replacement therapy (RRT) five years after diagnosis. Maternal inheritance of the missense variant c.520C > G was confirmed by genotyping the proband's sister and niece, both showing gastrointestinal symptoms, mild neuropathic pain and depression. The 22 year old heterozygote proband's daughter, had neuropathic pain, heat intolerance and gastrointestinal discomfort. She recently underwent kidney biopsy due to mild proteinuria. On light microscopy, glomeruli showed enlarged podocytes with foamy appearing microvacuoles and mild interstitial fibrosis, suggestive of early Fabry nephropathy. Four hemizygote relatives of the index case, related by a common grand-grand mother, two adult males in their sixties and two young males aged 20 and 21 year old, presenting with LVH, bradycardia, mitral valvular disease, proteinuria and depression in the former and episodic distal pain, heat intolerance and microalbuminuria in the later, are under ERT. Remarkably, in both branches of the family, four patients bearing the p.(Cys174Gly) mutation, two untreated females and two males under treatment show severe psychiatric manifestations.
Case 2
A kidney biopsy was performed in a 40 year old male with an 8 year history of proteinuria and progressive decrease of glomerular filtration rate. Light microscopy revealed focal and segmental glomerulosclerosis. Electron microscopy evaluation was not performed. The patient had no family history of renal disease. His mother and father died of coronary arterial disease at ages 65 and 68 years old respectively. The patient continued to progress to ESRD requiring RRT. At the age of 55, after 5 years of RRT, during a screen for FD in a hemodialysis unit, leukocyte α-Gal A activity was found to be 2.9 nmol/h/mg. Sequencing analysis of the GLA gene detected the c.1088G > A; p.(Arg363His) missense variant in exon 7. Plasma lyso-Gb3 value was 1.8 ng/ml or 2.3 nM (Normal Range: ≤ 0.9 ng/ml or 1.1 nM). Brain MRI did not show alterations. Echocardiographic studies showed mild LVH and ocular examination on slit lamp, revealed corneal deposits in the lower external quadrant of right eye. The patient notably did not manifest neuropathic pain, hypohidrosis nor angiokeratoma. His two obligate carrier daughters remain asymptomatic at the ages of 9 and 11 years. After diagnosis in the index case, the patient's elder brother (68 year old), also in dialysis for 3 years in another renal unit, was tested and found positive for p.(Arg363His). The patient's only sister refused testing, but she referred to have LVH and bradyarrhythmia. She underwent pacemaker implantation before FD diagnosis in the index case. Genotyping was extended to his brother's children. The obligate carrier 30 year old niece harbored the heterozygous mutation and underwent thorough evaluation for skin, renal, cardiac, ophthalmologic and neurologic alterations. She showed impaired perception of temperature changes, indicative of small fibers compromise, assessed by quantitative sensory testing (QST) as the only alteration that could be related to FD. A 65 year old cousin from the maternal branch of the index case, hemizygous for p.(Arg363His) is under dialysis treatment for 2 years and is a candidate for renal transplantion. He showed minimal QST alterations with no other organ systems involved. The index case and his male relatives are under ERT with agalsidase beta, 1 mg/kg, every other week.
3.2. Structural analysis on mutant AGAL_HUMAN proteins with C174G and R363H
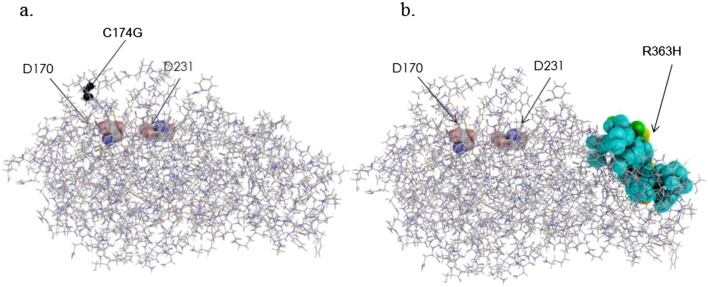
The ASA and RMSD values for each mutant were summarized in Table 1. The results of coloring of the mutant GLA proteins were shown in Fig. 1. C174G: C174, a low conserved residue across species [23], is located on the loop between β-strand (166–170) and α-helix (176–195) of the TIM barrel domain, and is exposed to solvent (Table 1). The cysteine residue is free and does not involve a disulfide bond. C174 is near the catalytic residues (D170 and D231) and N139, a N-glycosylation site. The predicted 3D structural change caused by C174G is very small but it may influence the activity and/or the stability of the enzyme (Fig. 1a). Since this mutation was detected in a single family in Argentina, we analyzed C174G with different in silico tools for prediction of pathogenicity. The results of predictions are shown in Table 2. Analysis of stability of mutant C174G enzyme, employing FoldX3.0, based on 3gxp structural model, shows that this mutation reduces protein stability by 3.49 kcal/mol [36]. I-Mutant Suite SVM2 ddG value prediction was − 1.23 Kcal/mol (large decrease in stability). SDM ddG prediction was: -3.03Kcal/mol (highly destabilizing): may cause protein malfunction and disease.
