Abstract
Ensuring the health of aquatic ecosystems and identifying species at risk from the detrimental effects of environmental contaminants can be facilitated by integrating analytical chemical analysis with carefully selected biological endpoints measured in tissues of species of concern. These biological endpoints include molecular, biochemical and physiological markers (i.e. biomarkers) that when integrated, can clarify issues of contaminant bioavailability, bioaccumulation and ecological effects while enabling a better understanding of the effects of non-chemical stressors. In the case of contaminant stressors, an understanding of chemical modes of toxicity can be incorporated with diagnostic markers of aquatic animal physiology to help understand the health status of aquatic organisms in the field. Furthermore, new approaches in functional genomics and bioinformatics can help discriminate individual chemicals, or groups of chemicals among complex mixtures that may contribute to adverse biological effects. While the use of biomarkers is not a new paradigm, such approaches have been underutilized in the context of ecological risk assessment and natural resource damage assessment. From a regulatory standpoint, these approaches can help better assess the complex effects from coastal development activities to assessing ecosystem integrity pre- and post-development or site remediation.
Keywords: fish, metals, oil, pesticides, assays, gene expression
INTRODUCTION
The input of anthropogenic contaminants to natural water systems has the potential to affect aquatic ecosystem health. Such inputs include controlled discharges, stormwater runoff and fugitive emissions, along with extreme events such as oil spills. When ecosystem degradation is identified, it is often difficult to determine whether effects are due to contaminants as opposed to non-chemical stressors such as turbidity, lack of oxygen, or lack of food due to changed in species composition resulting from habitat loss or over fishing.
Exposure to environmental contaminants can affect the survival of aquatic organisms via numerous mechanisms, including direct toxicity (both short- and long-term). More often the effects are more subtle, ultimately modulating organism fitness (Connon et al. 2009). Standardised laboratory measures of growth, reproduction and survival can be an unreliable estimation of the many indirect stressor effects in the field. More subtle changes in normal physiological function, such as appropriate reproductive behaviour, resilience to disease, and prey-capture abilities, may better indicate impacts on longer-term organism survival, reproductive output and ultimately, ecosystem health (Scholtz et al., 2012).
In order to better manage contaminants, it is desirable to be able to predict changes in ecosystem composition and function, as well as organism health, given a known set of environmental parameters and contaminant concentrations. It is also advantageous to have the ability to avoid losses in ecosystem structure and function before they occur, as opposed to conducting a retrospective analysis once an organism or system function has declined. However, this proposition is far more challenging than it seems, largely because of ecosystem complexity (for example, regular changes in the species composition and health of organisms in the field with tidal and seasonal cycles) and the presence of multiple stressors.
To further complicate environmental risk assessments, many contaminant exposure episodic in nature. Impacts such as oil spills, stormwater runoff following floods or heavy rains, release of antifoulants following ship groundings, release of material due to resuspension following dredging etc., are all likely to have a large influence on the health of organisms and their ability to recover (e.g. Scholz et al. 2011; Ward et al. 2013), even if they contribute minimally to overall contaminant concentrations over a larger spatial and temporal scale.
LIMITATIONS OF CURRENT METHODOLOGIES
The reductionist approach that is necessary to work with organisms in a laboratory setting may impair our ability to detect these sub-lethal, chronic impacts. Obviously, there are logistical constraints with the size and number of organisms that can be held in the laboratory. Complex behaviours, such as spawning aggregation or migration, cannot be reproduced in the laboratory. Long-term studies often pose logistical challenges as well. For example, determining the ultimate effects of chemical exposures that occur at larval phases on the reproductive output of a fish that takes years to reach sexual maturity are often outside the scope of a typical short-term research studies and also require extensive aquatic animal husbandry facilities to raise fish from larval to adult stages. In the field, organisms can move and potentially avoid contaminant hot-spots, whereas in the laboratory they are constrained to the test vessel (Ward et al. 2013). Furthermore, the ability to mimic natural stressors (e.g. hypoxia with tidal cycles, predator/ prey interaction, predator avoidance and food limitation) and complex natural exposure scenarios (e.g. stormwater-driven pulses, exposure to weathering oil following a spill, etc.) is extremely challenging, and often impossible. To further complicate laboratory studies, often only a small representative component of the spectrum of environmental species can be successfully maintained under laboratory conditions, making it necessary to use surrogate species in laboratory tests (Simpson and Spadaro 2011; Kennedy et al. 2009). While surrogate species are often chosen because they are sensitive to toxicants in standardized laboratory tests, these surrogate species may have very different life histories and abilities to deal with chemical and non-biological stressors relative to the target aquatic species of concern.
Traditional ecotoxicity tests are designed to be rapidly and inexpensively conducted, to be easily replicated, and to have obvious ecological significance (Fisher and Hook 2002). These tests may typically involve an examination of acute toxicity, or more chronic effects on growth and reproduction (Simpson and Spadaro 2011; Kennedy et al. 2009). They are often designed for short-lived, fast-growing species, and are typically conducted over a few days for acute tests, with many chronic tests typically 10-28 days. While these tests certainly provide valuable information, they do not mimic the environmental scenarios discussed above. In particular, they may not be predictive where exposure is to either low-level contaminant concentrations or to episodic, very high concentrations, and effects are subtle in the way they effect physiology but nevertheless, decrease the organisms’ fitness (Connon et al. 2009). To predict the subtle effects described above, it is necessary to move beyond the existing paradigm of standardized laboratory tests and beyond many of the traditional test endpoints. Here the use of molecular, biochemical or physiological biomarkers can provide unique insights into organism health.
BIOMARKER-BASED APPROACHES TO EVALUATING DEGRADED ECOSYSTEMS
Increasingly, environmental toxicologists are borrowing from the field of medicine to predict ecosystem health and function. Biomarkers are defined as detectable biochemical and tissue-level changes that indicate altered physiology (Smit et al. 2009). For example, a physician may analyse thyroid, kidney and liver function by combining a suite of biometric measurements and blood tests, as well as functional testing, and in some cases, imaging and pathology analysis. A similar multivariate biomarker approach involving multiple biological and physiological measurements, as well as analytical chemistry where appropriate, can also be used to assess the health of indicator species within ecosystems.
Biomarker approaches are not new, with several biomarkers being in use for decades (Stegeman 1978; Roesijadi 1980; Lee et al. 1981), although the acceptance of these approaches by regulatory agencies has been inconsistent. Water quality guidelines (e.g. ANZECC/ARMCANZ 2000) typically recommend that biomarker studies are best used to indicate exposure, as it is difficult to extrapolate between test results and ecosystem effects, even though they acknowledge that biomarker assays may be the best indicator of chronic stress (Hyne and Maher 2003). However, biomarkers, in general, have not yet been fully exploited in the context of an integrated monitoring system, and there are additional under-utilised advantages to incorporating a biomarker-based approach. Carefully chosen biomarkers may be the best approach to identify an early response to contaminants (Broeg et al. 2005) and are much more sensitive for identifying organism stress than whole animal responses (Smit et al. 2009), although it is not always clear the origin of the stress, and there are often multiple stressors present. Biomarker responses can be measured in organisms collected from, or deployed in field sites, to integrate the effects of chemical and non-chemical stressors, reducing the need for complex laboratory exposure scenarios. To achieve these goals, however, it is important that a biomarker-based approach links mechanistic knowledge with a consideration of whole-animal physiology and population-level effects (reviewed in Connon et al. 2011).
In the following sections, the utility of different types of biomarkers that help to better understand environmental stressor effects on organism health is described.
CLASSES OF BIOMARKERS AND THEIR APPLICATION TO AQUATIC ECOSYSTEMS
Biomarkers have been classified by the extent that they reflect exposure to environmental stressors, or adverse health effects from contaminant exposures. Some biomarkers can also indicate susceptibility to adverse outcomes from environmental contaminants, although these have not been developed or incorporated in ecological assessment frameworks due to the fact that they largely rely on well-defined genetic databases and the incorporation of epidemiological studies, which are areas of future development in the field of aquatic toxicology. However, biomarkers that reflect both exposure and biological effects have been widely incorporated in field and laboratory studies, although the examples are few (Taylor and Maher 2010).
Exposure Biomarkers
Biomarkers of exposure to single or multiple contaminants with similar modes of action (Table 1) can show an early response to contaminants and are typically specific to a particular class of contaminants, e.g. biliary fluorescent aromatic compounds (FACs) for exposure to oil, or the induction of the egg-yolk precursor protein vitellogenin (vtg) for environmental estrogens (Broeg et al. 2005).
Table 1.
Examples of biomarkers that have been used to assess aquatic contaminants
| Category | Measurement and Indication | References |
|---|---|---|
|
Biomarkers of exposure
| ||
| Bile fluorescent aromatic compounds (FACs) | Metabolites of polycyclic aromatic hydrocarbons measured in the bile of fish, can reflect exposure to oil | Myers et al. 1991; Myers et al. 1994 |
| Cytochrome P4501A mRNA or protein | An inducible isoform of the cytochrome p450 family that is measured in various tissues of fish and bivalves exposed to oil, and other chemicals that are Ah receptor agonists | Collier et al. 1996; Roberts et al. 2004 |
| Ethoxyresorufin-o-deethylase (EROD), Aryl hydrocarbon hydroxylase | Catalytic activities of CYP1A enzyme described above | Huggett et al. 2003; Martinez-Gomez et al. 2009 |
| Vitellogenin (vtg) | Egg yolk precursor protein induced on exposure to a broad range of estrogenic compounds | Folmar et al. 1996; Denslow et al. 2004 |
| Metallothioneins | Metal-binding proteins that are induced on exposure to certain metals (Cd, Hg) | Roberts et al. 2005; Sakuragui et al. 2013; Williams and Gallagher 2013 |
| Biomarkers of biological effects | ||
| Heat-shock proteins (i.e. HSP 90) | Proteins that are induced in tissues of aquatic organisms as a generalized response to stress, including exposure to chemicals, hypoxia, and temperature | Downs et al.2006 |
| Markers of oxidative stress (heme oxygenase, superoxide dismutase, glutathione, catalase, lipid peroxidation) | Enzymes, cofactors in metabolic products that can be quantitatively altered on exposure to pollutants. Oxidative stress is an adverse cellular and tissue level response to a variety of contaminants and non-chemical stressors | Ahmad et al. 2006; Nogueira et al. 2013; Patil and David 2013; Pereira et al. 2013; Williams and Gallagher 2013 |
| Markers of lysosomal membrane stability | Sub-cellular organelles containing hydrolytic enzymes that are sensitive to toxicity leading to membrane rupture and leakage (metals) | Ringwood et al. 2004; Edge et al. 2012 |
| Condition indices (hepatosomatic index, gonadal indices) | Decreases in organ weight relative to whole body weight can reflect organ toxicity or disease | Johnson et al. 2008a; Blazer et al. 2012 |
| Circulating hormone levels | Levels of circulating hormones can be used to measure normal sexual and reproductive behaviour | Blazer et al. 2012 |
| DNA damage measures (induction of DNA repair enzymes, presence of PAH-DNA adducts) | DNA damage is a reflection of exposure and genotoxic effects | Balk et al. 2011 |
| Triglyceride levels, IGF1, | The levels of lipids (such as triglycerides) and of growth hormones (such as IGF1) can be used as a metric of the animals energy reserves | Balk et al. 2011 |
|
Biomarkers that integrate both chemical exposures and biological effects | ||
| Acetylcholinesterase (AChE) | An enzyme that hydrolyzes neurotransmitters that is inhibited by exposure to carbamate and organophosphate pesticides | Laetz et al. 2009 |
| Tissue transcriptome | Microarray and RNA sequencing of tissue transcriptome can identify cellular pathways perturbed by chemical and environmental stressors | Connon et al. 2012; Uren et al. 2013 |
One of the most common biomarkers in this category is the measurement of the induction of a cytochrome P450 1A (i.e. CYP1A) at the messenger RNA, protein, or catalytic activity levels, as biomarkers of exposure to components of oil (Whyte et al. 2000; Lee and Anderson, 2005). Furthermore, the presence of some PAH metabolites excreted in the bile of fish (e.g. bile FACs) has also been used successfully to track the temporal and spatial effects of oil pollution (Aas et al. 2000). Oil biomarkers are particularly important as some components of oil can be rapidly metabolized in aquatic organisms (Whyte et al. 2000) or undergo environmental breakdown. However, elevations in oil exposure indices such as CYP1A activity have not been linked to higher level biological effects (Lee and Anderson 2005). Accordingly, measures of CYP1A as well as biliary FACs would have most predictive power in a regulatory context when used as part of a suite of biomarkers, including those discussed below that are more intricately linked to physiological and higher-level effects.
