Abstract
Because of the recent awareness that vancomycin doses should aim to meet a target area under the concentration-time curve (AUC) instead of trough concentrations, more aggressive dosing regimens are warranted also in the pediatric population. In this study, both neonatal and pediatric pharmacokinetic models for vancomycin were externally evaluated and subsequently used to derive model-based dosing algorithms for neonates, infants, and children. For the external validation, predictions from previously published pharmacokinetic models were compared to new data. Simulations were performed in order to evaluate current dosing regimens and to propose a model-based dosing algorithm. The AUC/MIC over 24 h (AUC24/MIC) was evaluated for all investigated dosing schedules (target of >400), without any concentration exceeding 40 mg/liter. Both the neonatal and pediatric models of vancomycin performed well in the external data sets, resulting in concentrations that were predicted correctly and without bias. For neonates, a dosing algorithm based on body weight at birth and postnatal age is proposed, with daily doses divided over three to four doses. For infants aged <1 year, doses between 32 and 60 mg/kg/day over four doses are proposed, while above 1 year of age, 60 mg/kg/day seems appropriate. As the time to reach steady-state concentrations varies from 155 h in preterm infants to 36 h in children aged >1 year, an initial loading dose is proposed. Based on the externally validated neonatal and pediatric vancomycin models, novel dosing algorithms are proposed for neonates and children aged <1 year. For children aged 1 year and older, the currently advised maintenance dose of 60 mg/kg/day seems appropriate.
INTRODUCTION
In the pediatric population, drugs are often administered in an off-label or unlicensed manner (1). While pediatric dosing regimens are mostly empirically derived using extrapolations based on body weight (2, 3), it has been shown before that dosing in children should be guided by the understanding of developmental changes in the pharmacokinetic and/or pharmacodynamic relation of drugs (2, 4, 5). More specifically, translation of results from pharmacokinetic modeling studies has been shown to result in individualized dosing guidelines for many different drugs in pediatric clinical practice (5–9).
The antibacterial agent vancomycin is commonly used to treat infections caused by Gram-positive organisms, like methicillin-resistant Staphylococcus aureus (MRSA) and amoxicillin-resistant Enterococcus species, in both adults and pediatric patients (10). In neonates, MRSA infection is relatively rare, and coagulase-negative staphylococci are the most commonly identified infectious organisms necessitating vancomycin treatment (11, 12). For vancomycin, it has been shown that bacterial killing of S. aureus is best predicted by the area under the concentration-time curve over 24 h (AUC24) divided by the MIC for the infecting pathogen (AUC24/MIC) (13–15). In adult patients, an AUC24/MIC of ≥400 resulted in a superior clinical and bacteriological response (16, 17). Because of the recent awareness that vancomycin doses should aim to meet a target AUC or exposure instead of trough concentrations (18), more aggressive dosing regimens are warranted also in the pediatric population. Depending on the population and the dosing frequency, target trough concentrations that can be used to adjust the dose in an individual child in clinical practice (therapeutic drug monitoring [TDM]) can be derived that will lead to the target AUC24 (19–21).
The exposure or AUC of vancomycin is dependent on the primary pharmacokinetic parameter total body clearance, which is known to vary widely between infants and children of different ages, especially in the heterogeneous group of preterm neonates (19, 20, 22–36). Despite the large number of proposed vancomycin dosing regimens currently available for neonates and infants (10, 11, 37–41), studies reporting on the results of therapeutic drug monitoring show that the target trough concentrations are difficult to attain in clinical practice (42–45). In accordance with reported clinical practice in adults, continuous dosing has also been investigated in neonates, infants, and children (31, 34, 46–49).
In previous reports, the developmental changes in vancomycin pharmacokinetics have been characterized in both neonates and children with normal renal function (defined as serum creatinine two to three times higher than the age-related reference value) (22, 23). Both models were based on a very large number of patients and internally validated (22, 23). In order to further investigate the predictive performance of these models, an external validation procedure with external data sets is needed (2–5). This external validation is necessary to allow the use of the model for deriving model-based dosing algorithms (3–5).
The aim of the current study is therefore to evaluate the predictive performance of the previously published neonatal and pediatric pharmacokinetic models (22, 23) against an external vancomycin data set containing preterm and term neonates and infants. The model is then used to evaluate currently used dosing regimens (37–40) in terms of target exposure. Based on the results of these simulations, a model-based vancomycin dosing algorithm for intermittent and continuous dosing will be derived that can be used in prospective clinical trials to optimize vancomycin treatment for neonates, infants, and children.
MATERIALS AND METHODS
Original models.
Previously, population models both for neonates (22) and for infants and children (23) have been developed in which the developmental changes in vancomycin pharmacokinetics in neonates and children were characterized. In the neonatal model (22), birth body weight (bBW), postnatal age (PNA), and the coadministration of ibuprofen (with ibuprofen set at a value of 1 in the case of ibuprofen coadministration and a value of 0 in the case of no coadministration) were identified as covariates for clearance (equation 1) while current body weight (cBW) was found as a covariate for the central volume of distribution (equation 2). In these equations, Cli represents the clearance in the ith individual, Vc,i is the central volume of distribution in the ith individual, Qi is the intercompartmental clearance in the ith individual, and Vp,i is the peripheral volume of distribution in the ith individual.
| (1) |
| (2) |
| (3) |
| (4) |
In the pediatric model (23), cBW was identified as a covariate for both clearance (equation 5) and the central volume of distribution (equation 6).
| (5) |
| (6) |
| (7) |
| (8) |
External model validation and evaluation.
