Abstract
The genetic structures involved in the dissemination of blaCMY-2 carried by Proteus mirabilis isolates recovered from different gull species in the South of France were characterized and compared to clinical isolates. blaCMY-2 was identified in P. mirabilis isolates from 27/93 yellow-legged gulls and from 37/65 slender-billed gulls. It was carried by a conjugative SXT/R391-like integrative and conjugative element (ICE) in all avian strains and in 3/7 human strains. Two clinical isolates had the same genetic background as six avian isolates.
TEXT
CMY-2 and its derivatives are the most widespread plasmid-mediated cephalosporinases (AmpC) in Proteus mirabilis (1, 2), and they are mainly found in plasmids from incompatibility (Inc) groups A/C and I1 (1, 3). blaCMY-2 mobilization by an integrative and conjugative element (ICE) in P. mirabilis was described recently in Japan and Spain (4, 5). Several studies focused on the characterization of AmpC-producing P. mirabilis isolates from food-producing animals and pets (6–8). Migratory birds can act as reservoirs and play an important role in the dissemination of these resistance genes (9). This study investigated the occurrence and the molecular structures supporting the spread of blaCMY-2 in P. mirabilis isolates from different gull species in the South of France. Human isolates from the same geographical region were used for comparison.
(Preliminary results of this research were presented at the 34th Réunion Interdisciplinaire de Chimiothérapie Anti-Infectieuse [RICAI], Paris, France, 27 to 28 November 2014.)
In April 2012, fecal samples were collected from 93 juvenile nonfledged yellow-legged gulls (Larus michahellis) and from 65 slender-billed gulls (Chroicocephalus genei) breeding in the island of Carteau in Port-Saint-Louis and in the Giens peninsula (France), respectively. A cotton swab was rotated inside the bird cloacae and was immediately inoculated in tryptic soy broth (Thermo Fisher Scientific). After a 24-h incubation at 37°C, broths were subcultured on chromID ESBL agar biplates (bioMérieux, Marcy l'Etoile, France) and were examined after 24 and 48 h. P. mirabilis was identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker Daltonics, Bremen, Germany). Susceptibility to amoxicillin, cefalotin, cefoxitin, cefotaxime, ceftazidime, cefepime, and imipenem was tested using the disk diffusion method on Mueller-Hinton agar and was interpreted following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints (version 5.0) (http://www.eucast.org/clinical_breakpoints/). The criteria used to select suspected AmpC producers were described previously (1). Extended-spectrum beta-lactamase (ESBL) production was excluded using the double-disc synergy test (10). Seven P. mirabilis human clinical isolates with AmpC phenotypes collected in 2012 in the same geographical region were used for comparison. Strain typing was performed by repetitive sequence-based PCR (rep-PCR) using the DiversiLab system (bioMérieux, France) as described previously (11).
The presence of genes encoding AmpC was assessed by multiplex PCR as previously described (12). Plasmids were typed using the PCR-based replicon-typing method (PBRT) (13) and the plasmid relaxase gene-typing method (PRaseT) (14). Primers targeting the highly conserved relaxase gene of the ICE belonging to the SXT/R391 family were designed as previously described (14). ICE integration at the 5′ end of the chromosomal prfC gene was investigated using primers to amplify the ORF_96 gene at the ICE 3′ extremity, degenerate primers to cover P. mirabilis and Escherichia coli prfC gene sequences, and primers that amplify both regions (overlapping PCR) (4, 5) (see Table S1 in the supplemental material). E. coli J53 was used as a negative control. All PCR products were sequenced bidirectionally using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) and an Applied Biosystems 3730xl capillary sequencer. Sequences were identified using the BLAST program and the NCBI database. Mating experiments were performed at 37°C using P. mirabilis and azide-resistant Escherichia coli J53 strains with a donor to recipient ratio of 1:1. Transconjugants were selected on Drigalski agar (Bio-Rad) containing 200 mg/liter sodium azide and 20 mg/liter cefoxitin. To investigate the possible chromosomal location of blaCMY-2, I-CeuI-restricted DNA from selected strains and transconjugants was separated by pulsed-field gel electrophoresis (PFGE). Pulse ramps were 90 to 150 s for 24 h and 90 to 120 s for 6 h at 170 V. Southern blotting and hybridization using a blaCMY-2 probe and a probe for the highly specific integrase of the SXT/R391-like ICE were carried out as previously described (15, 16).
