Abstract
Background
Late-onset Pompe disease (LOPD) is a metabolic myopathy caused by mutations in GAA and characterized by proximal muscle weakness and respiratory insufficiency. There is evidence from clinical studies that enzyme replacement therapy (ERT) with human recombinant alpha-glucosidase improves motor performance and respiratory function in LOPD.
Objective
We analyzed quantitative muscle MRI data of lower limbs to evaluate the effects of long-term ERT on muscle parameters.
Methods
Three symptomatic LOPD patients who received ERT for five years and four untreated presymptomatic LOPD patients were included in the study. T1-weighted MRI images were used to determine volumes of thigh and lower leg muscles. In addition, mean gray values of eight individual thigh muscles were calculated to assess the degree of lipomatous muscle alterations.
Results
We detected a decrease in thigh muscle volume of 6.7% (p < 0.001) and an increase in lower leg muscle volume of 8.2% (p = 0.049) after five years of ERT. Analysis of individual thigh muscles revealed a positive correlation between the degree of lipomatous muscle alterations at baseline and the increase of gray values after five years of ERT (R2 = 0.68, p < 0.001). Muscle imaging in presymptomatic patients showed in one case pronounced lipomatous alteration of the adductor magnus muscle and mild to moderate changes in further thigh muscles.
Conclusions
The results demonstrate that fatty muscle degeneration can occur before clinical manifestation of muscle weakness and suggest that mildly affected muscles may respond better to ERT treatment than severely involved muscles. If these findings can be validated by further studies, it should be discussed if muscle alterations detected by muscle MRI may be an objective sign of disease manifestation justifying an early start of ERT in clinically asymptomatic patients in order to improve the long-term outcome.
Abbreviations: LOPD, late onset Pompe disease; ERT, enzyme replacement therapy; rhGAA, human recombinant alpha-glucosidase; MRI, magnetic resonance imaging
Keywords: Pompe disease, Myopathy, MRI, Muscle imaging, Muscle volume, Enzyme replacement therapy
1. Introduction
Pompe disease (OMIM #232300), also known as glycogen storage disease type 2 or acid maltase deficiency, is a rare autosomal recessive disorder caused by mutations in GAA that lead to a deficiency of lysosomal acid alpha-glucosidase [1]. In late-onset Pompe disease (LOPD), skeletal muscle is the most affected tissue. The clinical phenotype of LOPD is characterized by slowly progressive proximal muscle weakness involving axial and respiratory muscles that is associated with significant morbidity and reduced life expectancy [2], [3], [4], [5], [6], [7]. Since 2006, an enzyme replacement therapy (ERT) with human recombinant alpha-glucosidase (rhGAA) is available for the treatment of Pompe disease (alglucosidase alfa, Myozyme®, Lumizyme®, Genzyme Corporation, a Sanofi company, Cambridge MA, USA). Clinical studies showed that motor performance, respiratory function and fatigue improved or stabilized in at least two-thirds of LOPD patients who received ERT over 6–36 months [8], [9], [10], [11], [12], [13], [14], [15]. The functional outcome seems to be best when ERT starts timely, i.e. at an early clinical stage of LOPD [12], [16]. This, by implication, suggests that disease progression has an influence on the efficacy of ERT.
An important feature of disease progression in LOPD and many other inherited myopathies is the replacement of muscle fibers by fat cells and connective tissue. This fatty alteration can be detected by magnetic resonance imaging (MRI) because it provides a high soft tissue contrast that allows a noninvasive assessment of striated muscles regarding shape, volume and tissue architecture [17]. Previous MRI studies in LOPD [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28] revealed changes in quantitative muscle MRI parameters with ERT treatment for between six and 24 months [24], [26]. Here we present quantitative muscle MRI data, including assessment of lipomatous alterations in individual thigh muscles, from three LOPD patients who received ERT over a time period of five years and from four untreated presymptomatic patients. The main aim was to investigate if the response to ERT regarding prevention of progressive fatty muscle degeneration depends on the degree of pre-existing alterations.
2. Materials and methods
2.1. Patients
Muscle MRI data from seven LOPD patients (diagnosis confirmed by detection of GAA deficiency and genetic analysis) were evaluated with approval of the ethics committee of the Ruhr-University Bochum. Three patients (IDs 1–3, see Table 1 for details) received an i.v. ERT with rhGAA derived from Chinese hamster ovary cells (Myozyme®, Genzyme) in a dosage of 20 mg/kg body weight every other week for at least five years. Four untreated patients were clinically presymptomatic (IDs 4–7 in Table 1). They presented with an elevated serum creatine kinase level but had a normal forced vital capacity and normal muscle strength in manual muscle testing.
Table 1.