Model validation using external data sets was performed using NONMEM, version 7.3 (Icon Development Solutions, Hanover, MD). Tools like PsN, version 4.2.0 (University of Uppsala, Sweden) (50), and R, version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria), were used to visualize data and evaluate the model. External validation was performed with an external data set containing TDM data which were not used for model development (42). This external data set (42) consisted of both (pre)term neonates and young infants (PNA up to 169 days; n = 206). An overview of the internal (model-building) (23, 24, 51) and external (42) data sets is given in Table 1.
TABLE 1.
Overview of the internal data sets used to develop the original neonatal and pediatric models and of the external data set used for the external validation of the original neonatal and pediatric modelsa
| Parameter | Median value (range) for the parameter |
|||
|---|---|---|---|---|
| Neonatal model |
Pediatric model |
|||
| Internal data set | External data set | Internal data set | External data set | |
| No. of patients | 273 | 191 | 429 | 206 |
| Gestational age (weeks) | 29 (23–34) | 32 (24–41) | NRb | NR |
| Postmenstrual age (weeks) | 30 (24–38) | 34 (25–44) | NR | NR |
| Postnatal age (days) | 14 (1–28) | 8 days (1–31) | 16 (1–6,205) | 8 (1–169) |
| Current body weight (g) | 1,170 (415–2,630) | 1,780 (420–4,680) | 1,800 (415–85,000) | 1,870 (420–5,000) |
| Birth body weight (g) | 1,140 (385–2,550) | 1,775 (420–4,680) | NR | NR |
| Ibuprofen coadministration (no. [%] of patients) | 23 (8.4) | 13 (6.8) | NR | NR |
For the external validation, previously published pharmacokinetic models (22, 23) were used to simulate each of the observations of the data sets 1,000 times. Normalized prediction distribution errors (NPDE) (52, 53) were computed for each of the data sets. The NPDE were statistically and visually evaluated for trends and bias. The NPDE were expected to follow the normal distribution, N(0, 1).
Simulations.
Concentration-time profiles were simulated in neonates and children for different dosing regimens reported in the Dutch Children's Formulary (37), British National Formulary for Children (BNFc) (38), the regimen proposed by the Infectious Diseases Society of America (IDSA) (39), and the meningitis regimen of the NeoFax manual (40) (see Table S1 in the supplemental material), using the parameter estimates from the original models (22, 23). Simulations were performed in six representative individuals (gestational ages [GA] of 24, 34, and 40 weeks and a PNA of 2 weeks and infants/children aged 6 months, 4, and 12 years (see Table S2). Infusion duration was 1 h (maximum, 10 mg/min), and treatment duration was 7 days. The AUC24 on day 7 was calculated noncompartmentally. For intermittent dosing, a peak concentration was simulated 1 h after the end of infusion, and a trough concentration (Ctrough) was simulated just before the next dose was given (10). For continuous dosing, a peak concentration (1 h after the loading dose) and, every 12 h, a steady-state concentration (Css) were simulated. Simulations were performed excluding the interindividual and residual variability. Based on the results, a model-based dosing algorithm was designed for neonates, infants, and children without renal dysfunction (defined as serum creatinine two to three times higher than the age-related reference value, similar to that of the original models [22, 23]), aiming for a target AUC24/MIC of >400 (17) without peak concentrations exceeding 40 mg/liter.
RESULTS
External validation of the neonatal model.
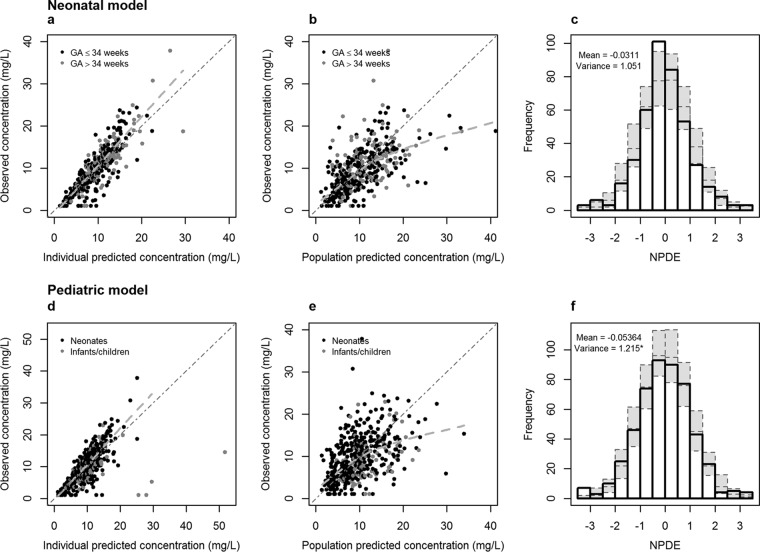
In Fig. 1a and b, the observed concentrations versus individual and population predicted concentrations are given for the neonatal model for the 191 neonates. Since the original model was built on vancomycin data from neonates with GA of ≤34 weeks, a distinction was made for neonates with GA of ≤34 weeks or >34 weeks. Extrapolation to neonates with GA of >34 weeks was evaluated because the original model used information on neonates with GA of >34 weeks from neonates receiving amikacin (22). The results of these data, together with the results of the NPDE analysis (mean, −0.0311; variance, 1.051) (Fig. 1c), show that the neonatal model can predict the median concentrations accurately and without bias in both preterm and term neonates. No clear trend was observed between the NPDE versus time and NPDE versus predicted concentrations for neonates with GA of ≤34 weeks or >34 weeks (Fig. 2), indicating that no time-dependent or concentration-dependent bias is present when predictions are made for new patients not included in the model-building data set.
FIG 1.
Results of the external validation of the models: observed versus individual predicted concentrations (a and d) and observed versus population predicted concentrations (b and e) for the neonatal model (black dots, preterm neonates; gray dots, term neonates) and the pediatric model (black dots, postnatal age of <31 days; gray dots, postnatal age of >30 days). The dotted lines represent the regression lines. The histograms (c and f) show the distributions of the normalized prediction distribution errors (NPDE) for, respectively, the neonatal model and the pediatric model, with mean and variance of NPDE analysis. The gray dotted boxes reflect the variance of the NPDE. *, variance is significantly different from 1 (P < 0.01).