Table 1.
The ASA and RMSD values for the amino acid substitutions.
| Genotype | ASA (Å2) | RMSD (Å) |
|---|---|---|
| C174G | 33.1 | 0.008 |
| R363H | 28.9 | 0.085 |
Fig. 1.
Coloring of the atoms in the three-dimensional structure of GLA proteins influenced by amino acid substitutions, C174G (a) and R363H (b). The backbone of GLA is shown as a line and the catalytic residues (D170 and D231) are exhibited as a CPK model. The atoms of the substituted amino acid residues are indicated as small black spheres and the influenced atoms as large spheres. The colors of the influenced atoms show the distances between the wild type and mutant ones as follows: 0.15 Å ≤ cyan < 0.30 Å, 0.30 Å ≤ green < 0.45 Å, 0.45 Å ≤ yellow < 0.60 Å, 0.60 Å ≤ orange < 0.75 Å, and red ≥ 0.75 Å.
Table 2.
Results obtained with different Predictive in Silico Algorithms.
| C174G | R363H | |||
|---|---|---|---|---|
| Prediction Tool | Score | Prediction | Score | Prediction |
| MutationTaster (Range0-1) | 0.708 | Disease Causing | 0.999 | Polymorphism |
| SIFT cutoff < 0.05 | 0.092 | Tolerated | 0.465 | Tolerated |
| PolyPhen2 (Range 0–1) | 0.043 | Benign | 0.014 | Benign |
| PhD-SNP | RI:5 | Disease Mutation | RI:6 | Disease Mutation |
| V7 Method (Range 0–1) | 0.998 | Pathologic | 0.706 | Pathologic |
| V8 Method (Range 0–1) | 1.00 | Pathologic | 0.801 | Pathologic |
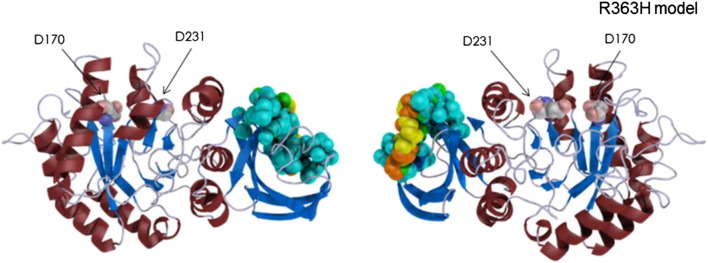
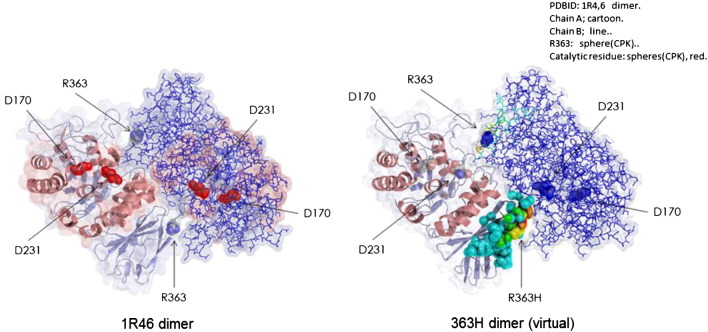
R363, a low conserved residue across species [23], is located on the β-strand (363–368) of the β-sandwich domain and is half-exposed to solvent (Table 1). Fig. 2 shows the atoms affected by aminoacid substitution at position 363. R363 is predicted to be one of the residues which constitute the dimer interface (Fig. 3). Predictions of pathogenicity for the p.(Arg363His) variant using different in silico tools are summarized in Table 2. Analysis of mutant p.(Arg363His) stability, employing FoldX3.0, that uses 3gxp structural model, shows a moderately reduction in protein stability (ddG: 1.05 kcal/mol). I-Mutant Suite SVM2 ddG value prediction (employing 1R46 model) was: -0.95 Kcal/mol, resulting in decrease of stability (reliability index: 4). SDM stability score (pseudo ddG) prediction was − 1,27 Kcal/mol (destabilizing mutation), but did not predict pathogenicity. The TANGO computer algorithm [18], that predicts aggregation nucleating sequences in proteins as well as the effect of mutations on the aggregation propensity of these regions, shows that R363H mutation increases the aggregation tendency of α-Gal A (score 1535; WT score 1471).