Characteristics of LOPD patients included in this study.
| Patient ID |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Gender | Man | Man | Woman | Man | Woman | Woman | Woman |
| Age | 39 | 51 | 48 | 26 | 22 | 27 | 19 |
| Muscle weakness | Yes | Yes | Yes | No | No | No | No |
| Age at onset [years] | 13 | 37 | 30 | – | – | – | – |
| Age at ERT onset [years] | 31 | 45 | 42 | – | – | – | – |
| Age at muscle MRI [years] | 33/38 | 45/50 | 42/47 | 25 | 22 | 26 | 18 |
| Able to walk without aid | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Requiring ventilator support | No | No | No | No | No | No | No |
2.2. Muscle imaging
Muscle MRI was performed on a 1.5 Tesla scanner with a four channel phased-array coil (Magnetom Symphony Quantum, Siemens Healthcare). Twelve axial slices were obtained through the thighs and lower legs using the following scanning parameters: T1-weighted spin-echo sequence, TR/TE of 500/20 ms, slice thickness of 10 mm, interslice gap of 150%, matrix of 512 × 512, two signal averages, and examination time of 2 min and 44 s. Symptomatic patients (ID 1–3) were examined twice at an interval of five years.
T1-weighted MRI images were used to determine muscle volumes and lipomatous muscle alterations. Slices of examinations at baseline and after five years of ERT were matched by three experienced investigators blinded to the patients' data to ensure that the same segments of thighs and lower legs were included in the analysis. Final measurements were performed by one investigator to prevent bias due to interobserver variability. Total volumes of skeletal muscles in eight consecutive axial slices through the thighs and in eight consecutive axial slices through the lower legs were measured using cellSens Dimension software, version 1.8 (Olympus Corporation, Muenster, Germany). The same slices were used for the assessment of lipomatous alterations of individual thigh muscles (quadriceps femoris, sartorius, gracilis, adductor longus, adductor magnus, biceps femoris, semitendinosus and semimembranosus) by calculating the mean gray values in muscle areas (11-bit grayscale, 2048 tones) with IMPAX software, Version 6.3.1.8000 (AGFA HealthCare N.V., Belgium). On T1-weighted images, the signal intensity of skeletal muscle tissue is much lower than that of fat tissue. The gray value of pixels is a unit-free parameter that depends on the brightness (black = 0, white = 2047). In our MRI setting, healthy muscle tissue has a mean gray value of about 230 and fat tissue a mean value of about 950. An increase of mean gray values of muscle tissue correlates with an increase of fatty replacement. A two-tailed paired t-test (equal variances assumed) at a significance alpha level of 0.05 was used for statistical analysis of differences between baseline and follow-up measurements. The correlation between mean gray values at baseline (of the eight individual thigh muscles in each patient) and changes of these gray values after ERT was assessed using Pearson's correlation coefficient.
3. Results
3.1. Muscle alterations at baseline in symptomatic patients
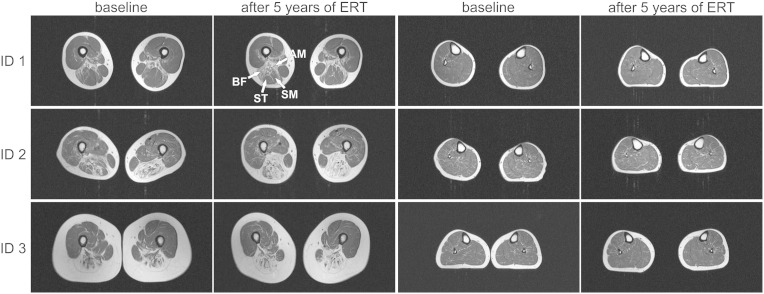
The pattern of muscle involvement with regard to lipomatous alterations was similar in all symptomatic LOPD patients (IDs 1–3). The semimembranosus, adductor magnus, biceps femoris and semitendinosus were the most affected muscles (Fig. 1, Fig. 2). The adductor longus was moderately involved and the gracilis, sartorius and quadriceps femoris showed only slight lipomatous alterations. Lower leg muscles were spared (ID 1) or only mildly involved (IDs 2 and 3).
Fig. 1.
Transverse T1-weighted muscle MRI images of three symptomatic LOPD patients (IDs 1–3) at baseline examination and after five years of ERT. The pattern of muscle involvement regarding fatty replacement is similar in all patients. On the thigh level (left columns), the semimembranosus (SM), adductor magnus (AM), biceps femoris (BF) and semitendinosus (ST) are most affected at baseline and also after five years of ERT. In lower legs (right columns), muscles show no (ID 1) or only mild lipomatous (IDs 2 and 3) alterations in both investigations.
