Abstract
As antibiotic resistance increases, there is a need for new therapies to treat infection, particularly in cystic fibrosis (CF), where Pseudomonas aeruginosa is a ubiquitous pathogen associated with increased morbidity and mortality. Bacteriophages are an attractive alternative treatment, as they are specific to the target bacteria and have no documented side effects. The efficacy of phage cocktails was established in vitro. Two P. aeruginosa strains were taken forward into an acute murine infection model with bacteriophage administered either prophylactically, simultaneously, or postinfection. The infective burden and inflammation in bronchoalveolar lavage fluid (BALF) were assessed at various times. With low infective doses, both control mice and those undergoing simultaneous phage treatment cleared P. aeruginosa infection at 48 h, but there were fewer neutrophils in BALF of phage-treated mice (median, 73.2 × 104/ml [range, 35.2 to 102.1 × 104/ml] versus 174 × 104/ml [112.1 to 266.8 × 104/ml], P < 0.01 for the clinical strain; median, 122.1 × 104/ml [105.4 to 187.4 × 104/ml] versus 206 × 104/ml [160.1 to 331.6 × 104/ml], P < 0.01 for PAO1). With higher infective doses of PAO1, all phage-treated mice cleared P. aeruginosa infection at 24 h, whereas infection persisted in all control mice (median, 1,305 CFU/ml [range, 190 to 4,700 CFU/ml], P < 0.01). Bacteriophage also reduced CFU/ml in BALF when administered postinfection (24 h) and both CFU/ml and inflammatory cells in BALF when administered prophylactically. A reduction in soluble inflammatory cytokine levels in BALF was also demonstrated under different conditions. Bacteriophages are efficacious in reducing both the bacterial load and inflammation in a murine model of P. aeruginosa lung infection. This study provides proof of concept for future clinical trials in patients with CF.
INTRODUCTION
Antimicrobial resistance in general has been flagged as a major global health risk by the World Health Organization (1), with the rising incidence of multidrug-resistant Gram-negative bacteria, such as Pseudomonas aeruginosa, being of particular concern. Pseudomonas aeruginosa is a ubiquitous, Gram-negative bacterium that opportunistically infects patients with chronic suppurative lung diseases, such as cystic fibrosis (CF), and is clearly associated with increased morbidity and mortality (2). Antimicrobial therapy is usually effective for eradicating the initial infection (3), but most patients ultimately become chronically infected as P. aeruginosa is both inherently resistant to many classes of antibiotics due to its efflux pump system (4) and rapidly develops mutation-based resistances in the presence of exposure to antimicrobial agents (5). Bacterial infection is closely associated with pulmonary inflammation in CF, and although there is increasing evidence that this paradigm may be simplistic (6), it is clear that neutrophilic inflammation causes lung injury (7) and that inflammation declines following antibiotic treatment of P. aeruginosa in CF (8). For CF patients, the failure of conventional antibiotics facilitates the development of chronic P. aeruginosa infection whereby originally free-floating (planktonic) organisms switch to a biofilm mode of growth (9). In addition to increasing antibiotic resistance (10), there are significant side effects associated with conventional antimicrobials, particularly when they are used repeatedly or over long periods of time. These include renal toxicity and ototoxicity, both of which are commonly encountered in adult clinics. There is thus an urgent need for novel antipseudomonal therapies for patients with CF.
Bacteriophages are naturally occurring viruses that specifically target bacterial cells (11). First described by Felix d'Herelle in 1917 (12), they were the focus of several therapeutic studies in the 1920s. However, these were run under conditions not comparable to modern standards and lacked suitable controls, and due to the low quality of some products, results were often inconsistent (13). For these reasons, coupled with the discovery of antibiotics in 1928 (14), widespread clinical use was mainly limited to Eastern Europe (12, 15).