AmpC enzymes were produced by 29% (27/93) of P. mirabilis isolates from yellow-legged gulls and by 56.9% (37/65) of isolates from slender-billed gulls. PCR analysis and sequencing showed that all 71 AmpC-producing P. mirabilis isolates (i.e., 64 avian and 7 human isolates) carried the blaCMY-2 gene.
Eleven clusters (named A to K) were recognized using rep-PCR (Fig. 1). Ten clusters contained exclusively avian isolates. Five clusters included only yellow-legged gull isolates (clusters A, B, C, D, and G), two clusters were comprised only of slender-billed gull isolates (clusters J and K), and three clusters included isolates from both species (clusters E, F, and I). Cluster H was comprised of two human and six avian P. mirabilis isolates.
FIG 1.
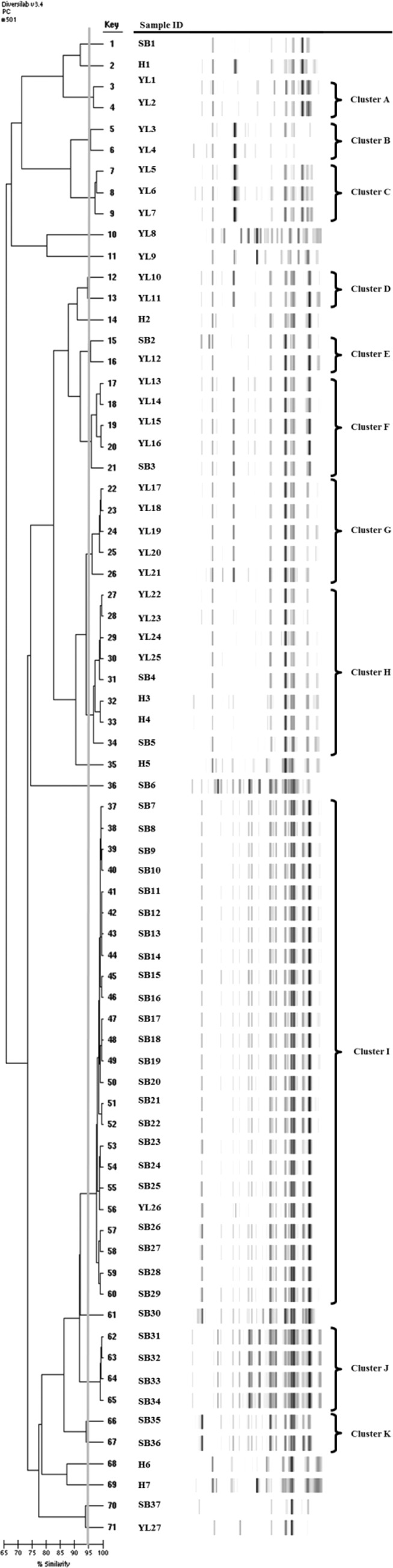
Dendrogram of P. mirabilis isolates carrying the blaCMY-2 genes. YL, yellow-legged gull isolates; SB, slender-billed gull isolates; H, human isolates.
In all avian isolates, PRaseT revealed the presence of an IncJ relaxase gene specific to the SXT/R391-like ICE family (17), while PBRT was negative. In the seven human isolates, the SXT/R391-like ICE (three strains), an IncA/C plasmid (two strains), or both elements (two strains) were detected. Mating experiments were performed using one randomly chosen P. mirabilis isolate for each clone defined by rep-PCR and all singletons. For cluster H, which includes human and avian isolates, one avian and the two human strains were chosen. The blaCMY-2 gene was successfully transferred to E. coli (average transfer frequency, 10−5 transconjugants/recipient). The blaCMY-2 and the IncJ relaxase genes were detected in all recipient cells, except for the two clinical isolates that carried only the IncA/C plasmid and the two human isolates with the IncA/C plasmid and the SXT/R391-like ICE in which only the IncA/C plasmid was detected, suggesting that it carried the resistance gene. PCR mapping of the blaCMY-2-containing region revealed a genetic structure similar to the one described by Harada et al. (4), which included a highly specific SXT/R391-like integrase, the IncJ relaxase, and a Tn10 composite transposon that carried the blaCMY-2 gene integrated in the ICE (see Fig. S1 in the supplemental material).