FIG 2.
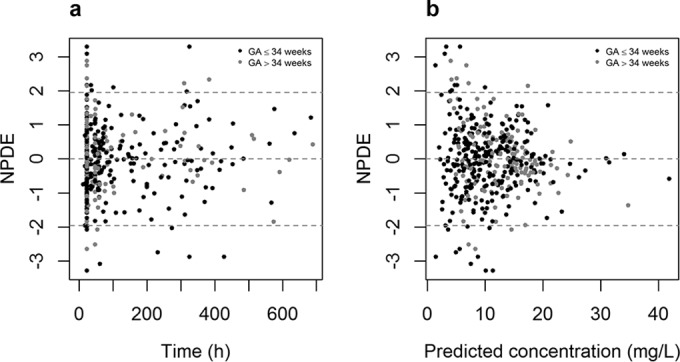
Results of the normalized prediction distribution error (NPDE) analysis of the neonatal model for individuals with GA of ≤34 and of >34 weeks versus time and versus predicted concentration.
External validation of the pediatric model.
In Fig. 1d and e, the observed concentrations versus individual and population predicted concentrations are given for the pediatric model for the 206 patients. The results of these figures and the NPDE analysis (mean, −0.0536; variance, 1.215) (Fig. 1f) show that the pediatric model can predict the median concentrations accurately.
Simulations of currently used dosing guidelines.
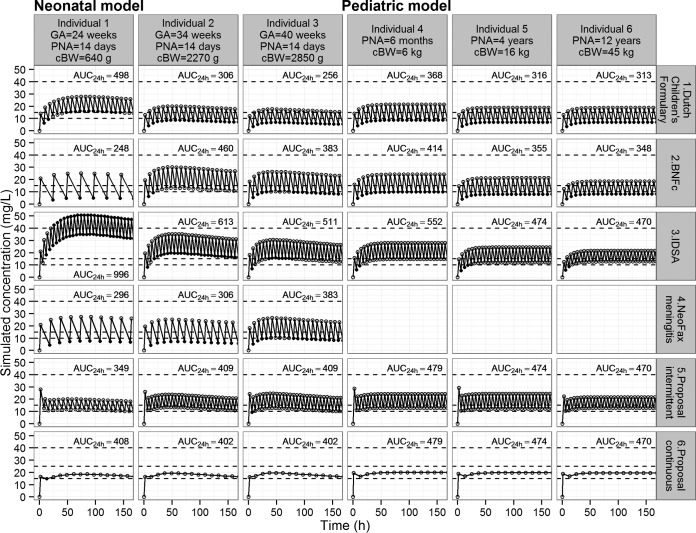
Figure 3 shows concentration-time profiles during vancomycin treatment for six representative individuals with current body weights varying between 0.640 kg and 45 kg following four different dosing regimens currently proposed, i.e., Dutch Children's Formulary (37), BNFc (38), IDSA (39), and NeoFax (40). In neonates, the dosing regimens as suggested by the Dutch Children's Formulary (37), BNFc (38), and NeoFax (40) generally led to an AUC24 below 400 mg · h/liter, except for some younger individuals (i.e., individual 1, with a cBW of 640 g) for the Dutch Children's Formulary (37) and individual 2 (cBW of 2.27 kg) for the BNFc regimen (38) (Fig. 3). After treatment according to the regimen suggested by IDSA (39), the AUC24 was above 400 mg · h/liter (Fig. 3). The concentrations in the smallest individual (individual 1), treated according to IDSA (39), exceeded 40 mg/liter (Fig. 3).
FIG 3.
Model-based predicted concentration-time profiles for six individuals. The Dutch Children's Formulary (37), British National Formulary for Children (BNFc) (38), Infectious Diseases Society of America (IDSA) (39), and NeoFax (meningitis regimen) (40; see also Table S1 in the supplemental material) dosing guidelines and model-based dosing algorithms (Table 2) were used. GA, gestational age; PNA, postnatal age; cBW, current body weight. The dotted lines indicate the target concentrations (10 to 15 mg/liter for trough concentrations upon intermittent dosing and 15 to 25 mg/liter for steady-state concentrations upon continuous dosing) and concentrations above which toxicity might occur (40 mg/liter). Concentrations outside the target and peak concentrations above 40 mg/liter are indicated by black dots. AUC24h, calculated area under the concentration-time curve (mg · h/liter) on the seventh day of treatment.
Individuals 4 to 6 treated according to the regimens in the Dutch Children's Formulary (37) and the BNFc (38) generally had AUC24 values below 400 mg · h/liter at day 7 (Fig. 3). Dosing as suggested by the IDSA (39) led to AUC24 values above 400 mg · h/liter for all individuals (Fig. 3).
Simulations to derive a model-based dosing regimen.
Using the externally validated neonatal and pediatric models, simulations were performed aiming for individualized dosing guidelines for neonates, infants, and children. The model-based dosing algorithm is anticipated to result in an AUC24 at day 7 above 400 mg · h/liter. Table 2 shows these model-based dosing algorithms, which are based on bBW, PNA, and the coadministration of ibuprofen for neonates and on cBW for infants and children. To calculate the dosages, cBW should be used for neonates, infants, and children. Compared to the currently used dosing regimens (37, 38, 40), the dosing frequency is increased to three to four doses/day for neonates and young infants to prevent high peak concentrations. For children aged 1 year and older, the dosing as suggested by IDSA (39), 60 mg/kg/day in four doses, is maintained.
TABLE 2.