Bacteriophages offer several advantages over conventional antibiotics: they are highly selective and so can be targeted against pathogenic bacteria without disturbing the resident bacterial flora; they multiply exponentially in the presence of host (bacterial) cells rather than decreasing in concentration over time, thereby potentially providing treatment targeted to the sites of need (12); they can adapt and mutate like bacteria, thereby potentially reducing the emergence of resistant bacterial strains (16, 17); and they appear to be relatively free of side effects (17). Bacteriophages are widely used in food preservation, being applied, for example, to the surfaces of preserved meats and cheeses (18, 19). Bacteriophages have been shown to be efficacious in vitro against P. aeruginosa in biofilms (20) and in vivo in murine models of P. aeruginosa septicemia: between 50 and 100% of mice infected with a lethal intraperitoneal dose of P. aeruginosa survived when administered a single intravenous (21) or intraperitoneal (22) dose of phage up to 1 h postinfection. Recent studies of acute lung infection in mice have used bioluminescent strains of P. aeruginosa to demonstrate phage efficacy; the bioluminescence decreased following administration of phage with an associated reduction in bacteria recovered from bronchoalveolar lavage fluid (BALF) and disease severity (as assessed by the histological analysis of lung tissue) in phage-treated mice compared with that in controls (23, 24). However, none of these studies investigated the impact of phage-targeted pseudomonal killing on lung inflammation. This is highly relevant as persistent neutrophilic inflammation has been associated with lung injury (25) and, even during periods of stability, CF patients with chronic P. aeruginosa infection have higher inflammatory indices than subjects without CF (26). The reduction in the bacterial load demonstrated in previous studies does not necessarily equate to attenuation of inflammatory damage. An important unanswered question is whether phage therapy itself induces a host inflammatory response either directly or secondary to phage-induced P. aeruginosa lysis (leading to release of toxins such as lipopolysaccharide [LPS]) or reduces the response by hastening bacterial clearance.
Although in vitro models suggest that bacteriophages can be deposited successfully in the human lung by nebulization (27), no studies of the efficacy in lung infection under strict regulatory criteria have been undertaken to date. However, a small randomized controlled trial in the United Kingdom reported that a single topical dose of phage reduced symptoms in patients with persistent P. aeruginosa ear infections refractory to multiple courses of antibiotics, with no reported adverse events (28). The safety of intravenous phage has also previously been reported in children (29).
Based on the previously published data, we considered the possibility that bacteriophages might be a useful treatment for P. aeruginosa in patients with CF. We hypothesized that such treatment would reduce the bacterial load as previously described and also thereby reduce inflammation and the detrimental downstream consequences thereof. In this study, we tested specifically designed anti-P. aeruginosa bacteriophage cocktails in a murine model of P. aeruginosa lung infection. P. aeruginosa strains assessed as being susceptible to bacteriophage cocktails in vitro were studied in vivo in order to determine if there were any immunological benefits of phage therapy. We assessed the effects on lung bacterial load, systemic spread of infection, and pulmonary inflammation and explored the potential both for treatment of infection and for prophylaxis.
MATERIALS AND METHODS
Ethics statement.
Female BALB/c mice (Harlan, United Kingdom) were housed in a specialized animal facility in accordance with European regulations. Food and drink were provided ad libitum. The work was prospectively approved by the United Kingdom Home Office and National Ethics Committee.
Bacteriophage isolation and cocktail selection.
Bacteriophages for this study were isolated by Special Phage Services Pty Ltd. (Sydney, Australia) from a variety of environmental sources in New South Wales, Australia, using different protocols as previously described (30). Three different bacteriophage cocktails: cocktail 1 (P. aeruginosa 24, P. aeruginosa 25, and P. aeruginosa 7), cocktail 2 (P. aeruginosa 39, P. aeruginosa 67, P. aeruginosa 77, and P. aeruginosa 119) and cocktail 3 (P. aeruginosa 3, P. aeruginosa 6, P. aeruginosa 10, P. aeruginosa 32, and P. aeruginosa 37) were selected based on their abilities to delay or inhibit the appearance of putative phage-resistant cells in liquid or solid media. Each bacteriophage was tested for its morphology and host spectrum of activity against P. aeruginosa strain PAO1 and 10 P. aeruginosa clinical isolates collected in Australia (see Table S1 in the supplemental material). The approximate molecular weight (MW) for each phage was also determined by pulsed-field electrophoresis (31), and each phage was shown to be different by restriction digest (data not shown).
In vitro phage susceptibility testing.