Induction of vtg in male or juvenile fish is a commonly used biomarker of exposure to environmental estrogens (e.g. Denslow et al. 2004; Folmar et al. 1996; Jobling et al. 1998). This induction can be measured both at the protein (Folmar et al. 1996; Jobling et al. 1998, Johnson et al. 2008a) and at the mRNA level (Roy et al. 2003). Increased vtg levels have been linked to pharmaceuticals in sewage (Folmar et al. 1996; Jobling et al. 1998; Roy et al. 2003), to overall anthropogenic impact (Johnson et al. 2008a), as well as to phytoestrogens in pulp and paper mill effluents (Denslow et al. 2004).
Metallothionein (MT) has known roles in routine physiological processes involving essential metals and in detoxifying non-essential metals such as cadmium (Amiard et al. 2006). Different MT isoforms can also be responsive to oxidative stress (Hook et al., 2006). MT mRNA levels in fish have been shown to vary along a contamination gradient (Tom et al. 1998), and also have been shown to correlate with bioavailable metals in fish caged along contamination gradients (Chesman et al. 2007). MT mRNA levels were a highly sensitive indicator of laboratory cadmium exposures in Coho salmon (Williams and Gallagher, 2013). Interestingly, another study of Coho salmon indicated that measuring MT mRNA levels in the olfactory system of salmon was more sensitive than in the liver, suggesting tissue-specific differences in MT induction (Espinoza et al. 2012).
Acetylcholinesterase (AChE) inhibition has long been used as a biomarker for exposure to, as well as effects of, carbamate and organophophorus pesticides in both fish and invertebrates (reviewed in Fulton and Key 2001; Klumpp et al. 2002), although it is not always as effective a biomarker for molluscs due to avoidance behaviours (Cooper and Bidwell, 2006). AChE inhibition is a rare and in some ways ideal biomarker as it indicates both exposure and effects. Inhibition can be linked to both decreased swimming stamina and survival, although the exact relationships between these linkages tend to be species specific (Fulton and Key 2001). Exposure to pesticides and AChE inhibition are also thought to be contributing to the poor recovery of some Pacific salmon populations (Laetz et al. 2009).
Effects Biomarkers
Examples of effects biomarkers, i.e. indicators of physiological or biochemical changes as a consequence of exposure, are listed in Table 1. These indices may be direct measures (e.g. DNA damage, AChE inhibition), or indirect measures such as the impact on sub-cellular lysosomes. Theoretically, an organism with a quantitative change in an effects biomarker will undergo some loss of fitness. However, these associations can be difficult to demonstrate quantitatively.
Despite their advantages, a major disadvantage of effects biomarkers is that very few can be linked directly to exposures to specific classes of chemicals. However, there are some notable exceptions, including AChE activity, as well as the presence of PAH-DNA adducts (Table 1). However, as discussed later, effects biomarkers can be effectively incorporated with other diagnostic markers of fish health and also with analytical chemistry approaches to provide evidence for the contributions of chemical exposures.
Biomarkers of oxidative stress represent a unique sub-class of effects biomarkers that have been applied inconsistently in aquatic studies. Oxidative stress is part of the ageing process and occurs in all living organisms when reactive oxygen species (or their by-products) cause cell and tissue injury (Winston 1991; Kelly et al. 1998). It is well accepted that exposure to a broad range of environmental chemicals, including pesticides, metals, and PAHs increase the level of cellular oxidative stress in aquatic organisms (Winston 1991; Kelly et al. 1998; Livingstone 2001; Pandey et al. 2003; Banni et al. 2005; Farombi et al. 2007; Taylor and Maher 2010). Aquatic organisms contain a full complement of antioxidant enzymes which comprise antioxidant defenses, including enzymatic components (e.g. superoxide dismutase (SOD), catalase and glutathione peroxidases) as well as small molecule antioxidants (e.g. glutathione). Since antioxidant defences can be quantitatively altered on exposure to contaminants, this has led to their exploitation as biomarkers in the field (Livingstone 2001; Pandey et al. 2003; Banni et al. 2005; Farombi et al. 2007). A breakdown in the antioxidant detoxification procedures with the potential for higher order effects has also been observed for metals in bivalves (Taylor and Maher 2010).
Elevated antioxidant enzyme parameters associated with increased lipid peroxidation have been observed in the fish, Fundulus sp. exposed to complex mixtures of organic chemicals (Bacanskas et al. 2004). Similar results have been reported for bivalves (Banni et al. 2005). Oxidative stress-associated parameters were particularly sensitive biomarkers of contaminants in receiving waters of a hydroelectric power plant (Sakuraguiet al. 2013).
Because the response of the organism may be both xenobiotic- and also tissue-specific, it is also important to examine several endpoints related to oxidative stress in different tissues. For example, Otto and Moon (1996) compared brown bullhead (Ameriurus nebulosus) collected from a system contaminated with PCBs to bullhead collected from a relatively non-contaminated site. Fish from the contaminated site had extremely high PCB concentrations in muscle compared to fish from the non-contaminated site, and SOD activity was increased in the kidney of fish from the contaminated site. However, the activity of another antioxidant enzyme, catalase, was lower in the kidney, and there were differences in the trends of expression of other enzymes. Studies such as these highlight the importance of tissue selection when evaluating oxidative stress in feral organisms.
Protective antioxidant enzymes and their non-enzymatic cofactors (e.g. GSH) can be overwhelmed during high levels of tissue oxidative stress, which can lead to the accumulation of metabolic by-products reflecting oxidative damage. These metabolic by-products include oxidized pigments such as lipofuscin, peroxidized cell membranes and their ensuing membrane breakdown products (i.e. lipid peroxides, malondialdehyde)as well as oxidative DNA and protein damage. These secondary products of oxidative damage can initially compromise the ability of the organism to maintain normal metabolic processes, and under chronic oxidative stress, to the disease states mentioned above. Lipid peroxidation, in particular, has been routinely measured in field studies to reflect chemical-induced oxidative damage.
It is important to note that there are quantitative differences in the normal levels of these antioxidants, as well as their induction capabilities among the various aquatic species (Kelly et al. 1998). Accordingly, oxidative stress biomarkers need to be carefully validated under controlled laboratory conditions Also, oxidative stress biomarkers and other enzyme based biomarkers are known to exhibit a “bell shaped dose response curve” with increasing toxicant dose or exposure time (e.g. Marigomez et al., 2013) This should be taken into account when designing field experiments. Furthermore, these parameters are significantly affected by nutritional status, age, and other non-chemical factors.
Another commonly used effects biomarker is the measurement of the integrity and stability of lysosomal membranes detected by the uptake and retention of a cationic dye, neutral red. Dose-response relationships have been found for a range of aquatic contaminants, including both metals and organics (Moore et al. 2013). Lysosomal stability has been positively correlated with scope for growth, reproductive output, genotoxicity, total oxyradical scavenging capacity and protein synthesis and can be deemed as ecologically relevant (Ringwood et al., 2004). Edge et al. (2012), in a study of the Sydney rock oyster in contaminated estuaries containing both metals and PAHs, showed that lysosomal membrane stability was a more useful indicator of environmental stress than lipid peroxidation or concentrations of glutathione (GSH). Lysosomal stability correlated well with fertilisation, normal embryo development and estuary status.
Significant correlations were observed between lysosomal membrane stability and induction of the exposure marker MT in the digestive glands and gills and liposomal membrane stability, in mussels (Mytilus galloprovincialis) exposed to heavy meal contaminants (Domouhtsidou et al. 2004), but it was noted that other factors such as age, size and seasonal variation could contribute to MT induction. The aforementioned findings and those of other studies support the use of multiple biomarkers covering both exposure and effects, rather than the use of a single indicator.
Genotoxicity is another important effect that is commonly measured using biomarkers. For instance, DNA integrity (measured as strand breaks with the Comet Assay) was inversely correlated with total oxyradical scavenging capacity in mussels collected from a eutrophic lagoon in coastal Italy (Frenzilli et al., 2001). Both a spatial and temporal gradient was measured, with mussels having poor metrics in the areas with the worst water quality and in the summer, when temperatures were elevated. Both metrics were correlated with a loss of biodiversity in the area (Frenzilli et al., 2001). DNA damage (measured using the Comet Assay) was also found to correlate with concentrations of sediment contaminants in mussels deployed in San Diego Harbour, CA., USA (Steinert et al., 1998).
DNA damage can also be measured directly using DNA adducts. For instance, levels of DNA adducts were found to be elevated in fish captured near an aluminium smelter in western Norway (Aas et al., 2001). Adduct levels correlated to both EROD (indicating PAH exposure) as well as skin ulcers and fin erosion (Aas et al., 2001). \ DNA adducts were also used to measure the potential impacts of the Sea Empress oil spill (Harvey et al., 1999). DNA adducts were measured in a sponge, a mussel and several species of fish. Although elevated levels of adducts were not measured in either species of invertebrate, they were found in all three fish species. The return of these adduct to baseline levels after eighteen months indicated that fish populations were no longer under genotoxic threat (Harvey et al., 1999).