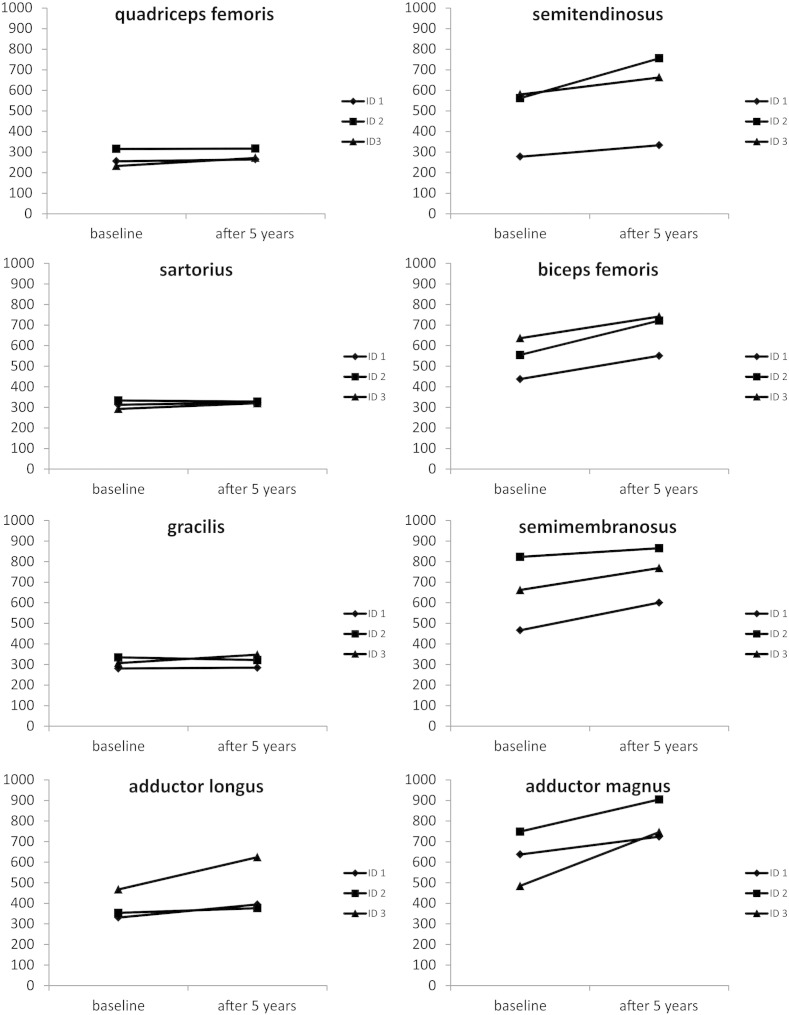
Fig. 2.
Changes of mean gray values in eight thigh muscles after five years of ERT. The mean gray values of unaffected or only mildly involved muscles (quadriceps femoris, sartorius, gracilis) showed only small changes after five years of ERT. In contrast, more affected muscles (adductor longus (ID 1), semitendinosus, biceps femoris, semimembranosus, adductor magnus) usually had higher gray values after five years of ERT indicating a clear increase of lipomatous muscle alterations.
3.2. Changes after five years of ERT treatment
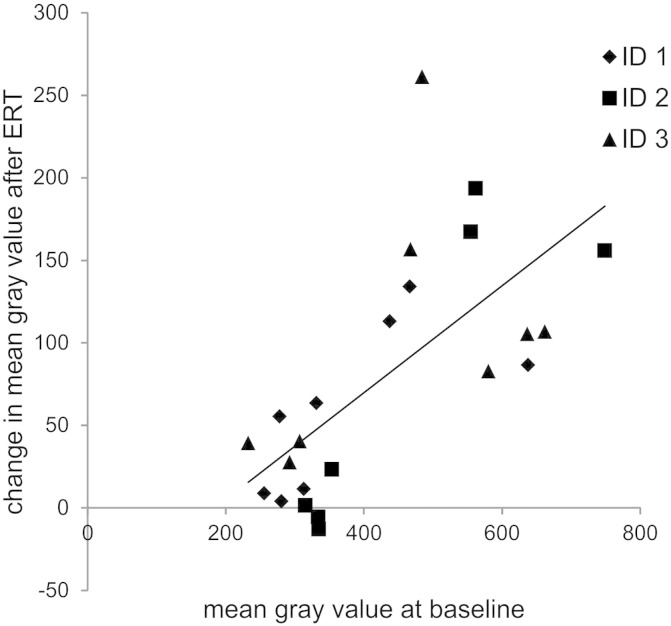
The volume of thigh muscles significantly decreased by 6.7% and that of lower leg muscles significantly increased by 8.2% compared to baseline measurements (Table 2). A slight decrease in total lower limb muscle mass (− 0.6%) was not statistically significant. Changes in mean gray values of selected thigh muscles were highly variable and we detected a significant positive correlation (R2 = 0.68, p < 0.001) between the mean gray values of individual muscles at baseline examination and the increase of these gray values after five years of ERT treatment (i.e. gray value after ERT minus gray value at baseline; Fig. 3). Muscles with mean gray values between 230 and 360 showed only slight changes whereas more affected muscles (mean gray values at baseline between 430 and 750) presented with a higher increase in mean gray values indicating a progression of lipomatous alterations. One value (of semimembranosus in Patient 2) was excluded from correlation analysis as the gray value at baseline was already in the range of fat tissue.
Table 2.
Changes in muscle MRI parameters in untreated and ERT-treated LOPD patients.
| Pichiecchio et al. [24] | Pichiecchio et al. [24] | Ravaglia et al. [26] | This study | |
|---|---|---|---|---|
| N | 4 | 9 | 11 | 3 |
| Duration | 5 years | 6 months | 18/24 months | 5 years |
| ERT with rhGAA | No | Yes | Yes | Yes |
| Changes in muscle volume [%] | ||||
| Lower limb | − 18.5 (p = 0.07) | 5.7 (p = 0.003) | 2.5 (p = 0.078) | − 0.6 (p = 0.76) |
| Thigh | − 24.8 (p = 0.07) | 8.1 (p = 0.051) | 5.3 (p = 0.035) | − 6.7 (p < 0.001) |
| Lower leg | − 1.3 (p = 0.14) | 2.7 (p = 0.008) | − 2.4 (p = 0.178) | 8.2 (p = 0.049) |
| Changes in intra-muscular fat [%] | ||||
| Lower limb | 8.4 (p = 0.068) | 2.8 (p = 0.021) | 3.3 (p = 0.001) | – |
| Thigh | 11.1 (p = 0.068) | 3.3 (p = 0.028) | 3.7 (p = 0.009) | – |
| Lower leg | 1.8 (p = 0.068) | 1.6 (p = 0.008) | 1.3 (p = 0.006) | – |
Fig. 3.