Proposed model-based vancomycin dosing algorithms for the neonatal and pediatric populationsa
| Clinical characteristic |
Intermittent-dosing regimen |
Continuous-dosing regimen |
||||||
|---|---|---|---|---|---|---|---|---|
| PNAb (days) | bBW (g)c | cBW (kg)d | Loading dose (mg/kg)e,f | Maintenance dose (mg/kg/day) (no. of doses)f | AUC24 (mg · h/liter)g |
Loading dose (mg/kg) | Maintenance dose (mg/kg/day)h | AUC24 (mg · h/liter) |
| 0–7 | ≤700 | 16 | 15 (3) | 264–301 | 10.5 | 25 | 440–502 | |
| 700–1,000 | 21 (3) | 332–450 | 25 | 395–536 | ||||
| 1,000–1,500 | 27 (3) | 349–490 | 27 | 349–484 | ||||
| 1,500–2,500 | 30 (4) | 339–530 | 30 | 339–530 | ||||
| >2,500 | 36 (4) | 354–482 | 36 | 354–482 | ||||
| 8–14 | ≤700 | 20 | 21 (3) | 331–590 | 10.5 | 25 | 394–703 | |
| 700–1,000 | 27 (3) | 341–475 | 32 | 404–563 | ||||
| 1,000–1,500 | 36 (3) | 390–610 | 36 | 390–610 | ||||
| 1,500–2,500 | 40 (4) | 380–504 | 40 | 380–504 | ||||
| >2,500 | 48 (4) | 390–633 | 48 | 390–633 | ||||
| 14–28 | ≤700 | 23i | 24 (3) | 337–444 | 10.5 | 30 | 421–556 | |
| 700–1,000 | 42 (3) | 407–610 | 42 | 407–610 | ||||
| 1,000–1,500 | 45 (3) | 385–571 | 45 | 385–571 | ||||
| 1,500–2,500 | 52 (4) | 311–657 | 52 | 311–657 | ||||
| >2,500 | 60 (4) | 357–572 | 60 | 357–572 | ||||
| >28 | <2.5 | 18 | 32 (4) | 385–471 | 13 | 32 | 385–471 | |
| 2.5–5.0 | 24 | 40 (4) | 385–468 | 40 | 385–468 | |||
| 5.0–10.0 | 27 | 52 (4) | 434–500 | 52 | 434–500 | |||
| >10.0 | 30 | 60 (4) | 463–502 | 60 | 463–502 | |||
This dosing algorithm (mg/kg current bodyweight) is based on a target AUC24/MIC of >400.
PNA, postnatal age.
bBW: birth body weight.
cBW, current body weight.
The maximum loading dose is 1,200 mg.
If ibuprofen is given and PNA is <28 days, decrease the dose by 2 mg/kg/dose.
AUC24 is the noncompartmental calculated area under the concentration-time curve over 24 h. Values are the expected daily AUC range for the different dosing groups.
If ibuprofen is given and the PNA is <28 days, decrease the maintenance dose by 6 mg/kg/day.
The loading dose is 26 mg/kg if the PNA is 21 to 28 days.
In neonates, time to steady state varied between approximately 155 h in a 1-week-old preterm neonate to 50 h in a 2-week-old term neonate (Fig. 3). For the pediatric population (>1 month of age, irrespective of GA), the data in Fig. 3 show that the concentration in young infants reaches steady state after approximately 45 h, which decreases to approximately 36 h in children aged 1 year and older. In order to reach steady state more rapidly, a loading dose (Table 2) is proposed. After implementation of the loading dose, the AUC24 on the first day of treatment will be above 400 mg · h/liter, which is not the case if no loading dose is given (Table 3). Similar results are obtained after implementation of a loading dose for continuous dosing (results not shown).
TABLE 3.
Expected AUC24 at 24h after start of treatment upon intermittent dosing without and with the proposed loading dose
| Individual no. | Agea | Expected AUC24 (mg · h/liter)b |
|
|---|---|---|---|
| Without loading dose | With loading dose | ||
| 1 | GA, 24 weeks; PNA, 14 days | 233 | 411 |
| 2 | GA, 34 weeks; PNA, 14 days | 337 | 440 |
| 3 | GA, 40 weeks; PNA, 14 days | 362 | 436 |
| 4 | PNA, 6 mo | 344 | 457 |
| 5 | PNA, 4 yr | 358 | 466 |
| 6 | PNA, 12 yr | 351 | 433 |
GA, gestational age; PNA, postnatal age.
AUC24, calculated area under the concentration-time curve over 24 h.
DISCUSSION
In this study, both neonatal (22) and pediatric (23) vancomycin models have been validated externally. Based on these models, currently used dosing regimens have been evaluated upon which a model-based dosing algorithm was proposed for intermittent and continuous administration of vancomycin in neonates and children. An AUC24/MIC of over 400 was the aim. The results show that for this AUC, the target Ctrough after intermittent dosing was 10 to 15 mg/liter, and the target Css after continuous dosing was 15 to 25 mg/liter.
In order to facilitate the development of rational drug dosing schemes in the pediatric population, new approaches are needed (2–5). At this moment, several pediatric pharmacokinetic models of vancomycin have been published (19–34, 36, 54–59), but only few individualized, model-based dosing algorithms are available (25, 27, 30, 34, 57, 59). In clinical practice, target concentrations and AUC24/MIC values are hard to achieve (42–45, 60). Simulations based on two models (22, 23), that are now validated internally and externally, showed, indeed, that adapted doses are needed, especially for neonates and young infants. In the neonatal model (22), clearance is dependent on birth body weight and postnatal age of the neonates and the coadministration of ibuprofen. In the pediatric model (23), the changes in clearance were described with a body weight-dependent exponent model based on current body weight. These clinical characteristics were taken into account in the developed model-based dosing algorithm (Table 2), and simulations showed that the target AUC24/MIC and Ctrough were reached (Fig. 3). According to several currently used dosing regimens (37, 38, 40), the daily dose should be administered in two to three doses/day. For neonates and infants, this was increased to three to four doses/day in the model-based dosing algorithm. Four doses/day are needed when high dosages are needed to remain within the target for Ctrough; otherwise these high dosages will increase the probability of concentrations exceeding 40 mg/liter.