Transcriptomic Biomarkers that Integrate Exposure and Effects
Incorporation of transcriptomic, or genome-enabled biomarkers, is becoming increasingly recognized as a powerful approach that can yield information on both exposure and pathways of injury (i.e. biological effects), and thus provide somewhat of a bridge between exposure and effects. Genomic approaches are widely accepted in the context of biomarker generation (reviewed in Hook, 2010), however, these transcriptional approaches need to be validated with a phenotypic anchor, i.e. clearly defined and measurable effects at organ, tissue, or physiological levels. For example, whole tissue transcriptome profiling has been effectively used for screening adverse outcomes associated with drug and chemical toxicity in mammalian studies (Hu et al. 2000; Martin et al. 2006). Microarray approaches may be particularly useful in determining the contributions of various classes of environmental contaminants toward sub-lethal toxicity in mixture scenarios (Tilton et al. 2011; Osborn and Hook, 2013; Hook et al., 2014).
Increasingly, microarray-based gene-expression profiling, or other methodologies that measure overall gene expression, are being used as “discovery” tools to characterize response in both laboratory and field studies. These profiling techniques have the advantage of being able to be used when the stressors are unknown and being able to identify the causative agent of decline in the organism's health, since any field results can be compared to results from controlled laboratory studies. They are also inherently multivariate, in that transcript levels of biomarkers can be assessed simultaneously with the transcripts for gene products more closely associated with fitness. The disadvantage of these techniques is that interpreting the data can be challenging, especially given that the No Observable Transcriptional Effects Level (NOTEL) is often far below the concentrations at which toxic effects are seen (Poynton et al. 2007; Poynton and Vulpe 2009), and that the annotation of many genomes is incomplete. Van Straalen and Feder (2012) considered transcriptomics as a ‘super-biomarker’ because of the wealth of information generated but noted the current difficulty in linking the measured gene expressions to ecologically relevant effects.
CHALLENGES ASSOCIATED WITH ESTABLISHING LINKAGES BETWEEN EXPOSURE AND EFFECTS
The use of a comprehensive suite of markers within a single target tissue can sometimes yield results that are difficult to evaluate biologically. For example, McFarland et al. (1999) investigated site differences in liver antioxidant parameters in brown bullhead fish in a PAH-contaminated and a control site in Ohio, USA. In the aforementioned study, the levels of the enzymes glutathione reductase, glutathione S-transferase, and glutathione peroxidase were measured, but no site differences were present. In contrast, other antioxidant parameters such as SOD, catalase, and total tissue glutathione levels appeared to correlate with environmental exposure to PAHs. In another study, 11 biochemical markers of aquatic pollutants were evaluated in the livers of chub caught at several sampling sites of a river containing various contaminants (Machala et al. 2001). The results were tested against measured concentrations of organochlorine compounds, PAHs, and heavy metals. The biochemical markers of oxidative stress, including in vivo lipid peroxidation and in vitro production of ROS, did not correlate with the concentrations of the contaminants, while glutathione-dependent enzymes formed a suitable battery of exposure biomarkers (Machala et al. 2001). These studies are among many that illustrate the complexities associated with drawing meaningful conclusions from differential antioxidant responses observed in the field. The section above summarizes the types of biomarkers that can be applied to the assessment of aquatic animal health. In the following sections, we provide some scenarios where these physiological markers have successfully been used in field scenarios.
DETERMINING THE SPATIAL AND TEMPORAL EXTENT OF CONTAMINATION
Biomarker-based approaches have been used to determine the spatial and temporal patterns of influence of a stressor in the field, i.e. extent of potential exposure. For instance, following a major oil spill, determining over what period of time the oil persists and whether organisms in the spill path were exposed is an important component of the ensuing risk assessment. Known oil exposure biomarkers, such as induction of CYP1A or EROD, are frequently used to assist in the assessment of hydrocarbon distribution and bioavailability.
Following a fuel oil spill in Micronesia, resource managers were uncertain as to whether the fuel oil partitioned into the water column, and if it did, if the effects of the fuel oil would be short-term or long-term. Downs et al. (2006) used protein-based biomarkers including heat-shock proteins, cytochrome p450s, heme oxygenase, SOD, catalase and DNA-repair enzymes that were indicative of exposure to fuel oil. The authors reported significantly different levels of expression between impacted and reference sites three months after the spill (Downs et al. 2006), indicating that there was sufficient fuel oil in the water column to have some impact on coral for at least three months.
Whether the oil released from the 1989 Exxon Valdez spill in Prince William Sound, Alaska, USA, caused long-term ecosystem damage, and if so, to what portions of the ecosystem, is still the subject of fierce debate (e.g. Incardona et al. 2012; Sellin Jefferies et al. 2013). Biomarker type responses were extensively used to assess these potential impacts. Aryl hydrocarbon hydroxylase levels were measured in Dolly Varden trout (Salvelinus malma) 120 and 460 days after the spill and were found to be elevated in fish collected from a heavily oiled site relative to those collected from a reference site (Collier et al. 1996). However, several years later, the oil response biomarkers were no longer elevated.
Hugget et al. (2003) collected fish from three operationally defined areas within Prince William Sound: (i) areas that were in the spill path and had oil deposited in the sediments at the time of the spill; (ii) areas in the spill path but had no oil deposited in the sediments at the time of the spill; and (iii) areas outside of the spill path. The researchers measured oil exposure biomarkers (EROD induction, CYP1A), immunohistochemistry, and bile FACs in near-shore and offshore fish species. These biomarkers were consistently found in the fish they collected, yet there were no significant difference in their levels between operational sites and other regions in the Gulf of Alaska. The data were used to argue that there was no ongoing exposure to Exxon-Valdez oil in Prince William Sound and that the exposure levels being measured in this study were due to other oil sources in the Gulf of Alaska, such as seeps and boat traffic.
Biomarkers levels were measured in the livers of demersal fish following the Prestige oil spill in Northern Spain (Martinez-Gomez et al. 2009) with the goal of determining the spatial and temporal extent of the impacts. EROD, GST, glutathione reductase and catalase activity were all measured, with EROD being the most sensitive. Patterns of biomarker induction matched the trajectory of spilled oil and patterns of oil deposition. In addition, levels of induction returned to baseline over a time period of two years at most sites (Martinez-Gomez et al. 2009). Two years after the spill, PAH concentrations were returning to background, but some sites still had a spill signature (Fernandez et al. 2010). To determine whether these concentrations of PAHs were having any biological effect, a variety of effects biomarkers were measured in mussels along a spatial gradient. At the spill sites, mussels had elevated oxidative stress markers, but no change in physiological markers that indicate changes in growth and reproductive capacity (Fernandez et al. 2010). Taken together, these data indicated that exposure to oil from the Prestige may have had an impact on fish health following the spill, but with a return to a normal physiological status within a few years.
Studies examining the spatial patterns of a stressor have not been confined only to oil. Microarray-based measures of gene expression have also been successfully used to distinguish contaminated field sites from relatively pristine ones in agricultural areas (Sellin Jeffries et al. 2012). Fathead minnows were caged in rivers having high concentrations of both agricultural pesticides and veterinary pharmaceuticals that have been shown to have anti-estrogenic properties. The patterns of hepatic gene expression were contrasted to minnows caged in rivers with low concentrations of these chemicals. The authors found that the patterns of gene expression could be used to differentiate high- and low-likelihood of impacts due to contaminant exposure. Microarray-based gene expression assays have also been used to differentiate between fish collected up- and downstream of a sewage treatment plant (Garcia–Reyero et al. 2008). These studies demonstrate the ability of transcriptomic biomarkers to differentiate between exposed and unexposed animals even when the exposures are to very low concentrations of complex mixtures. These gene expression patterns persisted despite the variability inherent in the transcriptome.
PREDICTING THE HEALTH OF ORGANISMS
Quantitative changes in biomarker levels have also been used to examine the health of organisms in contaminated waters. In these studies, the focus has been primarily but not exclusively on effects biomarkers. As a proof of concept, Broeg et al. (2005) used a multi-biomarker approach to differentiate amongst sites in the German Bight. They were able to distinguish both pristine and contaminated sites and to show a progression of disease from early to pathological stages in the demersal flounder Platichthys flesus. Their indices also correlated with other metrics of organism health such as immunological and lipid status. Dagnino et al. (2007) deployed caged mussels to a highly contaminated site near a refinery and used a series of effects-based biomarkers (oxidative stress markers, lipid accumulation and lysosomal stability, which has long been known to correlate with both exposure and effects of contaminants (see Ringwood et al. 1998)) to show a progression of deterioration in health with time. They also showed effects along a longitudinal gradient, using this series of biomarkers. This same series of biomarkers was recently used by Shaw et al. (2011) to categorize the health of mussels from the Tamar estuary in the UK. In addition, they also categorized stress using microarray-based analyses of gene expression. Both lysosomal stability and oxidative-stress markers were correlated with PAH concentrations in the sediment, and the number of genes with altered patterns of transcription was highest at the most contaminated sites. However, no changes in neutral lipids with contamination level were observed (Shaw et al. 2011).
A similar study in Southern Portugal transplanted mussels from a metal-contaminated site to a PAH-contaminated one and vice versa (Serafim et al. 2011). Over four weeks, the metal-responsive biomarkers decreased in the former and increased in the latter, whereas the PAH-responsive biomarkers showed the opposite trend. These studies demonstrate the utility and specificity of biomarkers in identifying relevant stressors and showing gradients of pollution. Multiple biomarkers, including several measures of oxidative stress, genotoxicity, lysomal integrity, MT and a vitellogenin like protein, were also used to predict the health status of two sites on the Basque coast (Marigomez et al., 2013). Integrative biomarker indices that considered all of these metrics were predictive of environmental health status.
Steelhead trout (Oncorhynchus mykiss) numbers have been declining in the Columbia River basin (USA), and there has been concern that increased exposure to contaminants (associated with the dams used for hydroelectric power generation on the river) is associated with decreased pathogen resistance (Connon et al. 2012). Previous work (Fiest et al. 2005) with sturgeon (Acipenser transmontanus) collected from this system had shown good reproductive success and low contaminant body burdens in fish from areas of the river that were free-flowing, but poor reproductive success and high contaminant concentrations were observed in fish collected from areas behind dams. Gonad size, circulating hormone levels, and triglycerides were all negatively correlated to contaminant levels (Fiest et al. 2005) suggesting that exposure to dam-associated contaminants could be impacting fish health. To test the hypothesis that contaminant exposure is directly influencing the health of these fish, Connon et al. (2012) measured immune response, general stress and contaminant-exposure biomarkers in out-migrating salmon collected at various field sites along the Columbia River. They found that gene expression profiles correlated to pathogen presence and that changes in gene expression could be used to separate fish into good, fair and poor condition. They suggested that these transcriptomic assays could be used as a tool to monitor fish health and to inform resource management.