Correlation between lipomatous alterations of thigh muscles at baseline and changes after five years of ERT. Statistical analysis of data presented in Fig. 2 revealed a significant correlation (R2 = 0.68, p < 0.001) between baseline gray values and increase of gray values after ERT.
3.3. Muscle imaging in presymptomatic patients
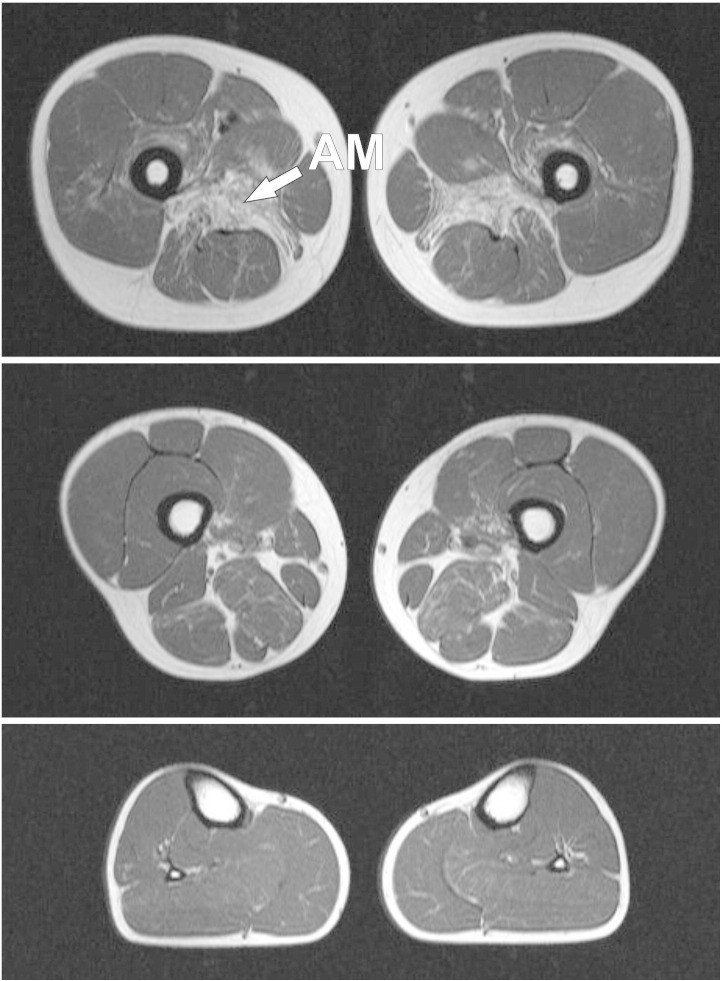
MRI in clinically asymptomatic Patients 5–7 showed no lipomatous muscle alterations (not shown). In contrast, muscle imaging in Patient 4 revealed an unexpected severe involvement of the adductor magnus muscle (mean gray value of 666). The vastus intermedius, adductor longus, semimembranosus and biceps femoris muscles showed mild to moderate lipomatous alterations. Lower leg muscles were not affected in this patient (Fig. 4).
Fig. 4.
Transverse T1-weighted muscle MRI images of a clinically presymptomatic patient. On the thigh level (upper and middle images), the adductor magnus muscles show severe fatty alterations. The vastus intermedius, adductor longus, semimembranosus and biceps femoris muscles are only mildly or moderately affected by lipomatous alterations. Lower leg muscles (lower image) are spared and show no pathologic changes.
4. Discussion
The deficiency of alpha-glucosidase in patients with Pompe disease leads to progressive muscle weakness associated with fatty muscle degeneration. It has been shown that ERT with rhGAA has a positive effect on clinical parameters including the 6-minute walk test, quantitative muscle testing and pulmonary function [8], [11], [12], [13], [29]. Most of the patients included in these studies were treated with ERT for six up to 36 months. Here we present for the first time muscle imaging data of LOPD patients who received long-term rhGAA treatment over a time period of five years.
MRI is a sensitive tool to detect lipomatous muscle alterations and to calculate muscle volumes in myopathies. Previous MRI studies in LOPD mainly focused on the pattern and degree of muscle involvement [18], [19], [21], [22], [30]. The findings in lower limb muscles in our three symptomatic LOPD patients were in accordance with results of these studies as we also detected fatty infiltration, particularly in the semimembranosus, semitendinosus, adductor magnus and biceps femoris muscles. This underlines that there is a typical pattern of muscle involvement in Pompe disease that differs from other myopathies [17] and may be helpful in differential diagnosis.