Fig. 2.
Representation of α-galactosidase A 3D R363H model. Secondary structures are shown as ribbons. The arrows indicate the catalytic residues. The influenced atoms depicted as large spheres are colored, showing the distances between the wild type and mutant, as indicated in Fig. 1.
Fig. 3.
Representation of wild type α-galactosidase A 1R46 dimer (left) and H363 dimer (virtual, right) with colored affected atoms, chain A shown as ribbon, chain B as lines.
4. Discussion
Recently, as a consequence of systematic screening programs for FD in low and high risk populations, an unexpected high frequency of late onset and unknown significance genetic variants in the GLA gene have been reported [6], [7]. A japanese survey over 21,170 neonates, estimated a prevalence of 1:7057 cases [24]. The overall frequency of such late onset variants is under continuous corrections, due to early interpretations of polymorphic and unknown significance variants as associated with deleterious mutations p.(Asp313Tyr); p.(Met296Ile); p.(Glu66Gln); p.(Ala143Thr); p.(Arg118Cys). In high risk population screenings, the prevalence of individuals with GLA variants is 0.62% [25]. Due to selection bias, the establishment of the pathogenic nature of previously unknown or rare mutations is important though challenging for therapeutic decisions, since symptoms may or may not be related to the new GLA variants. Detailing clinical and histologic manifestations is necessary for each new mutations identified in screening to aid in clinical decision making. In our first patient, follow-up for chronic kidney disease led to a second kidney biopsy that was consistent with segmental glomerulosclerosis. Electron microscopy, genetic studies and plasma Lyso-Gb3 confirmed the diagnosis of FD. Other interesting finding coming from the renal histology was the absence of endothelial inclusion or compromise of glomerular capillaries. Furthermore, Gb3 deposits were confined to the podocytes. These findings are similar to those recently reported for the non-classical variant in Taiwan, where endomyocardial biopsies showed absence of Gb3 deposits in the vascular endothelium, with intralysosomal inclusions restricted to cardiomyocytes [26]. Based on the results of the structural analysis, the predicted structural change caused by p.(Cys174Gly) is very small but may influence the activity and/or the stability of the enzyme (Fig. 1a), making it a potential candidate for rescue by pharmacological chaperones. Substitution of Cys174 for Arg174 was reported as causative of the classic form of FD [27]. The p.(Cys174Gly) missense mutation was described only in a single family of argentinean patients with admixed spanish and aborigin ancestors. Our data show that the p.(Cys174Gly) variant co-segregates with FD manifestations.
Case 2 was the first patient with the p.(Arg363His) mutation detected in Argentina. The renal biopsy was non-diagnostic but EM was not performed, thus highlighting the importance of ultrastructural examination with EM in all renal biopsies. The predicted structural change caused by p.(Arg363His) is moderate (Table 1), and it is thought that the structural change causes folding defect although it does not affect the catalytic site (Fig. 1b). This mutation detected in few families of spanish ancestors, showed marked phenotypic heterogeneity between male patients of different families and was initially reported as causing the classic form of FD [28]. However, asymptomatic adult male patients with p.(Arg363His) have also been described [29]. In a recent work, Lukas [30], employing a heterologous overexpression system in cultured HEK293H cells, tested the in vitro enzyme activity of p.(Arg363His). The mean residual enzyme activity in 8 experiments was 31.9 ± 2.9% of wild type activity. This is a similar enzymatic activity value as for the p.(Ala143Thr) variant, currently of questioned pathogenicity. Since the findings of low α-galactosidase A activity and a GLA variant cannot be directly related to FD [31], measurement of lyso-Gb3 was performed. The plasma lyso-Gb3 concentrations for p.(Cys174Gly) and p.(Arg363His) were within the pathologic but low concentration range, compatible with late onset FD. Recently, Niemann, et al. compared lyso-Gb3 values in patients showing the classic and non-classical variants. Threshold lyso-Gb3 value to separate both groups was established at 2.7 ng/ml (3.4 nM) [31]. Auray Blais et al. described 3 patients (2 females and 1 male) with the p.(Arg363His) mutation, presenting only subtle manifestations, who did not excrete measurable amounts of lyso-Gb3 in urine [32]. Despite our patient bearing the p.(Arg363His) mutation presented ESRD, lyso-Gb3 concentration in plasma was above normal range, but only slightly elevated: 1.8 ng/ml (2.3 nM). Lyso-Gb3 has been demonstrated to play a role in cell damage and progression in early onset FD [3]. Whether lyso-Gb3 is a pathogenic factor involved in the development of late onset FD is still under investigation.