BEHAVIOUR-BASED BIOMARKERS
Some studies indicate that biomarker responses could be used to make field-based measurements of endpoints that are difficult to capture in laboratory-based studies. In laboratory-based studies, exposure to the pesticide esfenvalerate at ng/L concentrations altered swimming behaviour in the delta smelt (Hypomesus transpacificus) (Connon et al. 2009). These changes could be correlated to changes concentrations of genes involved in neuromuscular processes, immune responses, apoptosis and redox and metal binding. Some genes, such as aspartoacylase and creatine kinase, could be mechanistically linked to the disrupted physiological processes (Connon et al. 2009). Exposures to environmentally relevant concentrations of copper were also shown to decrease swimming speeds in the endangered delta smelt (Connon et al. 2011). These behavioural changes also appeared to be related to changes in gene expression (Connon et al. 2011). The impact of low level exposures to certain contaminants, most notably pesticides and metals, has been linked to loss of olfactory ability in several fish species (Scholz et al. 2000; Tierney, Ross et al. 2007; Tierney et al. 2010; Baldwin et al. 2011).
Recently, Williams et al. (2013) linked molecular biomarker encoding olfactory receptors and signal transduction components to histological injury and loss of behavior in Coho salmon exposed to cadmium. This study is consistent with studies using the model organism zebrafish showing that olfactory antioxidant defense and signal transduction gene expression is quantitatively altered on exposure to the model trace metals copper and cadmium (Tilton et al. 2008; Wang et al. 2013). In the case of copper, the zebrafish study provided molecular insights into the ability of copper to impair sensory function in coho salmon, which has been linked with increased susceptibility to predation (McIntyre et al. 2008, 2012).
IDENTIFYING STRESSORS WITHIN COMPLEX SCENARIOS CONTRIBUTING TO ECOSYSTEM DECLINE
A combination of exposure and effects biomarkers can be used to identify the impacts of specific contaminant (e.g. Ernst and Peterson, 1994). A multiple biomarkers framework was used to determine whether specific contaminants were causing unknown species declines in waters of Puget Sound (USA), an urbanized estuary with limited circulation (Johnson et al. 2008b). The measures of total PAHs in sediments have been steadily increasing along with human population growth, and it was hypothesized that these increases are adversely impacting fish health. The multiple biomarker approach included determining levels of bile metabolites and CYP 1A activity to demonstrate exposure to PAHs, as well as showing that increased DNA adducts (which indicate the initiation of a cascade that can result in hepatic carcinogenesis) could all be correlated with increased PAHs in the sediments (Myers et al. 2003) and decreased fish health (reviewed in Johnson et al. 2008b). Over decades, this dataset has been successfully used to justify remediation and restoration efforts of several bays and waterways within Puget Sound (Johnson et al. 2008b) and the same biomarkers have been used to monitor the success of the remediation efforts. Analytical measures indicated that dredging sediments had removed PAH. Decreases in biomarker levels were indicative that bioavailable PAH concentrations decreased in a manner corresponding to the measured biomarkers (Myers et al. 2000, 2003). Since the measured PAH levels, biomarker levels and declines in fish reproductive status and increased tumour prevalence were all related, it was assumed that changes in biomarkers were predictive of PAH induced changes in fish health (reviewed in Johnson et al., 2008b).
Coral reefs are frequently subjected to multiple stressors, and are known to be in decline due to a variety of anthropogenic factors worldwide (Downs et al. 2012). Being able to predict which stressor was causing effects would enable remediation efforts to be prioritized. Multiple biomarkers were used to differentiate between chemical exposures and effects in corals from Guam (Downs et al. 2012). Coral tissue were collected from a series of sites separately influenced by herbicides and other pesticides, sedimentation, fuel and heat (at a shipyard), PCBs at a landfill site and antifoulants at a marina. Protein biomarker levels in corals from these sites were compared to a reference site and profiles indicative of the purported stressors were distinguished (Downs et al. 2012). The biomarker changes were consistent with known modes of action of the contaminants. This approach raises the possibility of using biomarkers in the equivalent of a toxicity identification and evaluation (TIE) procedure (Osborn and Hook, 2013, Hook et al., in review).
CHALLENGES IN USING BIOMARKER-BASED APPROACHES
The interpretation of biomarker studies is not without its challenges. For instance, the relationships between tissue body burden and biomarker levels can be confounded by age, sex and reproductive status (Hylland et al. 2009) which may necessitate either sampling at a reproductively inert time of the year or sampling juveniles. Also, some putatively contaminant-specific biomarkers have been shown to be induced by non-contaminant stressors. For instance, metallthionein in oysters has been shown to respond to other non metal stressors, including handling stress, anoxia and antibacterial compounds (reviewed in Amiard et al., 2006). Humic substances common to surface waters have also been shown to induce CYP1A in some fishes (Matsuo et al., 2006).
Furthermore, a conceptual framework for the progression of toxicant injury to aquatic organisms from initial injury through to death is provided in Figure 1 (see also Ankley et al., 2010; Brain and Brooks, 2012). At all of these levels, the cell/organism is trying to maintain homeostasis, so that all of these changes (except death) may ultimately be reversible. This reversibility in itself has applications for the use of biomarkers in environmental assessment. For instance, the lack of biomarker induction in fish years after the Valdez spill (e.g. Huggett et al., 2003) or lack of transcriptomic changes in mummichogs following remediation of a chromium contaminated shipyard (Roling et al. 2007) has been used to show that particular stressors were no longer compromising health.
Figure 1.
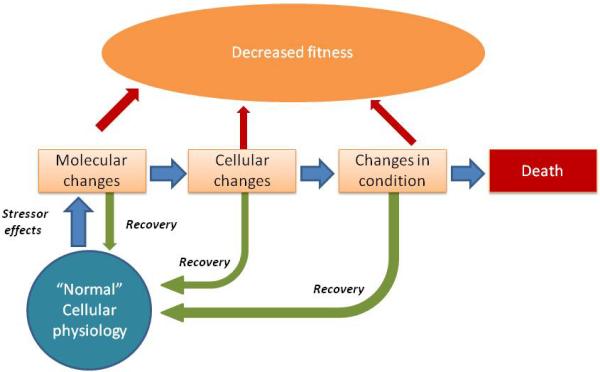
Conceptual model of a cellular response to a stressor to be evaluated using biomarkers
As shown in the framework depicted in Figure 1, organisms can undergo physiological changes to adjust to an external stressor, yet still maintain normal cellular function (termed ‘accommodation’, Nicholls et al. 2011). These changes are ’compensatory mechanisms’ that can mask toxicological impacts and complicate regulatory decisions. However, compensatory mechanisms have an associated energetic cost and can cause chronic stress and increase the organism's allostatic load (i.e. energetic demands or capacity to cope with stress). If the allostatic load is exceeded, the organism's health is negatively impacted (Nicholls et al. 2011).
Consequently, the reversibility of biomarker-like changes as a result of the maintenance of normal physiological function via compensatory mechanisms may compromise the overall fitness of the organism, even if the molecular and cellular changes return to a baseline state. An example of these costs is illustrated by a recent study by Kerabrum et al. (2102) in which juvenile fish were exposed to levels of crude oil relevant to levels encountered following an oil spill for a short time period (48-96 h), then allowed to depurate (recover) in clean water for a period of 4 weeks. At the end of this period, the condition of the fish was less than that of controls, even though the biomarker levels were decreasing to baseline levels. While in this case, elevations in exposure biomarkers were predictive of a long-term effect, a definite relationship cannot be made (Lee and Anderson 2005). It is often unknown whether a particular change is reversible, and what cost the maintenance of normal physiological function has to the organism. It has been suggested that a future challenge for the field will be to collect sufficient data from experimentally exposed and field-captured organisms to better differentiate between protective molecular responses and those that are indicative of an impending disease (Shaw et al. 2011).
Since biomarker induction following toxicological insult can be a dynamic process between injury and repair, choosing the proper timing for sampling of biomarker responses is critical. One of the chief advantages of biomarker responses is that they are generally rapid (Kerabrum et al. 2012), e.g. transcriptomic changes can be measured over a period of hours for example (Hook et al. 2008, Hook et al. 2010), while protein changes typically are slower to induce but longer lasting (Nahrang et al. 2009). This can be an advantage in that effects are seen early, however, some knowledge of the kinetics of the response, either from literature data or experiment, is desirable before using biomarkers an indicator of effects.
USE OF MULTIPLE BIOMARKERS
In this review, we are advocating a thoughtful approach measuring multiple biomarkers, ideally across multiple levels (as depicted in Figure 1). Measuring across multiple levels of function will allow better estimation of the stressors that are causing changes in fitness (exposure biomarkers), as well as the likely changes in cellular function and fitness (effects and condition biomarkers). Redundancy in measures would also likely decrease the probability of a false positive due to a spurious result in a single measure. Balk et al. (2011) used a multiple biomarkers framework to evaluate the health of fish collected near offshore oil and gas platforms. The authors noted elevations in oil exposure biomarkers (biliary PAH metabolites and EROD), changes in oxidative stress markers, and changes in lipid ratios. When integrated, these data indicate that the haddock and cod collected in areas proximate to oil and gas platforms have decreased fitness due to exposure to petroleum hydrocarbons.
As acknowledged by Bartell (2006), effects at the cellular and sub-cellular level, using one or several biomarkers are perceived to be ecologically difficult to interpret and their ecological significance is often not known or readily estimated. For example, the extent to which endocrine disruption in fish and invertebrates affects reproductive processes (and imposex in gastropods) leading to adverse ecologically significant impacts remains unclear in many studies (Arcand-Hoy and Benson 1998; Depledge and Billinghurst 1999; Matthiesson 2000; Hecker and Hollett 2000). It is, however, becoming increasingly apparent that certain biomarkers (e.g. behaviour and reproductive changes) can directly affect fitness which can be linked to survival. One particular biomarker, inhibition of brain AChE, has been linked to population level impacts in Pacific salmon (Laetz et al. 2009). Despite the ongoing efforts to restore salmon habitats, salmon returns have not increased to historic levelsThe levels of AChE in the area would indicate sufficiently decreased survival to explain the lack of recovery (Laetz et al., 2009).
The work of Taylor and Maher (2010) examining the effects of metals on benthic organisms has highlighted the value of using multiple biomarkers in an exposure-dose-response model to demonstrate the link between exposure and effect. The cascade of biomarkers shown in Figure 2 provides a link between molecular perturbations and higher order effects on the organism (e.g. Brain and Brooks, 2012).
Figure 2.

Flow diagram showing a cascade of interlinked cellular reactions which can occur in response to metal exposure (from Taylor and Maher, 2010 with permission from Nova Science Publishers Inc.)