blaCMY-2 chromosomal localization was demonstrated by Southern blotting in the avian strain YL11 and the human strain H3 and the corresponding transconjugants but not in the human strains H4 and H7 (Table 1). Hybridization using the ICE-specific probe revealed the presence of the ICE in strains YL11, H3, and H4 and in the transconjugants for strains YL11 and H3 (data not shown). ICE and blaCMY-2 colocalization on the same ≈565-kb I-CeuI fragment was observed in the strains YL11 and H3.
TABLE 1.
Antibiotic susceptibility testing of Proteus mirabilis isolatesa
| Isolate type | rep-PCR cluster | Genetic support of blaCMY-2 | No. isolates | Selected strains on which Southern blotting was performedb | Parental strain or transconjugantc |
Antibiotic resistance profile (inhibition zone diam, mmd) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMX | CTX | CF | FEP | FOX | CAZ | IPM | ||||||
| Avian | A | SXT/R391-like ICE | 2 | P | R (6) | S (23) | R (6) | S (29–30) | R (17) | I (19–21) | S (28–30) | |
| T | R (6) | R (13) | R (6) | S (30) | R (6) | R (9) | S (32) | |||||
| B | SXT/R391-like ICE | 2 | P | R (6) | I (19) | R (6) | S (24–27) | R (14–17) | I (19–20) | S (25–27) | ||
| T | R (6) | R (10) | R (6) | S (33) | R (6) | R (6) | S (30) | |||||
| C | SXT/R391-like ICE | 3 | P | R (6) | S (23–26) | R (6) | S (27–34) | S (19–21) | I (19–21) | S (25–28) | ||
| T | R (6) | R (10) | R (6) | S (32) | R (6) | R (6) | S (30) | |||||
| D | SXT/R391-like ICE | 2 | YL11 | P | R (6) | S (23–26) | R (6) | S (29–34) | R (17–18) | I (20–21) | S (26) | |
| T | R (6) | R (11) | R (6) | S (32) | R (6) | R (6) | S (31) | |||||
| E | SXT/R391-like ICE | 2 | P | R (6–12) | S (23–26) | R (6) | S (26–39) | R (18) | S/I (19–27) | S (24–29) | ||
| T | R (6) | R (16) | R (6) | S (34) | R (8) | R (12) | S (30) | |||||
| F | SXT/R391-like ICE | 5 | P | R (6) | S/I (19–26) | R (6) | S (27–34) | R (15–18) | S/I (19–24) | S (25–29) | ||
| T | R (6) | R (8) | R (6) | S (33) | R (6) | R (6) | S (32) | |||||
| G | SXT/R391-like ICE | 5 | P | R (6) | S/I (19–25) | R (6) | S (25–33) | R (10–18) | S/I (19–27) | S (27–30) | ||
| T | R (6) | R (6) | R (6) | S (32) | R (6) | R (6) | S (30) | |||||
| H | SXT/R391-like ICE | 6 | P | R (6–7) | S (23–28) | R (6) | S (28–39) | R (13–18) | S/I (19–30) | S (26–33) | ||
| T | R (6) | R (15) | R (6) | S (34) | R (9) | R (10) | S (32) | |||||
| I | SXT/R391-like ICE | 24 | P | R (6–10) | S (23–32) | R (6) | S (29–41) | R (8–18) | S/I (19–31) | S (24–31) | ||
| T | R (6) | R (9) | R (6) | S (37) | R (6) | R (8) | S (29) | |||||
| J | SXT/R391-like ICE | 4 | P | R (6–8) | S (25–32) | R (6) | S (32–41) | R (18) | S/I (21–32) | S (24–31) | ||
| T | R (6) | R (6) | R (6) | S (34) | R (6) | R (6) | S (30) | |||||
| K | SXT/R391-like ICE | 2 | P | R (6) | S (28–30) | R (6) | S (34–39) | RI (18) | S (28–29) | S (30–31) | ||
| T | R (6) | R (9) | R (6) | S (33) | R (6) | R (10) | S (32) | |||||