Although in our index patients the main compromised organ was the kidney, signs and symptoms affecting other organs, such as LVH, corneal deposits and VBD could be observed. The etiology of LVH could not be addressed due to the absence of a cardiac biopsy or advanced cardiac MRI techniques including delayed enhancement or T1 weighted imaging. It cannot be ruled out that LVH could be related to progression of CKD to ESRD per se. VBD is a very frequent finding in FD [33], and could be a marker of vascular smooth muscle cells compromise due Gb3 deposits at this level. Of note, this finding can be evident even in the absence of white matter ischemic lesions and has been proposed as an early marker of cerebrovascular damage [34]. Case 1 did not have hypertension before or at the time of cerebral MRI, thus VBD could be directly related to FD. Case 2 presented minimal cornea verticillata, a specific finding of FD in the absence of medications that can result in deposits in corneal epithelium, like chloroquine, hydroxichloroquine, amiodarone, indomethacin, or phenothiazines [35].
Despite the results of pathogenicity predictions for each of these two rare mutations were not coincident, there was specific clinical evidence of FD in both cases. Some α-Gal A mutants that retain biological activity in vitro, present reduced thermodynamic stability, increasing the unfolded protein fraction and the propensity to form aggregates [36]. Chronic exposure to destabilized proteins may induce changes in the expression of proteostatic network components and produce organ damage [36], [37], [38]. As shown in the results section, the p.(Cys174Gly) mutation reduces thermodynamic protein stability, increasing unfolded protein fraction and possibly promoting aggregation. We are considering the possibility of testing the mutant Gly174 cDNA construct in an in vitro overexpression system. This approach would provide objectivity about residual enzyme activity, characterize the damage to the enzyme, and responsiveness towards pharmacological chaperones. It has been shown that overexpressed enzyme activities of α-Gal A mutants in HEK293 cells exhibit the highest rate of correct disease severity predictor for mutations, compared to lyso-Gb3 and in silico algorithms [30]. This mutation is classified until present as a single nucleotide polymorphism at the dbSNP database of the NCBI (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi? rs = 181,562,963) and is absent from 1000 Genome Phase 3 release and ExAc Browsers. No other sequence variants were identified in the coding sequence and the 50 nucleotides adjacent to the splice sites and 5'UTR of initiation codon in the DNA of the index case. The Gly174 allele has not been identified in 182 healthy argentinean females tested.
The Arg363 residue is part of a gatekeeper sequence (amino acids GGPRS, positions 360 to 364) that counteracts the last, weak aggregation prone segment (amino acids YTIAVA, positions 365 to 370) present in the enzyme [18]. Computational analysis of proteomes showed strong enrichment of Arg at the flanks of stretches of hydrophobic aminoacids. Hsp70 chaperones preferentially bind positive charged Arg in gatekeepers and thereby shield proteins against aggregation. Gatekeeper mutations are several times more frequent in human disease mutants than in human polymorphisms [39], [40]. Removal of Arg363 gatekeeper residue may reduce efficiency of folding, could interfere with chaperone recruitment, and possibly promote aggregation in vivo, increasing disease susceptibility. Additionally, since Arg363 is predicted to constitute the dimer interface, its replacement by His could interfere in dimer interaction. In particular for the p.(Arg363His) variant, we speculate that individual proteostatic network capacity and other yet unknown genetic/constitutional and environmental factors, may Influence the development of symptoms in individuals bearing this mutation.