The primary aim of our study was to evaluate the effects of long-term ERT on quantitative MRI parameters. In addition to the measurement of muscle volumes in segments of upper and lower legs, we calculated the mean gray values of eight individual thigh muscles at baseline and after five years of ERT. These values correlate with the degree of lipomatous muscle alterations with higher values indicating more fatty degeneration. An advantage of using mean gray values compared to other approaches to assess intramuscular fat is that there is no need to set cut-off values, i.e. all pixels can be considered for analysis including those with a signal intensity between those defined for muscle and fat tissue. Limitations of our approach are that the percentage of intramuscular fat cannot be determined and that the interslice gap affects quantitative MR analysis. In addition, other MRI methods like proton-density weighted imaging, e.g. using the 3-point-Dixon technique, are nowadays established to separate fat and muscle tissue [31], [32], [33]. However, guidelines for skeletal muscle studies in neuromuscular diseases, including recommendations for MRI protocols, have been published after we already started our study [34]. We did not analyze gray values of lower leg muscles in detail because visual inspection revealed that these muscles were either not or only mildly affected in our LOPD patients. This is consistent with a previous MRI study which showed an involvement of the lower legs only at a very late disease stage [19].
We found only a slight and non-significant decrease in total lower limb muscle volume of less than 1% after five years of ERT. However, when we compared upper and lower leg muscles significant differences were detected between both regions. The more involved thigh muscles showed a significant decrease in muscle volume of about 7% whereas the muscle volume of spared or only mildly affected lower limb muscles increased significantly by about 8%. These findings suggest differences in the response to ERT treatment but the interpretation of our data, especially regarding the estimation of a treatment effect, is limited by the small number of patients and by the missing control group of LOPD patients without ERT. The latter has been an intrinsic problem in quite a few recently performed LOPD studies because nearly all symptomatic Pompe patients now receive rhGAA and because a placebo control cannot be justified due to ethical reasons. Thus, we were restricted to compare our findings with published data.
Pichiecchio et al. performed a quantitative muscle MRI study in four untreated LOPD patients. Follow-up measurements after five years, the same time period used in our study, revealed a markedly higher loss of muscle mass, as compared to our study, with an 18.5% decrease in lower limb muscle volume which was mainly caused by an atrophy of thigh muscles (see Table 2) [24]. The same group reported a 5.7% increase in lower limb muscle volume following 6 months of ERT and a 2.5% increase after 18 to 24 months of ERT in nine LOPD patients [24], [26]. The percentage of intramuscular fat increased by more than 8% in the untreated [24] and by about 3% in the ERT treated patients [24], [26]. The best therapeutic response was observed at the relatively spared anterior thigh level while intramuscular fat accumulation significantly progressed in spite of ERT, particularly in more compromised posterior thigh muscles [24].
Our systematic analysis of individual thigh muscles confirms and extends the aforementioned findings. We found a significant correlation between the degree of muscle alterations at baseline and the changes detected after five years of ERT. Thigh muscles that were not or only mildly affected at baseline showed only a slight or even no increase of mean gray values. This cannot be simply explained by the natural disease course with these muscles being spared, because this also pertained to the M. quadriceps, a muscle that typically shows a progression of lipomatous alterations early in the disease [19]. In contrast, thigh muscles that were more affected at baseline also showed a higher increase in gray values after five years of ERT. Taken together, the results of our study suggest that mildly affected muscles respond better to ERT than severely affected muscles. This is in accordance with findings from clinical studies indicating that the functional outcome is most likely best when ERT is started at an early stage of the disease [16], [35]. The reasons have not yet been elucidated but a relevant factor may be the increasing impairment of autophagy in skeletal muscle fibers of LOPD patients during disease progression [36], [37], as it has been recently shown that the compromised autophagic flux does not only play an essential role in the pathogenesis of Pompe disease but also seems to impair the efficacy of ERT [36], [37], [38].
Muscle MRI in our four presymptomatic LOPD patients revealed in one case an unexpected pronounced fatty degeneration of the adductor magnus muscle and mild to moderate lipomatous alterations in further thigh muscles. Considering the MRI findings in symptomatic LOPD patients, it may be possible that the efficacy of ERT would already be affected in these muscles with the risk of a further worsening before clinical manifestation of muscle weakness. This raises an important question: Would it be indicated to start ERT in this patient to improve the long-term outcome? Current guidelines recommend that ERT should only be started in symptomatic LOPD patients [39]. Presymptomatic patients without objective signs of muscle weakness should not be treated but examined every 6 months for proximal weakness and pulmonary function [39]. The number of patients investigated in our study is too small to make general conclusions but if the results can be validated by further studies, it should be discussed if lipomatous muscle alterations detected by MRI may be accepted as an objective sign of disease manifestation justifying an early start of ERT in LOPD.
In conclusion, MRI in our symptomatic LOPD patients showed a typical pattern of muscle involvement in lower limbs. Quantitative assessment of muscle volumes and lipomatous muscle alterations indicates that patients benefit from long-term ERT but also suggests that the efficacy of this treatment is affected by the pre-existing muscle involvement. Muscle imaging in presymptomatic patients revealed that fatty muscle degeneration can occur before clinical manifestation of weakness. Provided that the results of our study can be verified by further studies, they may have consequences for the management of presymptomatic LOPD patients in the future.
Conflict of interest
M.V. and R.A.K. received travel reimbursements and grants from Genzyme, a Sanofi company.