| Singletons | SXT/R391-like ICE | 7 | P | R (6) | S/R (16–29) | R (6) | S (27–37) | S/R (18–19) | S/I (19–27) | S (23–29) | ||
| T | R (6) | R (11–15) | R (6) | S (33–38) | R (6–8) | R (11–17) | S (31–33) | |||||
| Human clinical | H | IncA/C plasmide | 1 | H4 | P | R (6) | S (25) | R (6) | S (27) | R (18) | I (20) | S (25) |
| T | R (6) | R (6) | R (6) | S (33) | R (6) | R (6) | S (31) | |||||
| SXT/R391-like ICE | 1 | H3 | P | R (6) | S (23) | R (6) | S (32) | R (15) | I (20) | S (25) | ||
| T | R (6) | R (8) | R (6) | S (34) | R (6) | R (7) | S (32) | |||||
| Singletons | SXT/R391-like ICE | 2 | P | R (6) | S/I (19–23) | R (6) | S (25–35) | R (17–18) | I (19–21) | S (22–26) | ||
| T | R (6) | R (9–11) | R (6) | S (28–33) | R (6) | R (6–13) | S (29–32) | |||||
| Singleton | IncA/C plasmide | 1 | P | R (6) | I (19) | R (6) | S (25) | R (17) | I (19) | S (23) | ||
| T | R (6) | R (12) | R (6) | S (38) | R (6) | R (8) | S (32) | |||||
| Singleton | IncA/C plasmid | 2 | H7 | P | R (6) | S (21–23) | R (6) | S (34–37) | R (18) | I (20) | S (27–30) | |
| T | R (6) | R (15–16) | R (6) | S (32–35) | R (6) | R (6–10) | S (33) | |||||
Antibiotic susceptibility testing was performed and interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints (version 5.0) (http://www.eucast.org/clinical_breakpoints/). Mating experiments were performed using one randomly chosen representative P. mirabilis isolate for each clone defined by rep-PCR and all singletons.
Southern blotting with blaCMY-2 and ICE-specific integrase probes was performed after PFGE following DNA macrorestriction using the intronic endonuclease I-CeuI.
P, parental strain; T, transconjugant.
The range of the inhibition zone diameter for clustered isolates is indicated. AMX, amoxicillin; CF, cefalotin; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; FOX, cefoxitin; IPM, imipenem; R, resistant; S, susceptible; I, intermediate.
One strain also harbored a SXT/R391-like ICE that did not carry blaCMY-2.
Positive overlapping PCR amplification demonstrated that the ICE was integrated in the chromosome of each P. mirabilis isolate and the corresponding transconjugant. These data suggest that the ICE is involved in blaCMY-2 dissemination in all avian and in three human isolates. PCR analysis of ICE insertion sites in randomly chosen isolates for each clone of our collection and of their respective transconjugants gave the same result. As expected, (i) ORF_96 was present in P. mirabilis and its transconjugant but was absent in E. coli J53, (ii) prfC was present in P. mirabilis and E. coli J53 but could not be amplified in the transconjugant (due to disruption caused by the ICE insertion), and (iii) the overlapping PCR covering the ORF_96 and prfC genes was positive in P. mirabilis and its transconjugant but negative in E. coli J53. This confirmed the insertion of the blaCMY-2-carrying ICE at the 5′ end of prfC.