An AUC24/MIC of >400 has been associated with clinical success in adult patients with MRSA infections (16, 17). Whether this target is directly applicable to children has to be determined (60). For neonates, lower targets may suffice as most of the identified infectious organisms are coagulase-negative staphylococci instead of MRSA(11, 12). It has been reported that if the MIC is <1 mg/liter, 60 mg/kg/day seems necessary to reach an AUC24/MIC of >400 in children (20, 60–62).
In daily clinical practice the calculation of the AUC24 is not reliable on the basis of the TDM samples only. Therefore, Ctrough is mainly used as a surrogate predictor of AUC24 although the Ctrough is dependent on the dosing interval. One can debate what the proper Ctrough target range for neonates and infants is. IDSA recommends 15 to 20 mg/liter for severe infections after dosing two to three daily for adults and four times daily for children (39). Frymoyer et al. showed that a Ctrough of 7 to 11 mg/liter after once/twice-daily dosing is appropriate for reaching an AUC24/MIC of >400 in neonates (19). Other studies also showed that a Ctrough of around 8 mg/liter is appropriate for reaching the AUC24/MIC target in children (20, 21). The currently proposed dosing algorithm results in target trough concentrations between 10 and 15 mg/liter, with the data in Fig. 3 showing that the AUC24/MIC target of >400 is reached if the MIC is below 1 mg/liter.
Reaching the target AUC24/MIC in the first hours of treatment is associated with better outcomes (16). Studies in both adults (63) and neonates (34) show that higher concentrations are reached when a loading dose is given than with conventional dosing. By the implementation of a loading dose, steady-state concentrations can be reached sooner, especially in neonates where the elimination half-life is much longer than that in children and adults. At this moment, insufficient information is available regarding the safety of the administration of a vancomycin loading dose. Therefore, one could consider prolonging the infusion duration when a loading dose is given to reduce the risk of possible infusion reactions (17, 39). For intermittent dosing, the maximum loading dose is 1,200 mg to limit the infusion time to 2 h, allowing for the infusion line to be used for other drugs. The simulations of the model-based dosing algorithms have shown that the target concentrations for both intermittent and continuous dosing were reached within 24 h after administration of the loading dose (Table 3). The proposed loading dose, especially the loading dose for intermittent dosing, has to be tested in daily practice to confirm the safety of these dosages. For continuous dosing, a reduction in the loading dose compared to the loading dose suggested for intermittent dosing is proposed as the maintenance infusion starts immediately after the loading dose.
Continuous infusion of vancomycin has several advantages over intermittent infusion, for example, decreased pharmacokinetic variability and increased ease of TDM (18, 64). It has been shown in adults that continuous administration decreases the risk of nephrotoxicity compared to that with intermittent administration (64). However, although the number of neonates reaching the target concentrations increased with continuous dosing, TDM still seemed to be necessary (34). On the other hand, continuous vancomycin dosing might be problematic in neonatal clinical practice, especially in preterm neonates, because there may not be intravenous access available for this continuous infusion.
The data used for the external validation of the neonatal model consisted of both preterm and term neonates (42), and the model proved to be good in terms of predictive performance for both groups (Fig. 2). This is remarkable as the vancomycin data used for building the neonatal model consisted of preterm neonates only (GA up to 34 weeks) (Table 1) (51). However, for the vancomycin model (22), a neonatal amikacin covariate model (6) was used for extrapolation, for which data from both preterm and term neonates were available (6). This amikacin covariate model has been extrapolated not only to vancomycin but also to tobramycin, gentamicin, and netilmicin (22). For all other antibiotics, data were from both preterm and term neonates, and no prediction bias in term neonates could be observed for any of these antibiotics. Finally, an independent research group has successfully used the amikacin covariate model (6) to predict vancomycin clearance in both term and preterm neonates (36). Therefore, our conclusion that the model is valid for both preterm and term neonates seems justified. As shown in Table 1, similar percentages of the neonates included in model building (8.4%) and validation (6.8%) received ibuprofen. In the data set used for developing the amikacin covariate model, 13.5% received ibuprofen (6). Therefore, the proposed dosing algorithm is valid for both neonates receiving ibuprofen and those not receiving ibuprofen.
The original pediatric model was built using data from neonates, infants, children, and adolescents (Table 1). This model was developed to define a function to describe the maturation in glomerular filtration rate (GFR) (23). Adult GFR levels, expressed per unit of body surface area, are reached at approximately 6 to 12 months of age (65–68). External validation of this model was limited to neonates and young infants up to 6 months of age since no additional data were available. As the GFR approaches adult values at 6 to 12 months, the period where most developmental changes happen is covered by this external validation. For older infants and children, an additional external validation procedure should be performed. Based on these results, the proposed dosing algorithm should be adapted when necessary, with particular focus on infants older than 169 days.
In conclusion, both the neonatal and pediatric models could describe the data in the external data set well. This study shows that with currently used dosing regimens, the target AUC24/MIC and trough concentrations are hardly reached in neonates and young infants, whereas the model-based dosing algorithm results in the target exposure after both intermittent and continuous dosing. For children aged 1 year and older, the currently used dose of 60 mg/kg/day seems appropriate. In order to reach an AUC24/MIC of >400 on the first day of treatment, a loading dose should be administered. A prospective clinical study is warranted in order to validate the model-based dosing algorithm.