USE OF BIOMARKERS IN AN ASSESSMENT FRAMEWORK
The latest approaches to contaminant impact assessment in both waters and sediments have evolved significantly from the measurement of contaminant concentrations and toxicity as recommended, for example, in the Australian and New Zealand water quality guidelines (WQGs) (ANZECC/ARMCANZ, 2000). These guidelines suggest the use of hierarchical assessment frameworks which initially consider chemistry (i.e. whether contaminant concentrations exceed guideline trigger values (TVs)). If guideline concentrations were exceeded, then toxicity testing was recommended. These tests often measure sub-lethal impacts on growth and reproduction in sensitive surrogate species in laboratory culture. While this approach is possibly adequate for many assessments, where TVs are not exceeded, it may not be adequately protective in scenarios where: (i) multiple contaminants exert non-additive effects (ii) toxicological effects are observed in species of concern although individual toxicant concentrations are below guidelines, or (iii) unknown (emerging) compounds are contributing to the observed toxicity. Here further studies, such as the application of TIE procedures are required.
The goal of ecological assessments is to determine whether an ecosystem is healthy. Not surprisingly, we can generally use a combination of traditional approaches such as diversity indices and analytical chemistry to identify either healthy or highly impacted ecosystems, but it is assessments in the transition zone between these two extremes, namely the moderately impacted sites, are more problematic. It was recognised that in such cases simply measuring chemistry and ecotoxicity was insufficient and additional lines of evidence were required to better assess the health of an ecosystem, in a weight-of-evidence approach (Chapman et al. 2002; Burton et al. 2002).
Traditionally, the recommended additional lines of evidence included bioaccumulation and an ecological assessment of species diversity and abundance. Both involved comparisons with reference sites (as do chemical concentration measurements in cases where background concentrations are above guidelines). Bioaccumulation at best indicates whether there are bioavailable contaminants that are accumulating above background concentrations. However, it is well known that bioaccumulation alone cannot provide a direct link to toxic effects (e.g. Wallace and Lopez 1997; Rainbow 2002; Rainbow and Luoma 2011). Biodiversity studies look at whole system health as distinct from organism health, and new genomic techniques are permitting more comprehensive assessments than simply assessing macroinvertebrate populations as was done in the past (e.g. Chariton et al., 2010). Biodiversity measurements, however, indicate a longer-term response than the short-term evidence from toxicity testing. It may take time before chronic impacts translate into the disappearance of particular species of organisms and they are replaced by more tolerant species and possibly a new ecosystem structure develops. In many cases, it is the short-term impacts that need to be assessed, to initiate a rapid management response, and it is here that biomarkers may have a role.
A number of international jurisdictions are quite prescriptive about the role of biomarkers. Biomarker responses are typically seen at concentrations below those for toxicity, and while they may be indicative of some type of physiological impairment as a consequence of exposure to one or more contaminants or to some other non-chemical stressors, the significance of this effect on the health of the organism and the extrapolation of this to longer-term effects on ecosystem health are frequently difficult to demonstrate. Considerable care must therefore be exercised before biomarker data can be used in the derivation of water quality guidelines that are traditionally based on chronic toxicity thresholds for growth, reproduction and survival. The Canadian water quality guidelines (CCME 2007), for example, admit non-traditional endpoints (e.g. behaviour, predator avoidance, swimming ability, swimming speed, etc.) and those reflecting physiological/biochemical changes, including endocrine-disrupting ability, only if their ecological relevance can be demonstrated. This approach has also been proposed in the current revision of the Australian and New Zealand guidelines. Here the effects biomarkers in Table 1 need to be considered. To account for natural variability in organismal physiology, it would be ideal to compare any biomarker type responses to an established baseline of “normal” physiology. Optimizing baseline variables such as seasonality and sex differences increases the ability to discriminate nonchemical effects from those involving chemical stressors (Almeida et al., 2013).
Ecologically relevant effects have been defined as those that influence an organisms’ ecological competitiveness to an extent that they have a strong negative influence on survival, growth and reproductive ability (CCME 2007). As concluded by Lam (2009), for many biomarkers, links with ecosystem effects have not been adequately demonstrated. He concluded that the best use of biomarkers was to provide an early warning of impending environmental problems. Results for biomarkers that can be obtained rapidly (e.g. with 24-48 h of collection of organisms), makes them a potentially valuable component in an ecological assessment process. However, much of the literature (reviewed in McCarty and Munkittrick, 1996; Forbes et al., 2006) would indicate that in many cases decisions based on biomarker responses alone may result in incorrect decisions about health due to the high frequency of ‘false positive’ responses that cannot be explained and do not concur with any other lines of evidence (i.e. no toxicity, excellent abundance/diversity).
A scheme for evaluating multiple lines of evidence is shown in Figure 3. A reasonable approach is to use biomarkers as a supporting line of evidence to clarify issues associated with bioaccumulation or toxicity, although these responses are interrelated. The exposure biomarkers in Table 1 are used in a similar manner to bioaccumulation to provide evidence for exposure, while effects biomarkers need to be considered in relation to the ecological relevance of the response, both in the short term in relation to organism health and in the longer term with respect to ecosystem impacts, noting the difficulties in predicting the latter.
Figure 3.

A typical weight of evidence framework for water quality assessment (adapted from Humphrey et al. 2013)
To determine the suitability of any biomarker for use as a line of evidence, the following basic criteria have been proposed by other investigators (Svendsen et al. 2004; Huggett et al. 1992):
-
(i)
A clear dose-response relationship should be demonstrated for target contaminants, noting that in its application, the cause of response may include multiple stressors;
-
(ii)
The sensitivity should ideally be greater than that for effects on growth and reproduction
-
(iii)
Ecological relevance should be demonstrated;
-
(iv)
The effects of confounding non-chemical factors such as season, temperature, size, etc. should be understood;
-
(v)
Some knowledge of the chemical specificity is desirable to distinguish between non-specific biomarkers responding to a wide range of contaminants and those that are more specific to particular contaminants or contaminant classes;
-
(vi)
The applicability of the biomarker for the chosen test species needs to have been demonstrated;
-
(vii)
Time-response relationships for the biomarker need to be understood (how soon after exposure is the response seen and how long after exposure does the response persist);
-
(viii)
Methodological concerns such as reproducibility, robustness, ease of use.
Although the above criteria are relevant to the development and incorporation of biomarkers, the state of the science dictates that most of these criteria cannot currently be fully realized by use of a single biomarker (Ankley et al., 2010; Brain and Brooks, 2012). We have discussed that there are very few effects or exposure biomarkers at the biochemical or physiological levels that have clear effects at the population, let alone the ecosystem, levels. Accordingly, most biomarkers would likely fail the ecological relevance criterion. However, as discussed, there exist several biomarkers, such as clearly impaired behaviours (i.e. swimming, feeding, predator-avoidance, prey selection), reproductive impairment, and AChE inhibition that have been associated with loss of organism fitness. Many can be seen as indications of decreased health of individuals, which would impact the health of populations and systems.
Similarly, quantifying disturbances at the ecological level are problematic due to natural ecosystem variation over time, as well as there are likely no truly undisturbed ecosystems due to atmospheric deposition of contaminants, global climate change, etc. What is reasonable, however, is for the biomarker under consideration (and, in particular, effects biomarkers) to have a reasonable linkage to adverse effects of the organism level.
While it is essential to clarify any biomarker with respect to time of onset and permanence of response, it is important to consider that a particular biomarker that responds rapidly to chemical exposure, but then rapidly returns to baseline with cessation of exposures, can be extremely valuable in reflecting recent exposures. For example, gene transcriptional responses (i.e. heme oxygenase, metallothionein, CYP1A) in many aquatic species can occur rapidly upon chemical exposure (i.e. within 12 hours), but may then decline. This phenomenon is also observed with adaptive responses associated with oxidative stress, such as induction of tissue glutathione, whose tissue levels can rapidly return to homeostatic levels (Meister, 1974). Arguably, when used in conjunction with more long-lasting responses (i.e. liver DNA adducts, pre-neoclassic or neoplastic liver lesions) and also analytical chemistry, biomarkers in this category can provide the regulator with a more informed perspective of environmental degradation.
Non-chemical stressor effects are an extremely important consideration. In reality, however, even among the myriad of biomarkers that have been either used or proposed for use in aquatic toxicology, it can be argued that none have been fully characterized with respect to effects of non-chemical stressors such as salinity, temperature, seasonality, food deprivation, etc. While the use of single biomarkers may not provide a holistic measure of ecosystem health (e.g. Lee and Anderson 2005), this problem can be overcome by integrating multiple biomarkers or by integrating biomarkers with other chemical or toxicological effects data, will add strength to any inference. Integrating class-specific contaminant exposure biomarkers with effects biomarkers in a single assessment can predict overall organism health, and also identify causative agents. While these approaches would not address the reversibility of deleterious effects if the stressor is removed, or the capacity for organisms to avoid the stressors, they would still provide insight to a weight of evidence environmental risk assessment
A framework for using biomarker data as part of a line of evidence assessment of contaminant impacts is given in Figure 4.
Figure 4.

Framework for evaluating biomarker results
CONCLUSIONS
Often, the use of analytical chemistry and the results of toxicity tests will provide assessors with the necessary tools to assess environmental risk from the effects of discharges, spills, dredging or other perturbations of water quality. For subtle toxicological effects, low level episodic exposures, combinations of natural and contaminant stressors, or situation with high societal scrutiny, these metrics alone may not be sufficient to predict longer term effects on ecosystem health. While evidence of bioaccumulation is indicative of contaminant bioavailability, measures of short-term impacts on organism fitness can provide an early warning of impending broader effects on ecosystem health that can trigger early management intervention. Biomarkers of both exposure and effects can provide evidence of physiological and biochemical impacts at the organism level, however, the demonstration that these impacts have broader ecological relevance is proving to be a challenge, although proving useful as a monitoring tool to assess the spatial distribution of contaminant impacts and the rate at which systems recover as management actions are implemented.
The strength of the inferences from biomarker studies can be enhanced by integrating the responses from multiple biomarkers that encompass specificity to different contaminant classes as well as reflecting combined impacts on organism fitness. Their greatest value is as a line of evidence to supplement the more conventional measures of chemistry, ecotoxicology and bioaccumulation in a weight of evidence prediction of ecological risk. Few biomarkers are at a stage of development that they could be considered for use in water quality guideline development on their own.
ACKNOWLEDGMENTS
The ideas for this paper were conceived while EPG was the recipient of a Distinguished Visiting Scholar Fellowship from CSIRO Land and Water. EPG was supported in part by the University of Washington Superfund Research Program, Grant #: NIEHS P42ES004696. This manuscript was improved by careful reading by Anthony Chariton and Stuart Simpson (CSIRO, Land and Water), as well as by two anonymous reviewers.
Footnotes
DISCLAIMER—The peer-review process for this article was managed by the Editorial Board without the involvement of G. Batley.