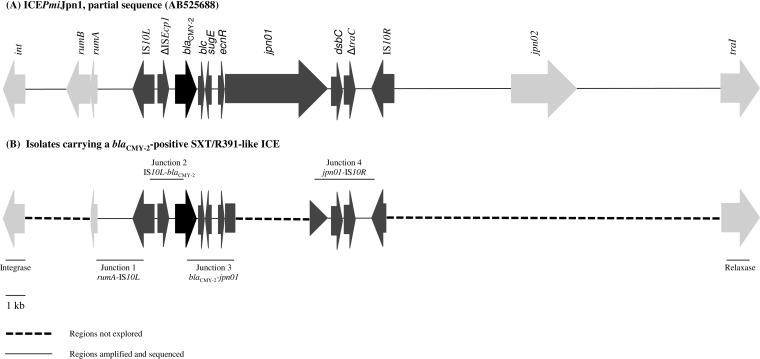
P. mirabilis isolates carrying blaCMY-2 were described in dogs (6), cats (18), and chickens (19). Mata et al. recently highlighted the increasing prevalence of blaCMY-2-carrying SXT/R391-like ICEs in P. mirabilis human isolates in Spain between 1999 and 2007 (5). In their study, the blaCMY-2 genetic environment was similar to the one observed in our avian P. mirabilis isolates. A similar structure was described in the ICEPmiJpn1 element recovered from a P. mirabilis clinical isolate in Japan in 2006 (Fig. 2). Our study is the first description of such a structure in P. mirabilis isolates in France and tends to confirm the hypothesis about the role of ICEs in blaCMY-2 dissemination worldwide (5). The finding that the ICE was the exclusive genetic support of the resistance gene in all avian isolates, which were classified in different clusters, suggests that it may play an important role in the spread of antibiotic resistance genes among these birds and in marine ecosystems. Although the biotic/abiotic reservoirs of resistant strains of P. mirabilis continue to be poorly known, the presence of similar clones in avian and human isolates in our study may suggest exchanges between different ecosystems (wildlife versus human). Moreover, P. mirabilis has already been described as a shuttle species between human and animal guts and water bodies (20).
FIG 2.
Genetic organization of the SXT/R391-like ICE carrying blaCMY-2 in P. mirabilis. The same molecular structure was observed in all 64 blaCMY-2-positive avian strains and in 3/7 (42.9%) human strains. This structure was similar to that of ICEPmiJpn1 (GenBank accession no. AB525688). Light gray arrows represent the conserved genes of the ICE. Dark gray arrows represent genes carried by the Tn10 composite transposon. Black arrows represent the blaCMY-2 gene. Thin black lines represent the different primers used to explore this region. (A) Partial sequence of ICEPmiJpn1 used as a template for PCR mapping. (B) Schematic representation of the regions amplified and sequenced in this study.
As ICEs from the SXT/R391-like family are widespread in environmental strains of Proteus spp., Vibrio spp., Photobacterium spp., and Shewanella spp. (21), the insertion of beta-lactam resistance genes in this structure is worrying and should be monitored. Further studies are needed to assess the presence of beta-lactam-resistant ICE-positive strains (P. mirabilis and other clinically relevant bacteria) in the environment and in wildlife microbiota.
Supplementary Material
ACKNOWLEDGMENT
We thank Elisabetta Andermarcher for assistance in preparing and editing the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01654-15.
REFERENCES
- 1.Mata C, Miró E, Rivera A, Mirelis B, Coll P, Navarro F. 2010. Prevalence of acquired AmpC beta-lactamases in Enterobacteriaceae lacking inducible chromosomal ampC genes at a Spanish hospital from 1999 to 2007. Clin Microbiol Infect 16:472–476. doi: 10.1111/j.1469-0691.2009.02864.x. [DOI] [PubMed] [Google Scholar]
- 2.Empel J, Baraniak A, Literacka E, Mrówka A, Fiett J, Sadowy E, Hryniewicz W, Gniadkowski M. 2008. Molecular survey of β-lactamases conferring resistance to newer β-lactams in Enterobacteriaceae isolates from Polish hospitals. Antimicrob Agents Chemother 52:2449–2454. doi: 10.1128/AAC.00043-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada S, Ishii Y, Saga T, Tateda K, Yamaguchi K. 2010. Chromosomally encoded blaCMY-2 located on a novel SXT/R391-related integrating conjugative element in a Proteus mirabilis clinical isolate. Antimicrob Agents Chemother 54:3545–3550. doi: 10.1128/AAC.00111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mata C, Navarro F, Miró E, Walsh TR, Mirelis B, Toleman M. 2011. Prevalence of SXT/R391-like integrative and conjugative elements carrying blaCMY-2 in Proteus mirabilis. J Antimicrob Chemother 66:2266–2270. doi: 10.1093/jac/dkr286. [DOI] [PubMed] [Google Scholar]