Supplementary Material
ACKNOWLEDGMENTS
This study was performed within the framework of Top Institute Pharma project number D2-501. K.A. is supported by the Fund for Scientific Research, Flanders (Fundamental Clinical Investigatorship 1800214N), and by an IWT-SBO project (130033). J.N.V.D.A. is supported by NIH (K24DA027992, R01HD048689, and U54HD071601) and the European Commission (TINN [223614] and TINN2 [260908], and Neurosis [223060]). C.A.J.K. is supported by the Innovational Research Incentives Scheme (Vidi grant, June 2013) of the Dutch Organization for Scientific Research.
Funding Statement
The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01968-15.
REFERENCES
- 1.Cuzzolin L, Atzei A, Fanos V. 2006. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf 5:703–718. doi: 10.1517/14740338.5.5.703. [DOI] [PubMed] [Google Scholar]
- 2.Knibbe CAJ, Danhof M. 2011. Individualized dosing regimens in children based on population PKPD modelling: are we ready for it? Int J Pharm 415:9–14. doi: 10.1016/j.ijpharm.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 3.De Cock RFW, Piana C, Krekels EHJ, Danhof M, Allegaert K, Knibbe CAJ. 2011. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol 67(Suppl 1):5–16. doi: 10.1007/s00228-009-0782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ince I, de Wildt SN, Tibboel D, Danhof M, Knibbe CAJ. 2009. Tailor-made drug treatment for children: creation of an infrastructure for data-sharing and population PK-PD modeling. Drug Discov Today 14:316–320. doi: 10.1016/j.drudis.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Admiraal R, van Kesteren C, Boelens JJ, Bredius RGM, Tibboel D, Knibbe CAJ. 2014. Towards evidence-based dosing regimens in children on the basis of population pharmacokinetic pharmacodynamic modelling. Arch Dis Child 99:267–272. doi: 10.1136/archdischild-2013-303721. [DOI] [PubMed] [Google Scholar]
- 6.De Cock RF, Allegaert K, Schreuder MF, Sherwin CM, de Hoog M, van den Anker JN, Danhof M, Knibbe CA. 2012. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet 51:105–117. doi: 10.2165/11595640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Krekels EHJ, Tibboel D, de Wildt SN, Ceelie I, Dahan A, van Dijk M, Danhof M, Knibbe CAJ. 2014. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet 53:553–563. doi: 10.1007/s40262-014-0135-4. [DOI] [PubMed] [Google Scholar]
- 8.Bartelink IH, van Kesteren C, Boelens JJ, Egberts TCG, Bierings MB, Cuvelier GDE, Wynn RF, Slatter MA, Chiesa R, Danhof M, Knibbe CAJ. 2012. Predictive performance of a busulfan pharmacokinetic model in children and young adults. Ther Drug Monit 34:574–583. doi: 10.1097/FTD.0b013e31826051bb. [DOI] [PubMed] [Google Scholar]
- 9.Valitalo PAJ, van den Anker JN, Allegaert K, de Cock RFW, de Hoog M, Simons SHP, Mouton JW, Knibbe CAJ. 2015. Novel model-based dosing guidelines for gentamicin and tobramycin in preterm and term neonates. J Antimicrob Chemother 70:2074–2077. doi: 10.1093/jac/dkv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EuroCept BV. 2010. Summary of product characteristics: Vancocin. EuroCept BV, Ankeveen, the Netherlands. [Google Scholar]
- 11.de Hoog M, Mouton JW, van den Anker JN. 2004. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet 43:417–440. doi: 10.2165/00003088-200443070-00001. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesh MP, Placencia F, Weisman LE. 2006. Coagulase-negative staphylococcal infections in the neonate and child: an update. Semin Pediatr Infect Dis 17:120–127. doi: 10.1053/j.spid.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Smirnova MV, Lubenko IY, Portnoy YA, Zinner SH, Firsov AA. 2007. Concentration-response relationships as a basis for choice of the optimal endpoints of the antimicrobial effect: daptomycin and vancomycin pharmacodynamics with staphylococci in an in vitro dynamic model. Int J Antimicrob Agents 29:165–169. doi: 10.1016/j.ijantimicag.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42:S35–9. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 15.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 17:479–501. doi: 10.1016/S0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 16.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 17.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 18.Stockmann C, Roberts JK, Yu T, Constance JE, Knibbe CA, Spigarelli MG, Sherwin CM. 2014. Vancomycin pharmacokinetic models: informing the clinical management of drug-resistant bacterial infections. Expert Rev Anti Infect Ther 12:1371–1388. doi: 10.1586/14787210.2014.966081. [DOI] [PubMed] [Google Scholar]
- 19.Frymoyer A, Hersh AL, El-Komy MH, Gaskari S, Su F, Drover DR, Van Meurs K. 2014. Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob Agents Chemother 58:6454–6461. doi: 10.1128/AAC.03620-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le J, Bradley JS, Murray W, Romanowski GL, Tran TT, Nguyen N, Cho S, Natale S, Bui I, Tran TM, Capparelli EV. 2013. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J 32:e155–63. doi: 10.1097/INF.0b013e318286378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frymoyer A, Guglielmo BJ, Hersh AL. 2013. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant staphylococcal infections. Pediatr Infect Dis J 32:1077–1079. doi: 10.1097/INF.0b013e318299f75c. [DOI] [PubMed] [Google Scholar]