The authors state that they have no conflicts of interest.
REFERENCES
- Aas E, Baussant T, Balk L, Liewnborg B, Andersen OK. PAH metabolites in bile, cytochrome p4501A and DNA adducts as environmental risk parameters for chronic oil exposure: a laboratory experiment with Atlantic cod. Aquat Toxicol. 2000;51:241–258. doi: 10.1016/s0166-445x(00)00108-9. [DOI] [PubMed] [Google Scholar]
- Aas E, Beyer J, Jonsson G, Reichert WL, Andersen OK. Evidence of uptake, biotransformation and DNA binding of polyaromatic hydrocarbons in Atlantic cod and corking wrasse caught in the vicinity of an aluminium works. Mar Environ Res. 2001;52:213–229. doi: 10.1016/s0141-1136(00)00269-5. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Pacheco M, Santos MA. Anguilla anguilla L. oxidative stress biomarkers: an in situ study of freshwater wetland ecosystem (Pateira de Fermentelos, Portugal). Chemosphere. 2006;65:952–962. doi: 10.1016/j.chemosphere.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Alemeida C, Pereira CG, Gomes T, Cardoso C, Bebianno MJ. Genotoxicity in two bivalve species from a coastal lagoon in the South of Portugal. Mar Environ Res. 2013;89:29–38. doi: 10.1016/j.marenvres.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Amiard J-C, Amiard-Triquet C, Barka S, Pellerin J, Rainbow JS. Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol. 2006;76:160–202. doi: 10.1016/j.aquatox.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptualframework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- ANZECC/ARMCANZ Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council, Agriculture and Resource Management Council of Australia and New Zealand, Canberra. 2000 [Google Scholar]
- Balk L, Hylland K, Hansson T, Berntssen MHG, Beyer J, Jonsson G, Melbye A, Grung M, Torstensen BE, Borseth JF, Skarphedinsdottir H, Klungsoyr J. Biomarkers in natural fish populations indicate adverse biological effects in offshore oil production. Plos One. 2011;6:e19735. doi: 10.1371/journal.pone.0019735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacanskas LR, Whitaker J, Di Guilio RT. Oxidative stress in two populations of killifish (Fundulus heteroclitus) with differing contaminant exposure histories. Mar Environ Res. 2004;58:597–601. doi: 10.1016/j.marenvres.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Baldwin DH, Tatara CP, Scholz NL. Copper-induced olfactory toxicity in salmon and steelhead: extrapolation across species and rearing environments. Aquat Toxicol. 2011;101:295–297. doi: 10.1016/j.aquatox.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Banni M, Jebali J, Daubeze M, Clerandau C, Guerbej H, Narbonne JF, Bousetta H. Monitoring pollution in Tunisian coasts: application of a classification scale based on biochemical markers. Biomarkers. 2005;10:105–116. doi: 10.1080/13547500500107497. [DOI] [PubMed] [Google Scholar]
- Blazer VS, Iwanowicz LR, Henderson H, Mazik PM, Jenkins JA, Alvarez DA, Young JA. Reproductive endocrine disruption in smallmouth bass (Micropterus dolomieu) in the Potomac River basin: spatial and temporal comparisons of biological effects. Environ Monit Assess. 2012;184:4309–4334. doi: 10.1007/s10661-011-2266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain RA, Brooks BW. Brooks BW, Huggett DB, editors. Considerations and criteria for the incorporation of mechanistic sub-lethal endpoints into environmental risk assessment for biologically active compounds. Human Pharmaceuticals in the Environment: Current and Future Perspectives. 2012:304. [Google Scholar]
- Broeg K, Westernhagen Hv Zander S, Korting W, Koehler A. The “bioeffect assessment index” (BAI) A concept for the quantification of effects of marine pollution by an integrated biomarker approach. Mar Poll Bull. 2005;50:495–503. doi: 10.1016/j.marpolbul.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Burton GA, Chapman PM, Smith EP. Weight-of-evidence approaches for assessing ecosystem impairment. Hum Ecol Risk Assess. 2002;8:1657–1673. [Google Scholar]
- Chapman PM, McDonald BG, Lawrence GS. Weight-of-evidence issues and frameworks for sediment quality and other assessments. Hum Ecol Risk Assess. 2002;8:1489–1515. [Google Scholar]
- Chariton AA, Court LN, Hartley DM, Colloff MJ, Hardy CM. Ecological assessment of estuarine sediments by pyrosequencing eukaryotic ribosomal DNA. Front Ecol Environ. 2010;8:233–238. [Google Scholar]
- Chesman BS, O'Hara S, Burt GR, Langston WJ. Hepatic metallotheionein and total oxyradical scavenging capacity in Atlantic cod Gadus morhua caged in open sea contamination gradients. Aqua Toxicol. 2007;84:310–320. doi: 10.1016/j.aquatox.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Collier TK, Krone CA, Krahn MM, Stein JE, Chan S-L, Varanasi U. Petroleum exposure and associated biochemical effects in subtidal fish after the Exxon Valdez oil spill. Amer Fish Soc Symp. 1996;18:671–683. [Google Scholar]
- Connon RE, Geist J, Pfieff J, Loguinov AV, D'Abronzo LS, Wintz H, Vulpe CD, Werner I. Linking mechanistic and behavioural responses to sublethal esfenvalerate exposure in the endangered delta smelt; Hypomesus transpacificus (Fam. Osmeridae). BMC Genomics. 2009;10:608. doi: 10.1186/1471-2164-10-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connon RE, Beggel S, D'Abronzo LS, Giest JP, Pfieff J, Longuinov AV, Vulpe CD, Werner I. Linking molecular biomarkers with higher level condition indicators to identify effects of copper exposures on the endangered delta smelt (Hypomesus transpacificus). Environ Toxicol Chem. 2011;30:290–300. doi: 10.1002/etc.400. [DOI] [PubMed] [Google Scholar]
- Connon RE, D'Abronzo LS, Hotstetter NJ, Javidmehr A, Roby DD, Evans AF, Loge FJ, Werner I. Transcription profiling in environmental diagnostics: health assessments in Columbia River basin steelhead (Oncorhynchus mykiss). Environ Sci Technol. 2012;46:6081–6087. doi: 10.1021/es3005128. [DOI] [PubMed] [Google Scholar]
- Cooper NL, Bidwell JR. Cholinesterase inhibition and impacts on behavior of the Asian clam, Corbicula fluminea, after exposure to an organophosphate insecticide. Aquat Toxicol. 2006;76:258–267. doi: 10.1016/j.aquatox.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Dagnino A, Allen JI, Moore MN, Broeg K, Canesi L, Viarengo A. Development of an expert system for the integration of biomarker responses in mussels into an animal health index. Biomarkers. 2007;12:155–172. doi: 10.1080/13547500601037171. [DOI] [PubMed] [Google Scholar]
- Denslow ND, Kochera J, Sepulveda MS, Gross T, Holm SE. Gene expression fingerprints of largemouth bass (Micropterus salmoides) exposed to pulp and paper mill effluents. Mutat Res. 2004;552:19–34. doi: 10.1016/j.mrfmmm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Domouhtsidou GP, Dailianis S, Kaloyianni M, Dimitriadis VK. Lysosomal membrane stability and metallothionein content in Mytilus galloprovincialis (L.), as biomarkers: Combination with trace metal concentrations. Mar Pollut Bull. 2004;48:572–586. doi: 10.1016/j.marpolbul.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Downs CA, Richmond RH, Mendiola WJ, Rougee L, Ostrander GK. Cellular physiological effects of the MV Kyowa Violet fuel-oil spill on the hard coral, Porites lobata. Environ Toxicol Chem. 2006;25:3171–3180. doi: 10.1897/05-509r1.1. [DOI] [PubMed] [Google Scholar]
- Downs CA, Ostrander GK, Rougee L, Rongo T, Knutson S, Williams DE, Mendiola W, Holbrook J, Richmond RH. The use of cellular diagnostics for indentifying sub-lethal stress in reef corals. Ecotoxicol. 2012;21:768–782. doi: 10.1007/s10646-011-0837-4. [DOI] [PubMed] [Google Scholar]
- Edge KJ, Johnston EL, Roach AC, Ringwood AH. Indicators of environmental stress: cellular biomarkers and reproductive responses in the Sydney rock oyster (Saccostrea glomerata). Ecotoxicol. 2012;21:1415–1425. doi: 10.1007/s10646-012-0895-2. [DOI] [PubMed] [Google Scholar]
- Espinoza HM, Williams CM, Gallagher EP. Effect of cadmium on glutathione S-transferase and metallothionein gene expression in coho salmon liver, gill and olfactory tissues. Aquat Toxicol. 2012;110-111:37–44. doi: 10.1016/j.aquatox.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farombi EO, Adelowo OA, Ajimoko YR. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African catfish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health. 2007;4:158–165. doi: 10.3390/ijerph2007040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist GW, Webb MAH, Gundersen DT, Foster EP, Schreck CB, Maule AG, Fitzpatrick MS. Evidence of detrimental effects of environmental contaminants on growth and reproductive physiology of White Sturgeon in impounded areas of the Columbia River. Environ Health Perspect. 2005;113:1675–1682. doi: 10.1289/ehp.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez B, Albentosa M, Vinas L, Franco A, Gonzalez JJ, Campillo JA. Integrated assessment of water quality of the Costa da Morte (Galicia, NW Spain) by means of mussel chemical, biochemical and physiological parameters. Ecotoxicol. 2010;19:735–750. doi: 10.1007/s10646-009-0450-y. [DOI] [PubMed] [Google Scholar]
- Fisher NS, Hook SE. Toxicology tests with aquatic animals need to consider the trophic transfer of metals. Toxicol 181. 2002;182:531–536. doi: 10.1016/s0300-483x(02)00475-4. [DOI] [PubMed] [Google Scholar]
- Folmar LC, Denslow ND, Rao V, Chow M, Crain DA, Enblom J, Marcino J, Guillette LJ. Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyorinus carpio) captured near a major metropolitan sewage treatment plant. Environml Health Perspect. 1996;104:1096–1101. doi: 10.1289/ehp.961041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes VE, Palmqvist A, Bach L. The use and misuse of biomarkers in ecotoxicology. Environmental Toxicology and Chemistry. 2006;25:272–280. doi: 10.1897/05-257r.1. [DOI] [PubMed] [Google Scholar]