- 6.Harada K, Niina A, Shimizu T, Mukai Y, Kuwajima K, Miyamoto T, Kataoka Y. 2014. Phenotypic and molecular characterization of antimicrobial resistance in Proteus mirabilis isolates from dogs. J Med Microbiol 63:1561–1567. doi: 10.1099/jmm.0.081539-0. [DOI] [PubMed] [Google Scholar]
- 7.Wong MHY, Wan HY, Chen S. 2013. Characterization of multidrug-resistant Proteus mirabilis isolated from chicken carcasses. Foodborne Pathog Dis 10:177–181. doi: 10.1089/fpd.2012.1303. [DOI] [PubMed] [Google Scholar]
- 8.Seiffert SN, Tinguely R, Lupo A, Neuwirth C, Perreten V, Endimiani A. 2013. High prevalence of extended-spectrum-cephalosporin-resistant Enterobacteriaceae in poultry meat in Switzerland: emergence of CMY-2- and VEB-6-possessing Proteus mirabilis. Antimicrob Agents Chemother 57:6406–6408. doi: 10.1128/AAC.01773-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnedahl J, Drobni P, Johansson A, Hernandez J, Melhus A, Stedt J, Olsen B, Drobni M. 2010. Characterization, and comparison, of human clinical and black-headed gull (Larus ridibundus) extended-spectrum beta-lactamase-producing bacterial isolates from Kalmar, on the southeast coast of Sweden. J Antimicrob Chemother 65:1939–1944. doi: 10.1093/jac/dkq222. [DOI] [PubMed] [Google Scholar]
- 10.Jarlier V, Nicolas MH, Fournier G, Philippon A. 1988. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis 10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 11.Hawser SP, Badal RE, Bouchillon SK, Hoban DJ, Hackel MA, Biedenbach DJ, Goff DA. 2014. Susceptibility of gram-negative aerobic bacilli from intra-abdominal pathogens to antimicrobial agents collected in the United States during 2011. J Infect 68:71–76. doi: 10.1016/j.jinf.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Pérez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Compain F, Poisson A, Le Hello S, Branger C, Weill FX, Arlet G, Decré D. 2014. Targeting relaxase genes for classification of the predominant plasmids in Enterobacteriaceae. Int J Med Microbiol 304:236–242. doi: 10.1016/j.ijmm.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet R, Marchandin H, Chanal C, Sirot D, Labia R, De Champs C, Jumas-Bilak E, Sirot J. 2002. Chromosome-encoded class D beta-lactamase OXA-23 in Proteus mirabilis. Antimicrob Agents Chemother 46:2004–2006. doi: 10.1128/AAC.46.6.2004-2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teyssier C, Marchandin H, Siméon De Buochberg M, Ramuz M, Jumas-Bilak E. 2003. Atypical 16S rRNA gene copies in Ochrobactrum intermedium strains reveal a large genomic rearrangement by recombination between rrn copies. J Bacteriol 185:2901–2909. doi: 10.1128/JB.185.9.2901-2909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Böltner D, MacMahon C, Pembroke JT, Strike P, Osborn AM. 2002. R391: a conjugative integrating mosaic comprised of phage, plasmid, and transposon elements. J Bacteriol 184:5158–5169. doi: 10.1128/JB.184.18.5158-5169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hordijk J, Schoormans A, Kwakernaak M, Duim B, Broens E, Dierikx C, Mevius D, Wagenaar JA. 2013. High prevalence of fecal carriage of extended spectrum β-lactamase/AmpC-producing Enterobacteriaceae in cats and dogs. Front Microbiol 4:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reich F, Atanassova V, Klein G. 2013. Extended-spectrum β-lactamase- and AmpC-producing enterobacteria in healthy broiler chickens, Germany. Emerg Infect Dis 19:1253–1259. doi: 10.3201/eid1908.120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sosa V, Schlapp G, Zunino P. 2006. Proteus mirabilis isolates of different origins do not show correlation with virulence attributes and can colonize the urinary tract of mice. Microbiology 152:2149–2157. doi: 10.1099/mic.0.28846-0. [DOI] [PubMed] [Google Scholar]
- 21.Burrus V, Marrero J, Waldor MK. 2006. The current ICE age: biology and evolution of SXT-related integrating conjugative elements. Plasmid 55:173–183. doi: 10.1016/j.plasmid.2006.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.