In conclusion, our patients present two rare non-classical variants of FD diagnosed through FD screenings in renal disease units. Continued screening in high risk populations such as stroke, hypertrophic cardiomyopathy, and patients on dialysis is likely continue to yield new non-classical variants of FD. This may cause therapeutic decision dilemmas for treating physicians. Lyso-Gb3 concentrations were within the pathogenic, low concentration range, compatible with late onset FD cases. The in silico analysis of p.(Cys174Gly) and p.(Arg363His), employing different tools, suggest that enzyme destabilization and possibly aggregation may play a role in chronic organ damage.
Take-home message.
Screening programs for FD in high risk populations are important as FD is a treatable condition.
Compliance with Ethics Guidelines
Conflict of Interest
Politei Juan has received speaker honorarium from Genzyme, Shire and Amicus Serebrinsky G, Calvo M, Fernandez S, Saito S, Ohno K, Wallace E, Warnock D, Sakuraba H declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation of Labgen, Buenos Aires, Argentina and with the Helsinki Declaration of 1975, as revised in 2000.
Informed consent was obtained from all patients for being included in the study.
The informed consents are available if journal need them.
Details of the contributions of individual authors:.
Author contributions.
G.S. performed the genetic analysis and the bioinformatics, JP, D.W. E.W, MC and SF performed clinical studies, H.S., S.S. and K.O. performed the structural studies and analyzed the data; G.S.; J.P, D.W, E.W and H.S. wrote the paper.
References
- 1.Germain D.P. Fabry disease. Orphanet J. Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desnick R.J., Ioannou Y.A., Eng C.M. α-Galactosidase A Deficiency: Fabry Disease. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic Bases of Inherited Disease. 8th ed. MacGraw-Hill; New York: 2001. pp. 3733–3774. [Google Scholar]
- 3.Aerts J.M., Groener J.E., Kuiper S., Donker-Koopman W.E., Strijland A., Ottenhoff R., van Roomen C., Mirzaian M., Wijburg F.A., Linthorst G.E., Vedder A.C., Rombach S.M., Cox-Brinkman J., Somerharju P., Boot R.G., Hollak C.E., Brady R.O., Poorthuis B.J. Elevated globotriaosylsphingosine is a hallmark of fabry disease. Proc. Natl. Acad. Sci. U. S. A. 2008;105(8):2812–2817. doi: 10.1073/pnas.0712309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutin M., Auray-Blais C. Multiplex tandem mass spectrometry analysis of novel plasma lyso-Gb₃-related analogues in fabry disease. Anal. Chem. 2014;86(7):3476–3483. doi: 10.1021/ac404000d. [DOI] [PubMed] [Google Scholar]
- 5.Meikle P.J., Hopwood J.J., Clague A.E., Carey W.F. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 6.Spada M., Pagliardini S., Yasuda M., Tukel T., Thiagarajan G., Sakuraba H., Ponzone A., Desnick R.J. High incidence of later-onset fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006;79(1):31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien Y.H., Lee N.C., Chiang S.C., Desnick R.J., Hwu W.L. Fabry disease: incidence of the common later-onset α-galactosidase a IVS4 + 919G → a mutation in Taiwanese newborns–superiority of DNA-based to enzyme-based newborn screening for common mutations. Mol. Med. 2012;18:780–784. doi: 10.2119/molmed.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakao S., Takenaka T., Maeda M., Kodama C., Tanaka A., Tahara M., Yoshida A., Kuriyama M., Hayashibe H., Sakuraba H. An atypical variant of fabry's disease in men with left ventricular hypertrophy. N. Engl. J. Med. 1995;333(5):288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- 9.Nakao S., Kodama C., Takenaka T., Tanaka A., Yasumoto Y., Yoshida A., Kanzaki T., Enriquez A.L., Eng C.M., Tanaka H., Tei C., Desnick R.J. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a "renal variant" phenotype. Kidney Int. 2003;64(3):801–807. doi: 10.1046/j.1523-1755.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 10.Houge G., Tøndel C., Kaarbøe O., Hirth A., Bostad L. and Svarstad Fabry or not Fabry –a question of ascertainment European Journal of Human Genetics (2011) 19, 1111. [DOI] [PMC free article] [PubMed]