- Grenzilli G, Nigro M, Scarcelli V, Gorbi S, Regoli F. DNA integrity and total oxyradical scavenging capacity in the Mediterranean mussel, Mytilus galloprovincialis: a field study in a highly eutrophicated coastal lagoon. Aquat Toxicol. 2001;53:19–32. doi: 10.1016/s0166-445x(00)00159-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Reyero N, Adelman I, Liu L, Denslow N. Gene expression profiles of fathead minnows exposed to surface waters above and below a sewage treatment plant in Minnesota. Mar Environ Res. 2008;66:134–136. doi: 10.1016/j.marenvres.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón-Pérez MI, Romero-Bañuelos CA, Toledo-Ibarra GA, Rojas-García E, Medina-Diaz IM, Robledo-Marenco ML, Vega-López A. Evaluation of pollution in Camichin estuary (Mexico): pro-oxidant and antioxidant response in oyster (Crassostrea corteziensis). Comp Biochem Physiol A Mol Integr Physiol. 2013;165:476–82. doi: 10.1016/j.cbpa.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Harvey JS, Lyons BP, Page TS, Stewart C, Parry JM. An assessment of the genotoxic impact of the Sea Empress oil spill by the measurement of DNA adduct levels in selected invertebrate and vertebrate species. Mutat Res. 1999;441:103–114. doi: 10.1016/s1383-5718(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Hook SE, Skillman AD, Small JA, Schultz IR. Gene expression patterns in Rainbow Trout, Onorhynchus mykiss, exposed to a suite of model toxicants. Aquatic Toxicology. 2006;77:372–385. doi: 10.1016/j.aquatox.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook SE, Skillman AD, Small JA, Schultz IR. Temporal Changes in Gene Expression in Rainbow Trout Exposed to Ethynyl Estradiol. CompBiochemiPhysiol. 2007;145:73–85. doi: 10.1016/j.cbpc.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook SE. Promise and progress in environmental genomics: a status report on the applications of microarray studies in ecologically relevant fish species. J Fish Biol. 2010;77:1999–2022. doi: 10.1111/j.1095-8649.2010.02814.x. [DOI] [PubMed] [Google Scholar]
- Hook SE, Osborn HL, Spadaro DA, Simpson SL. Assessing mechanisms of toxicant response in the amphipod Melita plumulosa through transcriptomic profiling. AquatToxicol. 2014;146:247–257. doi: 10.1016/j.aquatox.2013.11.001. http://dx.doi.org/10.1016/j.aquatox.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Hu JS, Durst M, Kerb R, Truong V, Ma JT, Khurgin E, Balaban D, Gingeras TR, Hoffman BB. Analysis of drug pharmacology towards predicting drug behavior by expression profiling using high-density oligonucleotide arrays. Ann N Y Acad Sci. 2000;919:9–15. doi: 10.1111/j.1749-6632.2000.tb06862.x. [DOI] [PubMed] [Google Scholar]
- Huggett RJ, Stegeman JJ, Page DS, Parker KR, Woodin B, Brown JS. Biomarkers in fish from Prince William Sound and the Gulf of Alaska: 1999-2000. Environ Sci Technol. 2003;37:4043–4051. doi: 10.1021/es0342401. [DOI] [PubMed] [Google Scholar]
- Huggett RJ, Kimerle RA, Mehrl PM, Bergman HL, Dickson KL, Fava JA, McCarthy JF, Parrish R, Dorn PB, McFarland V, Lahvis G. Introduction. In: Huggett RJ, Kimerle RA, Mehrle PM, Bergman HL, editors. Biomarkers: Biochemical, Physiological, and Histological Markers of Anthropogenic Stress. Lewis Publishers; Boca Raton, FL, USA: 1992. [Google Scholar]
- Hylland K, Ruus A, Grung M, Green N. Relationships between physiology, tissue contaminants, and biomarker responses in Atlantic Cod (Gadus morhua L.). J Toxicol Environ Health Part A. 2009;72:226–233. doi: 10.1080/15287390802539129. [DOI] [PubMed] [Google Scholar]
- Hyne RV, Maher WA. Invertebrate biomarkers: links to toxicosis that predict population decline. Ecotoxicol Environ Saf. 2003;54:366–374. doi: 10.1016/s0147-6513(02)00119-7. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Vines CA, Linbo TL, Myers MS, Sloan CA, Anulacion BF, Boyd D, Collier TK, Morgan S, Cherr GN, Scholz NL. Potent phototoxicity of marine bunker oil to translucent herring embryos after prolonged weathering. Plos One. 2012;7:e30116. doi: 10.1371/journal.pone.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environ Sci Technol. 1998;32:2498–2506. [Google Scholar]
- Johnson LL, Lomax DP, Myers MS, Olson OP, Sol SY, O'Neill SM, West J, Collier TK. Xenoestrogen exposure and effects in English sole (Parophyrys vetulus). Aquat Toxicol. 2008a;88:29–38. doi: 10.1016/j.aquatox.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Johnson LL, Arkossh MR, Bravo CF, Collier TK, Krahn MM, Meador JP, Myers MS, Reichert WL, Stein JE. The effects of polycyclic aromatic hydrocarbons in fish from Puget Sound, Washington. In: Di Giulio RT, Hinton DE, editors. The Toxicology of Fishes. CRC Press; Boca Raton, FL, USA: 2008b. pp. 877–923. [Google Scholar]
- Kelly KA, Havrilla CM, Brady TC, Abramo KH, Levin ED. Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ Health Perspect. 1998;106:375–384. doi: 10.1289/ehp.98106375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AJ, Steevens JA, Lotufo GR, Farrar JD, Reiss MR, Kropp RK, Kropp RK, Doi J, Bridges TS. A comparison of acute and chronic toxicity methods for marine sediments. Mar Environ Res. 2009;68:118–127. doi: 10.1016/j.marenvres.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Kerambrun E, Le Floch S, Sanchez W, Guyon HT, Meziane T, Henry F, Amara R. Responses of juvenile sea bass, Dicentrarchus labrax, exposed to acute concentrations of crude oil, as assessed by molecular and physiological biomarkers. Chemosphere. 2012;87:692–702. doi: 10.1016/j.chemosphere.2011.12.059. [DOI] [PubMed] [Google Scholar]
- Klumpp DW, Humphrey C, Huasheng H, Tao F. Toxic contaminants and their biological effects in coastal waters of Xiamen, China. II. Biomarkers and embryo malformation rates as indicators of pollution stress in fish. Mar Pollut Bull. 2002;44:761–769. doi: 10.1016/s0025-326x(02)00054-1. [DOI] [PubMed] [Google Scholar]
- Laetz CA, Baldwin DA, Collier TK, Herbert V, Stark JD, Schol NL. The synergistic toxicity of pesticide mixtures: implications for risk assessment and the conservation of endangered Pacific salmon. Environ Health Perspect. 2009;117:348–353. doi: 10.1289/ehp.0800096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RF, Singer SC, Page DS. Response of cytochrome p 450 systems in marine crab and polychaetes to organic pollutants. Aquat Toxicol. 1981;1:355–365. [Google Scholar]
- Lee RF, Anderson JW. Significance of cytochrome P450 systems responses and levels of bile fluorescent aromatic compounds in marine wildlife following oil spills. Mar Pollut Bull. 2005;50:705–723. doi: 10.1016/j.marpolbul.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Livingstone DR. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull. 2001;42(8):656–666. doi: 10.1016/s0025-326x(01)00060-1. [DOI] [PubMed] [Google Scholar]
- Machala M, Dusek L, Hilscherova K, Kubinova R, Jurajda P, Neca J, Ulrich R, Gelnar M, Studnickova Z, Holoubek I. Determination and multivariate statistical analysis of biochemical responses to environmental contaminants in feral freshwater fish Leuciscus cephalus, L. Environ Toxicol Chem. 2001;20:1141–1148. [PubMed] [Google Scholar]
- Marigomez I, Zorita I, Izagirre U, Ortiz-Zarragoitia M, Navarro P, Etxebarria N, Orbea A, Soto M, Cajaraville MP. Combined use of native and caged mussels to assess biological effects of pollution through the integrative biomarker approach. AquatToxicol136. 2013;13:32–48. doi: 10.1016/j.aquatox.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Martin R, Rose D, Yu K, Barros S. Toxicogenomics strategies for predicting drug toxicity. Pharmacogenomics. 2006;7:1003–1016. doi: 10.2217/14622416.7.7.1003. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez C, Fernandez B, Valdes J, Campillo JA, Bendicto J, Sanchez F, Vethaak AD. Evaluation of three-year monitoring with biomarkers in fish following the Prestige oil spill (N Spain). Chemosphere. 2009;74:613–620. doi: 10.1016/j.chemosphere.2008.10.052. [DOI] [PubMed] [Google Scholar]
- Matsuo AYO, Woodin BR, Reddy CM, Stegemann JJ. Humic substances and crude oil induce cytochrome P450 1A expression in the Amazonian fish species Colossoma macropomum (Tambaqui) Environ Sci Technol. 2006;40:2851–2858. doi: 10.1021/es052437i. [DOI] [PubMed] [Google Scholar]
- McCarty LS, Munkittrick KR. Environmental biomarkers in aquatic toxicology: fiction, fantasy, or functional? Hum Ecol Risk Assess. 1996;2:268–274. [Google Scholar]
- McIntyre JK, Baldwin DH, Meador JP, Scholz NL. Chemosensory deprivation in juvenile coho salmon exposed to dissolved copper under varying water chemistry conditions. Environ Sci Technol. 2008;42:1352–1358. doi: 10.1021/es071603e. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione, metabolism and function via the gamma glutamyl cycle. Life Sci. 1974;15:177–90. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- Moore MN, Viarengo AG, Somerfield PJ, Sforzini S. Linking lysosomal biomarkers and ecotoxicological effects at higher biological levels. In: Amiard-Triquet C, Amiard JC, Rainbow PS, editors. Ecological Biomarkers: Indicators of Ecotoxicological Effects. CRC Press; Boca Raton, FL, USA: 2013. pp. 107–130. [Google Scholar]
- Myers M, Anulacion BF, French B, Hom T, Collier TK. Biomarker and histopathologic responses in flatfish following site remediation in Eagle Harbor, WA. Mar Environ Res. 2000;45:47–60. [Google Scholar]
- Myers MS, Johnson LL, Collier TK. Establishing the causal relationship between polycyclic aromatic hydrocarbon (PAH) exposure and hepatic neoplasms and neoplasia-related liver lesions in English sole (Pleuronectes vetulus). Hum Ecol Risk Assess. 2003;9:67–94. [Google Scholar]
- Nahrgang J, Camus L, Gonzalez P, Goksoyr A, Christainsen A, Hop H. PAH biomarker responses in polar cod (Boreogadus saida) exposed to benzo(a)pyrene. Aquat Toxicol. 2009;94:309–319. doi: 10.1016/j.aquatox.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Nicholls JW, Breen M, Denver RJ, DiStefano JJ, Edwards JS, Hoke RA, Volz DC, Zhang X. Predicting chemical impacts on vertebrate endocrine systems. Environ Toxicol Chem. 2011;30:39–51. doi: 10.1002/etc.376. [DOI] [PubMed] [Google Scholar]