Acknowledgments
This work was supported by Genzyme, a Sanofi company (grant number LSD0126-2012). Genzyme was not involved in the study design, in the collection, analysis and interpretation of data, in the writing of the paper or in the decision to submit the article for publication.
We thank Lauren Haag for proofreading the manuscript.
Contributor Information
Kai Michael Gruhn, Email: kai.gruhn@bergmannsheil.de.
Christoph Malte Heyer, Email: christoph.heyer@rub.de.
Anne-Katrin Güttsches, Email: anne-katrin.guettsches@bergmannsheil.de.
Robert Rehmann, Email: robert.rehmann@bergmannsheil.de.
Volkmar Nicolas, Email: volkmar.nicolas@rub.de.
Tobias Schmidt-Wilcke, Email: tobias.schmidt-wilcke@bergmannsheil.de.
Martin Tegenthoff, Email: martin.tegenthoff@rub.de.
Matthias Vorgerd, Email: matthias.vorgerd@rub.de.
Rudolf Andre Kley, Email: rudolf.kley@rub.de.
References
- 1.Kishnani P.S., Steiner R.D., Bali D., Berger K., Byrne B.J., Case L.E., Case L., Crowley J.F., Downs S., Howell R.R., Kravitz R.M., Mackey J., Marsden D., Martins A.M., Millington D.S., Nicolino M., O'Grady G., Patterson M.C., Rapoport D.M., Slonim A., Spencer C.T., Tifft C.J., Watson M.S. Pompe disease diagnosis and management guideline. Genet. Med. 2006;8:267–288. doi: 10.1097/01.gim.0000218152.87434.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkel L.P.F., Hagemans M.L.C., van Doorn P.A., Loonen M.C.B., Hop W.J.C., Reuser A.J.J., van der Ploeg A.T. The natural course of non-classic Pompe's disease; a review of 225 published cases. J. Neurol. 2005;252:875–884. doi: 10.1007/s00415-005-0922-9. [DOI] [PubMed] [Google Scholar]
- 3.van der Beek N.A.M.E., de Vries J.M., Hagemans M.L.C., Hop W.C.J., Kroos M.A., Wokke J.H.J., de Visser M., van Engelen B.G.M., Kuks J.B.M., van der Kooi A.J., Notermans N.C., Faber K.G., Verschuuren J.J.G.M., Reuser A.J.J., van der Ploeg A.T., van Doorn P.A. Clinical features and predictors for disease natural progression in adults with Pompe disease: a nationwide prospective observational study. Orphanet J. Rare Dis. 2012;7:88. doi: 10.1186/1750-1172-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Beek N.A.M.E., van Capelle C.I., van der Velden-van Etten K.I., Hop W.C.J., van den Berg B., Reuser A.J.J., van Doorn P.A., van der Ploeg A.T., Stam H. Rate of progression and predictive factors for pulmonary outcome in children and adults with Pompe disease. Mol. Genet. Metab. 2011;104:129–136. doi: 10.1016/j.ymgme.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Yang C., Chien Y., Lee N., Chiang S., Lin S., Kuo Y., Chen S., Jong Y., Hwu W. Rapid progressive course of later-onset Pompe disease in Chinese patients. Mol. Genet. Metab. 2011;104:284–288. doi: 10.1016/j.ymgme.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Karabul N., Berndt J., Kornblum C., Kley R.A., Wenninger S., Tiling N., Mengel E., Plöckinger U., Vorgerd M., Deschauer M., Schoser B., Hanisch F. Pregnancy and delivery in women with Pompe disease. Mol. Genet. Metab. 2014;112:148–153. doi: 10.1016/j.ymgme.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Byrne B.J., Kishnani P.S., Case L.E., Merlini L., Müller-Felber W., Prasad S., van der Ploeg A. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol. Genet. Metab. 2011;103:1–11. doi: 10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Bembi B., Pisa F.E., Confalonieri M., Ciana G., Fiumara A., Parini R., Rigoldi M., Moglia A., Costa A., Carlucci A., Danesino C., Pittis M.G., Dardis A., Ravaglia S. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J. Inherit. Metab. Dis. 2010;33:727–735. doi: 10.1007/s10545-010-9201-8. [DOI] [PubMed] [Google Scholar]
- 9.Angelini C., Semplicini C., Ravaglia S., Bembi B., Servidei S., Pegoraro E., Moggio M., Filosto M., Sette E., Crescimanno G., Tonin P., Parini R., Morandi L., Marrosu G., Greco G., Musumeci O., Di Iorio G., Siciliano G., Donati M.A., Carubbi F., Ermani M., Mongini T., Toscano A. Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J. Neurol. 2012;259:952–958. doi: 10.1007/s00415-011-6293-5. [DOI] [PubMed] [Google Scholar]
- 10.Regnery C., Kornblum C., Hanisch F., Vielhaber S., Strigl-Pill N., Grunert B., Müller-Felber W., Glocker F.X., Spranger M., Deschauer M., Mengel E., Schoser B. 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J. Inherit. Metab. Dis. 2012;35:837–845. doi: 10.1007/s10545-012-9451-8. [DOI] [PubMed] [Google Scholar]