- 11.Kobayashi M., Ohashi T., Fukuda T., Yanagisawa T., Inomata T., Nagaoka T., Kitagawa T., Eto Y., Ida H., Kusano E. No accumulation of globotriaosylceramide in the heart of a patient with the E66Q mutation in the α-galactosidase a gene. Mol. Genet. Metab. 2012;107(4):711–715. doi: 10.1016/j.ymgme.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Saito S., Ohno K., Sakuraba H. Comparative study of structural changes caused by different substitutions at the same residue on α–galactosidase. PLoS One. 2013;8 doi: 10.1371/journal.pone.0084267. e84267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz J.M., Cooper D.N., Schuelke M., Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods. 2014 Apr;11(4):361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P., Henikoff S., Ng P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4(7):1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 15.Adzhubei, I.A., Schmidt, S., Peshkin, L., Ramensky, V.E., Gerasimova, A., Bork, P., Kondrashov, A.S., Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods2010, 7:248–249. [DOI] [PMC free article] [PubMed]
- 16.Capriotti E, Calabrese R, Casadio R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics. 2006; 22(22):2729–34 [DOI] [PubMed]
- 17.Riera C., Lois S., Domínguez C., Fernandez-Cadenas I., Montaner J., Rodríguez-Sureda V., de la Cruz X. Molecular damage in Fabry disease: Characterization and prediction of alpha-galactosidase A pathological mutations. Proteins. 2014 doi: 10.1002/prot.24708. [DOI] [PubMed] [Google Scholar]
- 18.De Baerts G., Van Durme J., Reumers J., Maurer-Stroh S., Vanhee P., Dopazo J., Schymkowitz J., Rousseau F. SNPeffect 4.0: on-line prediction of molecular and structural effects of protein-coding variants. Nucleic Acids Res. 2012;40(1):D935–D939. doi: 10.1093/nar/gkr996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capriotti E, Fariselli P, Rossi I, Casadio R. A three-state prediction of single point mutations on protein stability changes. BMC Bioinformatics. 2008, 26 (9) Suppl 2:S6. [DOI] [PMC free article] [PubMed]
- 20.Topham CM, Srinivasan N and Blundell TL. Prediction of protein mutants based on structural environment-dependent amino acid substitution and propensity tables. Protein Eng. 1997, 10 (1): 7-21 [DOI] [PubMed]
- 21.Tanislav C., Kaps M., Rolfs A., Bottcher T., Lackner K., Paschke E. Frequency of fabry disease in patients with small-fibre neuropathy of unknown aetiology: a pilot study. Eur. J. Neurol. 2011;18:631–636. doi: 10.1111/j.1468-1331.2010.03227.x. [DOI] [PubMed] [Google Scholar]
- 22.H Mukdsi J, Gutiérrez S, Barrón B, Novoa P, Fernández S, de Diller AB, I Torres A, Formica RN Jr, Orías M. A renal variant of Fabry disease: A case with a novel Gal A hemizygote mutation. J Nephropathol. 2012;1(3):194–7. [DOI] [PMC free article] [PubMed]
- 23.http://www.ebi.ac.uk/pdbsum/1R46
- 24.Inoue T., Hattori K., Ihara K., Ishii A., Nakamura K., Hirose S. Newborn screening for fabry disease in Japan: prevalence and genotypes of fabry disease in a pilot study. J. Hum. Genet. 2013;58(8):548–552. doi: 10.1038/jhg.2013.48. [DOI] [PubMed] [Google Scholar]
- 25.Van der Tol L, Smid BE, Poorthuis BJ, Biegstraaten M, Deprez RH, Linthorst GE, Hollak CE. A systematic review on screening for fabry disease: prevalence of individuals with genetic variants of unknown significance. J. Med. Genet..2014;51(1):1–9. [DOI] [PubMed]
- 26.Hsu T.R., Sung S.H., Chang F.P., Yang C.F., Liu H.C., Lin H.Y., Huang C.K., Gao H.J., Huang Y.H., Liao H.C., Lee P.C., Yang A.H., Chiang C.C., Lin C.Y., Yu W.C., Niu D.M. Endomyocardial biopsies in patients with left ventricular hypertrophy and a common Chinese later-onset fabry mutation (IVS4 + 919G > a) Orphanet J. Rare Dis. 2014;9:96. doi: 10.1186/1750-1172-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng Y., Zhang W.M., Shi H.P., Wei M., Huang S.Z. Clinical manifestations and mutation study of 16 Chinese patients with fabry disease. Zhonghua Yi Xue Za Zhi. 2010;90(8):551–554. [PubMed] [Google Scholar]
- 28.Shabbeer J., Yasuda M., Luca E., Desnick R.J. Fabry disease: 45 novel mutations in the alpha-galactosidase a gene causing the classical phenotype. Mol. Genet. Metab. 2002;76(1):23–30. doi: 10.1016/s1096-7192(02)00012-4. [DOI] [PubMed] [Google Scholar]
- 29.3rd Update on Fabry Nephropathy. Mutations versus polymorphisms. Hong Kong, June 4–5, 2013.