- 22.De Cock RFW, Allegaert K, Sherwin CMT, Nielsen EI, de Hoog M, van den Anker JN, Danhof M, Knibbe CAJ. 2014. A neonatal amikacin covariate model can be used to predict ontogeny of other drugs eliminated through glomerular filtration in neonates. Pharm Res 31:754–767. doi: 10.1007/s11095-013-1197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Cock RFW, Allegaert K, Brussee JM, Sherwin CMT, Mulla H, de Hoog M, van den Anker JN, Danhof M, Knibbe CAJ. 2014. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi-physiological function for maturation in glomerular filtration. Pharm Res 31:2643–2654. doi: 10.1007/s11095-014-1361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NHG. 2007. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol 63:75–84. doi: 10.1111/j.1365-2125.2006.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capparelli EV, Lane JR, Romanowski GL, McFeely EJ, Murray W, Sousa P, Kildoo C, Connor JD. 2001. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J Clin Pharmacol 41:927–934. doi: 10.1177/00912700122010898. [DOI] [PubMed] [Google Scholar]
- 26.Giachetto GA, Telechea HM, Speranza N, Oyarzun M, Nanni L, Menchaca A. 2011. Vancomycin pharmacokinetic-pharmacodynamic parameters to optimize dosage administration in critically ill children. Pediatr Crit Care Med 12:e250–4. doi: 10.1097/PCC.0b013e3181fe4047. [DOI] [PubMed] [Google Scholar]
- 27.Grimsley C, Thomson AH. 1999. Pharmacokinetics and dose requirements of vancomycin in neonates. Arch Dis Child Fetal Neonatal Ed 81:F221-7. doi: 10.1136/fn.81.3.F221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Hoog M, Schoemaker RC, Mouton JW, van den Anker JN. 2000. Vancomycin population pharmacokinetics in neonates. Clin Pharmacol Ther 67:360–367. doi: 10.1067/mcp.2000.105353. [DOI] [PubMed] [Google Scholar]
- 29.Kimura T, Sunakawa K, Matsuura N, Kubo H, Shimada S, Yago K. 2004. Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob Agents Chemother 48:1159–1167. doi: 10.1128/AAC.48.4.1159-1167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marqués-Miñana M-R, Saadeddin A, Peris J-E. 2010. Population pharmacokinetic analysis of vancomycin in neonates. A new proposal of initial dosage guideline. Br J Clin Pharmacol 70:713–720. doi: 10.1111/j.1365-2125.2010.03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oudin C, Vialet R, Boulamery A, Martin C, Simon N. 2011. Vancomycin prescription in neonates and young infants: toward a simplified dosage. Arch Dis Child Fetal Neonatal Ed 96:F365-70. doi: 10.1136/adc.2010.196402. [DOI] [PubMed] [Google Scholar]
- 32.Seay RE, Brundage RC, Jensen PD, Schilling CG, Edgren BE. 1994. Population pharmacokinetics of vancomycin in neonates. Clin Pharmacol Ther 56:169–175. doi: 10.1038/clpt.1994.120. [DOI] [PubMed] [Google Scholar]
- 33.Yasuhara M, Iga T, Zenda H, Okumura K, Oguma T, Yano Y, Hori R. 1998. Population pharmacokinetics of vancomycin in Japanese pediatric patients. Ther Drug Monit 20:612–618. doi: 10.1097/00007691-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W, Lopez E, Biran V, Durrmeyer X, Fakhoury M, Jacqz-Aigrain E. 2013. Vancomycin continuous infusion in neonates: dosing optimisation and therapeutic drug monitoring. Arch Dis Child 98:449–453. doi: 10.1136/archdischild-2012-302765. [DOI] [PubMed] [Google Scholar]
- 35.Zhao W, Zhang D, Fakhoury M, Fahd M, Duquesne F, Storme T, Baruchel A, Jacqz-Aigrain E. 2014. Population pharmacokinetics and dosing optimization of vancomycin in children with malignant hematological disease. Antimicrob Agents Chemother 58:3191–3199. doi: 10.1128/AAC.02564-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W, Biran V, Jacqz-Aigrain E. 2013. Amikacin maturation model as a marker of renal maturation to predict glomerular filtration rate and vancomycin clearance in neonates. Clin Pharmacokinet 52:1127–1134. doi: 10.1007/s40262-013-0101-6. [DOI] [PubMed] [Google Scholar]
- 37.Nederlands Kenniscentrum voor Farmacotherpie bij Kinderen. 2013. Kinderformularium. Nederlands Kenniscentrum voor Farmacotherpie bij Kinderen, Rotterdam, the Netherlands. [Google Scholar]
- 38.Paediatric Formulary Committee. 2009. British national formulary for children. BMJ Group, London, United Kingdom. [Google Scholar]
- 39.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 40.Young TE, Mangum B, Thomson Reuters Clinical Editorial Staff . 2011. NeoFax, 24th ed PDR Network, Montvale, NJ. [Google Scholar]
- 41.Hey E, Northern Neonatal Network . 2011. Neonatal formulary 6; drug use in pregnancy and the first year of life, 6th ed BMJ Group, Chichester, United Kingdom. [Google Scholar]
- 42.Vandendriessche A, Allegaert K, Cossey V, Naulaers G, Saegeman V, Smits A. 2014. Prospective validation of neonatal vancomycin dosing regimens is urgently needed. Curr Ther Res Clin Exp 76:51–57. doi: 10.1016/j.curtheres.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eiland LS, English TM, Eiland EH. 2011. Assessment of vancomycin dosing and subsequent serum concentrations in pediatric patients. Ann Pharmacother 45:582–589. doi: 10.1345/aph.1P588. [DOI] [PubMed] [Google Scholar]
- 44.Broome L., So T-Y. 2011. An evaluation of initial vancomycin dosing in infants, children, and adolescents. Int J Pediatr 2011:470364. doi: 10.1155/2011/470364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frymoyer A, Guglielmo BJ, Wilson SD, Scarpace SB, Benet LZ, Hersh AL. 2011. Impact of a hospital-wide increase in empiric pediatric vancomycin dosing on initial trough concentrations. Pharmacotherapy 31:871–876. doi: 10.1592/phco.31.9.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKamy S, Chen T, Lee M, Ambrose PJ. 2012. Evaluation of a pediatric continuous-infusion vancomycin therapy guideline. Am J Health Syst Pharm 69:2066–2071. doi: 10.2146/ajhp120072. [DOI] [PubMed] [Google Scholar]