- 11.van der Ploeg A.T., Barohn R., Carlson L., Charrow J., Clemens P.R., Hopkin R.J., Kishnani P.S., Laforêt P., Morgan C., Nations S., Pestronk A., Plotkin H., Rosenbloom B.E., Sims K.B., Tsao E. Open-label extension study following the Late-Onset Treatment Study (LOTS) of alglucosidase alfa. Mol. Genet. Metab. 2012;107:456–461. doi: 10.1016/j.ymgme.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 12.van der Ploeg A.T., Clemens P.R., Corzo D., Escolar D.M., Florence J., Groeneveld G.J., Herson S., Kishnani P.S., Laforet P., Lake S.L., Lange D.J., Leshner R.T., Mayhew J.E., Morgan C., Nozaki K., Park D.J., Pestronk A., Rosenbloom B., Skrinar A., van Capelle C.I., van der Beek N.A., Wasserstein M., Zivkovic S.A. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N. Engl. J. Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 13.Strothotte S., Strigl-Pill N., Grunert B., Kornblum C., Eger K., Wessig C., Deschauer M., Breunig F., Glocker F.X., Vielhaber S., Brejova A., Hilz M., Reiners K., Müller-Felber W., Mengel E., Spranger M., Schoser B. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J. Neurol. 2010;257:91–97. doi: 10.1007/s00415-009-5275-3. [DOI] [PubMed] [Google Scholar]
- 14.Güngör D., de Vries J.M., Brusse E., Kruijshaar M.E., Hop W.C.J., Murawska M., van den Berg L.E., Reuser A.J., van Doorn P.A., Hagemans M.L., Marloes, Plug I., van der Ploeg A.T. Enzyme replacement therapy and fatigue in adults with Pompe disease. Mol. Genet. Metab. 2013;109:174–178. doi: 10.1016/j.ymgme.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Toscano A., Schoser B. Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J. Neurol. 2013;260:951–959. doi: 10.1007/s00415-012-6636-x. [DOI] [PubMed] [Google Scholar]
- 16.de Vries J.M., van der Beek N.A.M.E., Hop W.C.J., Karstens F.P.J., Wokke J.H., de Visser M., van Engelen B.G.M., Kuks J.B.M., van der Kooi A.J., Notermans N.C., Faber C.G., Verschuuren J.J.G.M., Kruijshaar M.E., Reuser A.J.J., van Doorn P.A., van der Ploeg A.T. Effect of enzyme therapy and prognostic factors in 69 adults with Pompe disease: an open-label single-center study. Orphanet J. Rare Dis. 2012;7:73. doi: 10.1186/1750-1172-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wattjes M.P., Kley R.A., Fischer D. Neuromuscular imaging in inherited muscle diseases. Eur. Radiol. 2010;20:2447–2460. doi: 10.1007/s00330-010-1799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alejaldre A., Díaz-Manera J., Ravaglia S., Tibaldi E.C., D'Amore F., Morís G., Muelas N., Vílchez J.J., García-Medina A., Usón M., Martínez García F.A., Illa I., Pichiecchio A. Trunk muscle involvement in late-onset Pompe disease: study of thirty patients. Neuromuscul. Disord. 2012;22(Suppl. 2):S148–S154. doi: 10.1016/j.nmd.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Carlier R., Laforet P., Wary C., Mompoint D., Laloui K., Pellegrini N., Annane D., Carlier P.G., Orlikowski D. Whole-body muscle MRI in 20 patients suffering from late onset Pompe disease: involvement patterns. Neuromuscul. Disord. 2011;21:791–799. doi: 10.1016/j.nmd.2011.06.748. [DOI] [PubMed] [Google Scholar]
- 20.de Jager A.E., van der Vliet T.M., van der Ree T.C., Oosterink B.J., Loonen M.C. Muscle computed tomography in adult-onset acid maltase deficiency. Muscle Nerve. 1998;21:398–400. doi: 10.1002/(sici)1097-4598(199803)21:3<398::aid-mus15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Del Gaizo A., Banerjee S., Terk M. Adult onset glycogen storage disease type II (adult onset Pompe disease): report and magnetic resonance images of two cases. Skelet. Radiol. 2009;38:1205–1208. doi: 10.1007/s00256-009-0797-4. [DOI] [PubMed] [Google Scholar]
- 22.Dlamini N., Jan W., Norwood F., Sheehan J., Spahr R., Al-Sarraj S., Anthony Hulse J., Hughes D., Champion M.P., Jungbluth H. Muscle MRI findings in siblings with juvenile-onset acid maltase deficiency (Pompe disease) Neuromuscul. Disord. 2008;18:408–409. doi: 10.1016/j.nmd.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Laloui K., Wary C., Carlier R., Hogrel J., Caillaud C., Laforêt P. Making diagnosis of Pompe disease at a presymptomatic stage: to treat or not to treat? Neurology. 2011;77:594–595. doi: 10.1212/WNL.0b013e318228c0ea. [DOI] [PubMed] [Google Scholar]
- 24.Pichiecchio A., Poloni G.U., Ravaglia S., Ponzio M., Germani G., Maranzana D., Costa A., Repetto A., Tavazzi E., Danesino C., Moglia A., Bastianello S. Enzyme replacement therapy in adult-onset glycogenosis II: is quantitative muscle MRI helpful? Muscle Nerve. 2009;40:122–125. doi: 10.1002/mus.21304. [DOI] [PubMed] [Google Scholar]