- 30.Lukas J. Characterization of novel α galactosidase A mutations in Fabry disease based on in vitro, in vivo, and pharmacological data. Dissertation. Jan Lukas Rostock 10.04.2013
- 31.Niemann M., Rolfs A., Störk S., Bijnens B., Breunig F., Beer M., Ertl G., Wanner C., Weidemann F. Gene mutations versus clinically relevant phenotypes: lyso-Gb3 defines fabry disease. Circ. Cardiovasc. Genet. 2014;7(1):8–16. doi: 10.1161/CIRCGENETICS.113.000249. [DOI] [PubMed] [Google Scholar]
- 32.Auray-Blais C., Ntwari A., Clarke J.T.R., Warnock D.G., Oliveira J.P., Young S.P., Millington D.S., Bichet D.G., Sirrs S., West M.L., Casey R., Hwu W.-L., Keutzer J.M., Zhang K.X., Gagnon R. How well does urinary lyso-Gb3 function as a biomarker in Fabry disease? Clinica Chimica Acta. 2010;411:1906–1914. doi: 10.1016/j.cca.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Fellgiebel A., Keller I., Marin D., Müller M.J., Schermuly I., Yakushev I., Albrecht J., Bellhäuser H., Kinateder M., Beck M., Stoeter P. Diagnostic utility of different MRI and MR angiography measures in fabry disease. Neurology. 2009;72(1):63–68. doi: 10.1212/01.wnl.0000338566.54190.8a. [DOI] [PubMed] [Google Scholar]
- 34.Politei J., Schenone A., Burlina A., Blanco M., Lescano S., Szlago M., Cabrera G. Vertebrobasilar dolichoectasia in fabry disease: the earliest marker of neurovascular involvement? JIEMS. 2014;2:1–6. [Google Scholar]
- 35.Sivley M.D. Fabry disease: a review of ophthalmic and systemic manifestations. Optom. Vis. Sci. 2013;90(2):e63–e78. doi: 10.1097/OPX.0b013e31827ec7eb. [DOI] [PubMed] [Google Scholar]
- 36.Siekierska A., De Baets G., Reumers J., Gallardo R., Rudyak S., Broersen K., Couceiro J., Van Durme J., Schymkowitz J., Rousseau F. α-galactosidase aggregation is a determinant of pharmacological chaperone efficacy on fabry disease mutants. J.Biol. Chem. 2012 Aug 17;287(34):28386–28397. doi: 10.1074/jbc.M112.351056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inagi R., Ishimoto Y., Nangaku M. Proteostasis in endoplasmic reticulum —new mechanisms in kidney disease. Nat. Rev. Nephrol. 2014;10:369–378. doi: 10.1038/nrneph.2014.67. [DOI] [PubMed] [Google Scholar]
- 38.Yue P., Li Z.L., Moult J. Loss of protein structure stability as a major causative factor in monogenic disease. J. Mol. Biol. 2005;353(2):459–473. doi: 10.1016/j.jmb.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Beerten J., Schymkowitz J., Rousseau F. Aggregation prone regions and gatekeeping residues in protein sequences. Curr. Top. Med. Chem. 2012;12(22):2470–2478. doi: 10.2174/1568026611212220003. [DOI] [PubMed] [Google Scholar]
- 40.Reumers J., Maurer-Stroh S., Schymkowitz J., Rousseau F. Protein sequences encode safeguards against aggregation. Hum. Mutat. 2009 Mar;30(3):431–437. doi: 10.1002/humu.20905. [DOI] [PubMed] [Google Scholar]