- 47.Pawlotsky F, Thomas A, Kergueris MF, Debillon T, Roze JC. 1998. Constant rate infusion of vancomycin in premature neonates: a new dosage schedule. Br J Clin Pharmacol 46:163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel AD, Anand D, Lucas C, Thomson AH. 2013. Continuous infusion of vancomycin in neonates. Arch Dis Child 98:478–479. doi: 10.1136/archdischild-2012-303197. [DOI] [PubMed] [Google Scholar]
- 49.Plan O, Cambonie G, Barbotte E, Meyer P, Devine C, Milesi C, Pidoux O, Badr M, Picaud JC. 2008. Continuous-infusion vancomycin therapy for preterm neonates with suspected or documented Gram-positive infections: a new dosage schedule. Arch Dis Child Fetal Neonatal Ed 93:F418-21. doi: 10.1136/adc.2007.128280. [DOI] [PubMed] [Google Scholar]
- 50.Lindbom L, Ribbing J, Jonsson EN. 2004. Perl-speaks-NONMEM (PsN): a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75:85–94. doi: 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Allegaert K, Anderson BJ, van den Anker JN, Vanhaesebrouck S, de Zegher F. 2007. Renal drug clearance in preterm neonates: relation to prenatal growth. Ther Drug Monit 29:284–291. doi: 10.1097/FTD.0b013e31806db3f5. [DOI] [PubMed] [Google Scholar]
- 52.Comets E, Brendel K, Mentré F. 2008. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the NPDE add-on package for R. Comput Methods Programs Biomed 90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Brendel K, Comets E, Laffont C, Laveille C, Mentré F. 2006. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23:2036–2049. doi: 10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamarre P, Lebel D, Ducharme MP. 2000. A population pharmacokinetic model for vancomycin in pediatric patients and its predictive value in a naive population. Antimicrob Agents Chemother 44:278–282. doi: 10.1128/AAC.44.2.278-282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim H-S, Chong YP, Noh Y-H, Jung J-A, Kim YS. 2014. Exploration of optimal dosing regimens of vancomycin in patients infected with methicillin-resistant Staphylococcus aureus by modeling and simulation. J Clin Pharm Ther 39:196–203. doi: 10.1111/jcpt.12123. [DOI] [PubMed] [Google Scholar]
- 56.Zhao W, Kaguelidou F, Biran V, Zhang D, Allegaert K, Capparelli EV, Holford N, Kimura T, Lo Y-L, Peris J-E, Thomson A, van den Anker JN, Fakhoury M, Jacqz-Aigrain E. 2013. External evaluation of population pharmacokinetic models of vancomycin in neonates: the transferability of published models to different clinical settings. Br J Clin Pharmacol 75:1068–1080. doi: 10.1111/j.1365-2125.2012.04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo Y-L, van Hasselt JGC, Heng S-C, Lim C-T, Lee T-C, Charles BG. 2010. Population pharmacokinetics of vancomycin in premature Malaysian neonates: identification of predictors for dosing determination. Antimicrob Agents Chemother 54:2626–2632. doi: 10.1128/AAC.01370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revilla N, Martín-Suárez A, Pérez MP, González FM, Fernández de Gatta MDM. 2010. Vancomycin dosing assessment in intensive care unit patients based on a population pharmacokinetic/pharmacodynamic simulation. Br J Clin Pharmacol 70:201–212. doi: 10.1111/j.1365-2125.2010.03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.James A, Koren G, Milliken J, Soldin S, Prober C. 1987. Vancomycin pharmacokinetics and dose recommendations for preterm infants. Antimicrob Agents Chemother 31:52–54. doi: 10.1128/AAC.31.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frymoyer A, Hersh AL, Benet LZ, Guglielmo BJ. 2009. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr Infect Dis J 28:398–402. doi: 10.1097/INF.0b013e3181906e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demirjian A, Finkelstein Y, Nava-Ocampo A, Arnold A, Jones S, Monuteaux M, Sandora TJ, Patterson A, Harper MB. 2013. A randomized controlled trial of a vancomycin loading dose in children. Pediatr Infect Dis J 32:1217–1223. doi: 10.1097/INF.0b013e3182a26774. [DOI] [PubMed] [Google Scholar]
- 62.Frymoyer A, Hersh AL, Coralic Z, Benet LZ, Joseph Guglielmo B. 2010. Prediction of vancomycin pharmacodynamics in children with invasive methicillin-resistant Staphylococcus aureus infections: a Monte Carlo simulation. Clin Ther 32:534–542. doi: 10.1016/j.clinthera.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosini JM, Laughner J, Levine BJ, Papas MA, Reinhardt JF, Jasani NB. 2015. A randomized trial of loading vancomycin in the emergency department. Ann Pharmacother 49:6–13. doi: 10.1177/1060028014556813. [DOI] [PubMed] [Google Scholar]
- 64.Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. 2012. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 67:17–24. doi: 10.1093/jac/dkr442. [DOI] [PubMed] [Google Scholar]
- 65.Alcorn J, McNamara PJ. 2002. Ontogeny of hepatic and renal systemic clearance pathways in infants: part II. Clin Pharmacokinet 41:1077–1094. doi: 10.2165/00003088-200241130-00005. [DOI] [PubMed] [Google Scholar]
- 66.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. 2003. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 67.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, Chatelut E, Grubb A, Veal GJ, Keir MJ, Holford NHG. 2009. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 68.Chen N, Aleksa K, Woodland C, Rieder M, Koren G. 2006. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol 21:160–168. doi: 10.1007/s00467-005-2105-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.