- 25.Pichiecchio A., Uggetti C., Ravaglia S., Egitto M.G., Rossi M., Sandrini G., Danesino C. Muscle MRI in adult-onset acid maltase deficiency. Neuromuscul. Disord. 2004;14:51–55. doi: 10.1016/j.nmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Ravaglia S., Pichiecchio A., Ponzio M., Danesino C., Saeidi Garaghani K., Poloni G.U., Toscano A., Moglia A., Carlucci A., Bini P., Ceroni M., Bastianello S. Changes in skeletal muscle qualities during enzyme replacement therapy in late-onset type II glycogenosis: temporal and spatial pattern of mass vs. strength response. J. Inherit. Metab. Dis. 2010;33:737–745. doi: 10.1007/s10545-010-9204-5. [DOI] [PubMed] [Google Scholar]
- 27.Vielhaber S., Brejova A., Debska-Vielhaber G., Kaufmann J., Feistner H., Schoenfeld M.A., Awiszus F. 24-months results in two adults with Pompe disease on enzyme replacement therapy. Clin. Neurol. Neurosurg. 2011;113:350–357. doi: 10.1016/j.clineuro.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Yonee C., Toyoshima M., Young S.P., Maruyama S., Higuchi I., Narita A., Maegaki Y., Nanba E., Ohno K., Kawano Y. Quantitative computed tomography for enzyme replacement therapy in Pompe disease. Brain Dev. 2012;34:834–839. doi: 10.1016/j.braindev.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Andreassen C.S., Schlütter J.M., Vissing J., Andersen H. Effect of enzyme replacement therapy on isokinetic strength for all major muscle groups in four patients with Pompe disease—a long-term follow-up. Mol. Genet. Metab. 2014;112:40–43. doi: 10.1016/j.ymgme.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Horvath J.J., Austin S.L., Case L.E., Greene K.B., Jones H.N., Soher B.J., Kishnani P.S., Bashir M.R. Correlation between quantitative whole-body muscle MRI and clinical muscle weakness in Pompe disease. Muscle Nerve. 2014 doi: 10.1002/mus.24437. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 31.Gloor M., Fasler S., Fischmann A., Haas T., Bieri O., Heinimann K., Wetzel S.G., Scheffler K., Fischer D. Quantification of fat infiltration in oculopharyngeal muscular dystrophy: comparison of three MR imaging methods. J. Magn. Reson. Imaging. 2011;33:203–210. doi: 10.1002/jmri.22431. [DOI] [PubMed] [Google Scholar]
- 32.Hiba B., Richard N., Hébert L.J., Coté C., Nejjari M., Vial C., Bouhour F., Puymirat J., Janier M. Quantitative assessment of skeletal muscle degeneration in patients with myotonic dystrophy type 1 using MRI. J. Magn. Reson. Imaging. 2012;35:678–685. doi: 10.1002/jmri.22849. [DOI] [PubMed] [Google Scholar]
- 33.Wokke B.H., Bos C., Reijnierse M., van Rijswijk C.S., Eggers H., Webb A., Verschuuren J.J., Kan H.E. Comparison of Dixon and T1-weighted MR methods to assess the degree of fat infiltration in Duchenne muscular dystrophy patients. J. Magn. Reson. Imaging. 2013;38:619–624. doi: 10.1002/jmri.23998. [DOI] [PubMed] [Google Scholar]
- 34.Hollingsworth K.G., de Sousa P.L., Straub V., Carlier P.G. Towards harmonization of protocols for MRI outcome measures in skeletal muscle studies: consensus recommendations from two TREAT-NMD NMR workshops, 2 May 2010, Stockholm, Sweden, 1–2 October 2009, Paris, France. Neuromuscul. Disord. 2012;22(Suppl. 2):S54–S67. doi: 10.1016/j.nmd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Park J., Kim H., Shin J., Choi Y., Kim D. Effect of enzyme replacement therapy in late onset Pompe disease: open pilot study of 48 weeks follow-up. Neurol. Sci. 2015;36:599–605. doi: 10.1007/s10072-014-2000-5. [DOI] [PubMed] [Google Scholar]
- 36.Raben N., Wong A., Ralston E., Myerowitz R. Autophagy and mitochondria in Pompe disease: nothing is so new as what has long been forgotten. Am. J. Med. Genet. C: Semin. Med. Genet. 2012;160C:13–21. doi: 10.1002/ajmg.c.31317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nascimbeni A.C., Fanin M., Masiero E., Angelini C., Sandri M. The role of autophagy in the pathogenesis of glycogen storage disease type II (GSDII) Cell Death Differ. 2012;19:1698–1708. doi: 10.1038/cdd.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nascimbeni A.C., Fanin M., Tasca E., Angelini C., Sandri M. Impaired autophagy affects acid α-glucosidase processing and enzyme replacement therapy efficacy in late-onset glycogen storage disease type II. Neuropathol. Appl. Neurobiol. 2015 doi: 10.1111/nan.12214. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 39.Cupler E.J., Berger K.I., Leshner R.T., Wolfe G.I., Han J.J., Barohn R.J., Kissel J.T. Consensus treatment recommendations for late-onset Pompe disease. Muscle Nerve. 2012;45:319–333. doi: 10.1002/mus.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]