- Osborn HL, Hook SE. Using transcriptomic profiles in the diatom Phaeodactylum tricornutum to identify and prioritize stressors. Aquat Toxicol. 2013;138:12–25. doi: 10.1016/j.aquatox.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Otto DM, Moon TW. Phase I and II enzymes and antioxidant responses in different tissues of brown bullheads from relatively polluted and non-polluted systems. Arch Environ Contam Toxicol. 1996;31:141–147. doi: 10.1007/BF00203918. [DOI] [PubMed] [Google Scholar]
- Pandey S, Parvez S, Sayeed I, Hague R, Bin-Hafeez B, Raisuddin S. Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ. 2003;309:105–115. doi: 10.1016/S0048-9697(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Patil VK, David M. Oxidative stress in freshwater fish, Labeo rohita as a biomarker of malathion exposure. Environ Monit Assess. 2013;184:10191–10199. doi: 10.1007/s10661-013-3323-z. [DOI] [PubMed] [Google Scholar]
- Pereira S, Pinto AL, Cortes R, Fontaínhas-Fernandes A, Coimbra AM. Gill histopathological and oxidative stress evaluation in native fish captured in Portuguese northwestern rivers. Ecotoxicol Environ Saf. 2013;90:157–166. doi: 10.1016/j.ecoenv.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Poyton HC, Vulpe CD. Ecotoxicogenomics: Emerging technologies for emerging contamiantns. J Amer Water Resourc Assoc. 2009;45:83–96. [Google Scholar]
- Poynton HC, Varshavsky JR, Chang B, Cavigiolio G, Chan S, Holman PS, Loguinov AV, Bauer DJ, Komachi K, Theil EC, Perkins EJ, Hughes O, Vulpe CD. Daphnia magna ecotoxicogenomics provides mechanistic insights into metal toxicity. Environ Sci Technol. 2007;41:1044–1050. doi: 10.1021/es0615573. [DOI] [PubMed] [Google Scholar]
- Rainbow PS. Trace metal concentrations in aquatic invertebrates: why and so what? Environl Pollut. 2002;120:497–507. doi: 10.1016/s0269-7491(02)00238-5. [DOI] [PubMed] [Google Scholar]
- Rainbow PS, Luoma SN. Metal toxicity , uptake and bioaccumulation in aquatic invertebrates – Modelling zinc in crustaceans. Aquat Toxicol. 2011;105:455–465. doi: 10.1016/j.aquatox.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Ringwood AH, Conners DE, Hoguet J. Effects of natural and anthropogenic stressors on lysosomal destabilization in oysters Crassostrea virginica. MarEcolProg Ser. 1998;166:163–171. [Google Scholar]
- Ringwood AH, Hoguet J, Keppler C, Gielazyn M. Linkages between cellular biomarker responses and reproductive success in oysters Crassostrea virginica. Mar Environ Res. 2004;58:151–155. doi: 10.1016/j.marenvres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Roling JA, Bain LJ, Gardea-Torresday J, Key PB, Baldwin WS. Using mummichog (Fundulus heteroclitus) arrays to monitor the effectiveness of remediation at a superfund site in Charleston South Carolina USA. Environ Toxicol Chem. 2007;26:1205–1213. doi: 10.1897/06-421r.1. [DOI] [PubMed] [Google Scholar]
- Roberts A P, Oris JT, Burton GA, Clements WH. Gene expression in caged fish as a first-tier indicator of contaminant exposure in streams. Environ Toxicol Chem. 2005;24:3092–3098. doi: 10.1897/05-137r.1. [DOI] [PubMed] [Google Scholar]
- Roesijadi G. Influence of copper on the clam Protothaca staminea: effects on gills and influence of copper binding proteins. Biol Bull. 1980;158:233–237. [Google Scholar]
- Sakuragui MM, Paulino MG, Pereira CD, Carvalho CS, Sadauskas-Henrique H, Fernandes MN. Integrated use of antioxidant enzymes and oxidative damage in two fish species to assess pollution in man-made hydroelectric reservoirs. Environ Pollut. 2013;178:41–51. doi: 10.1016/j.envpol.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Scholz NL, Truelove NK, French BL, Berejikian BA, Quinn TP, Casillas E, Collier TK. Diazinon disrupts antipredator and homing behaviors in chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 2000;57:1911–1918. [Google Scholar]
- Scholz NL, Myers MS, McCarthy SG, Labenia JS, McIntyre JK, Ylitalo GM, Rhodes LD, Laetz CA, Stehr CM, French BL, McMillan B, Wilson D, Reed L, Lynch KD, Damm S, Davis JW, Collier TK. Recurrent die-offs of adult Coho salmon returning to spawn in Puget Sound lowland urban streams. Plos One. 2011;12:e28013. doi: 10.1371/journal.pone.0028013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin Jeffries MK, Conoan NH, Cox MB, Sangster JL, Balsiger HA, Bridges AA, Cowman T, Knight LA, Bartlet-Hunt SL, Kolok AS. The anti-estrogenic activity of sediments from agriculturally intense watersheds: assessment using in vivo and in vitro assays. Aquat Toxicol. 2011;105:189–198. doi: 10.1016/j.aquatox.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin Jeffries MK, Mehinto AC, Carter BJ, Denslow ND, Kolok AS. Taking microarrays to the field: differential hepatic gene expression of caged fathead minnows from Nebraska watersheds. Environ Sci Technol. 2012;46:1877–1885. doi: 10.1021/es2039097. [DOI] [PubMed] [Google Scholar]
- Sellin Jeffries MK, Claytor C, Stubblefield W, Pearson WH, Oris JT. Quantitative risk model for polycyclic aromatic hydrocarbon photoinduced toxicity in Pacific herring following the Exxon Valdez oil spill. Environ Sci Technol. 2013;47:5450–5458. doi: 10.1021/es400759y. [DOI] [PubMed] [Google Scholar]
- Serafim A, Lopes B, Company R, Cravo A, Gomes T, Sousa V, Bebianno MJ. A multi-biomarker approach in cross-transplanted mussels Mytilus galloprovincialis. Ecotoxicol. 2011;20:1950–1974. doi: 10.1007/s10646-011-0737-7. [DOI] [PubMed] [Google Scholar]
- Shaw JP, Dondero F, Moore MN, Negri A, Dagnino A, Readman JW, Lowe DR, Frickers PE, Beesley A, Thain JE, Viarengo A. Integration of biochemical, histochemical and toxicogenomic indices for the assessment of health status of mussels from the Tamar Estuary, U.K. Mar Environ Res. 2011;72:13–24. doi: 10.1016/j.marenvres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Simpson SL, Spadaro DA. Performance and sensitivity of rapid sublethal sediment toxicity tests with the amphipod Melita plumulosa and copepod Nitocra spinipes. Environ Toxicol Chem. 2011;30:2326–2334. doi: 10.1002/etc.633. [DOI] [PubMed] [Google Scholar]
- Smit MGD, Bechmann RK, Hendriks AJ, Skadsheim A, Larsen BK, Baussant T, Bamber S, Sanni S. Relating biomarkers to whole-organism effects using species sensitivity distributions: a pilot study for marine species exposed to oil. Environ Toxicol Chem. 2009;28:1104–1109. doi: 10.1897/08-464.1. [DOI] [PubMed] [Google Scholar]
- Stegeman JJ. Influence of environmental contamination on cytochrome P450 mixed function oxygenases in fish- implications for recovery in wild harbor marsh. J Fish Res Board Can. 1978;35:668–674. [Google Scholar]
- Steinert SA, Streib-Montee R, Leather JM, Chadwick DB. DNA damage in mussels at sites in San Diego Bay. Mutat Res. 1998;399:65–85. doi: 10.1016/s0027-5107(97)00267-4. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Maher WA. Establishing metal exposure-dose-response relationships in marine organisms: Illustrated with a case study of cadmium toxicity in Tellina deltoidalis. In: Martorino L, Puopolo K, editors. New Oceanography Research Developments; Marine Chemistry, Ocean Floor Analyses, and Marine Phytoplankton; Nova Science Publishers; Hauppauge, New York, USA: 2010. pp. 1–57. [Google Scholar]
- Tierney KB, Baldwin DH, Hara TJ, Ross PS, Scholz NL, Kennedy CJ. Olfactory toxicity in fishes. Aquat Toxicol. 2010;96:2–26. doi: 10.1016/j.aquatox.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Tierney KB, Ross PS, Kennedy CJ. Linuron and carbaryl differentially impair baseline amino acid and bile salt olfactory responses in three salmonids. Toxicology. 2007;231:175–187. doi: 10.1016/j.tox.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Tilton F, Tilton SC, Bammler TK, Beyer R, Farin F, Stapleton PL, Gallagher EP. Transcriptional biomarkers of copper-induced olfactory injury in zebrafish. Environ Sci Technol. 2008;42:9404–9411. doi: 10.1021/es801636v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton FA, Tilton SC, Bammler TK, Beyer RP, Stapleton PL, Scholz NL, Gallagher EP. Transcriptional impact of organophosphate and metal mixtures on olfaction: copper dominates the chlorpyrifos-induced response in adult zebrafish. Aquat Toxicol. 2011;102:205–215. doi: 10.1016/j.aquatox.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom M, Moran O, Jakubov E, Cavar B, Rinkevic B. Molecular characterization of metallothionein-cDNA of Sparus aurata used for detecting heavy metal pollution along the Mediterranean coast of Israel. Mar Pollut Bull. 1998;36:131–137. [Google Scholar]
- Uren Webster TM, Bury N, van Aerle R, Santos EM. Global transcriptome profiling reveals molecular mechanisms of metal tolerance in a chronically exposed wild population of brown trout. Environ Sci Technol. 2013;47:8869–8877. doi: 10.1021/es401380p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Straalen NM, Feder ME. Ecological and evolutionary functional genomics – how can it contribute to the risk assessment of chemicals. Environ Sci Technol. 2012;46:3–9. doi: 10.1021/es2034153. [DOI] [PubMed] [Google Scholar]
- Wallace WG, Lopez GR. Bioavailability of biologically sequestered cadmium and the implications of metal detoxification. Mar Ecol Prog Ser. 1997;147:149–157. [Google Scholar]
- Ward D, Simpson S, Jolley D. Slow avoidance response to contaminated sediments elicits sublethal toxicity to benthic invertebrates. Environ Sci Technol. 2013;47:5947–5953. doi: 10.1021/es400152a. [DOI] [PubMed] [Google Scholar]
- Williams C R, Gallagher EP. Effects of cadmium on olfactory mediated behaviors and molecular biomarkers in coho salmon (Oncorhynchus kisutch). Aquat Toxicol 140. 2013;141C:295–302. doi: 10.1016/j.aquatox.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston GW. Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol C. 1991;100:173–176. doi: 10.1016/0742-8413(91)90148-m. [DOI] [PubMed] [Google Scholar]