Before our chosen bacterial isolates were used in vivo, their susceptibilities to the bacteriophage cocktails were initially confirmed using conventional plaque assays (32). PAO1, a well-described laboratory reference strain (33, 34), and five P. aeruginosa strains isolated from the sputa of adult inpatients with CF at the Royal Brompton Hospital, London, were tested against the three novel bacteriophage cocktails. Pure isolates were inoculated into 10 ml of tryptone soy broth (TSB) (Oxoid, United Kingdom) and cultured overnight at 37°C with agitation. The optical density (OD) of the broth was measured spectrophotometrically (Spectronic, United Kingdom) and adjusted to 0.1 (equivalent to approximately 1 × 108 CFU/ml) by dilution with sterile TSB. Then 100 μl of the diluted broth was added to 3 ml of semisolid agar (prepared by dissolving 3 g of TSB powder [Sigma, United Kingdom] and 0.4 g of agar [Sigma] in 100 ml of deionized water and autoclaving) that had been maintained at 55°C in a water bath before being poured onto Pseudomonas-specific agar (PSA) (Oxoid). After cooling, 10-μl aliquots of each bacteriophage cocktail (6.2 × 1010 PFU/ml undiluted and serially log10 diluted down to 10−6) were pipetted onto the prepared bacterial lawns and incubated overnight at 37°C. The cocktail that was most broadly efficacious with the lab strain PAO1 and the most susceptible strain isolated from CF patients (here termed the “clinical strain”) were taken forward for these proof-of-principle in vivo studies.
In vivo methodology.
Following overnight culture of the two selected bacterial strains in TSB, broth was centrifuged (Meadowrose Scientific, United Kingdom) at 2,000 × g at 4°C for 10 min, and the resultant cell pellet was resuspended in 10 ml of phosphate-buffered saline (PBS) (Gibco, United Kingdom). The OD was adjusted by dilution with PBS; the relationship between CFU/ml and OD was previously determined by serial dilution and colony counting according to the method of Miles et al. (35).
Adult BALB/c mice were anesthetized by isoflurane inhalation. In a pilot dose-finding study, 3 mice/group received 50 μl by nasal gavage (sniffing) of 1 × 109, 5 × 108,1 × 108, or 5 × 107 CFU/ml. Mice in the first 3 groups were either deceased or unwell at 24 h postinfection. Therefore, a maximum inoculum of 5 × 107 CFU/ml was selected for use in the initial experiments.
Mice were infected by intranasal sniffing initially with 50 μl of 5 × 107 CFU/ml (2.5 × 106 CFU; “low dose”); in later experiments where bronchoalveolar lavage (BAL) was carried out 24 h postinfection, we were able to apply 50 μl of 5 × 108 CFU/ml (2.5 × 107 CFU; “high dose”). Then 20 μl (1.2 × 109 PFU) of intranasal phage therapy or SM buffer (Teknova, USA) (controls) was administered either simultaneously, 24 h postinfection, or 48 h preinfection. BAL was carried out either 24 or 48 h postinfection using the following technique. Terminal general anesthesia was achieved by intraperitoneal administration of Hypnorm (Vetapharma, United Kingdom) and Hypnovel (Roche, United Kingdom). After cessation of circulation, the trachea was surgically exposed and cannulated with a 22-g Abbocath (Hospira, United Kingdom). BAL was performed by repeated instillation and aspiration of 500 μl of PBS (three cycles) via the Abbocath. Spleens were dissected and harvested into 500 μl of PBS.
Processing of samples.
A 100-μl aliquot of BALF was serially log10 diluted, and 5 10-μl drops were cultured overnight at 37°C on PSA plates according to the method of Miles et al. (35). Nonquantitative cultures on PSA were also performed on homogenized explanted spleens to determine systemic spread.
The remaining BALF was centrifuged at 4°C and 2,000 × g for 10 min, and 100-μl aliquots of supernatant were stored at −80°C for subsequent batch analysis of inflammatory cytokine levels. The cytokines were selected based on their inclusion in a commercially available multiplex enzyme-linked immunosorbent assay (ELISA) platform (Meso Scale Discovery [MSD] mouse proinflammatory 7-plex ultrasensitive assay). The remaining cell pellet was resuspended in 200 μl of PBS, 20 μl of this solution was added to 40 μl of trypan blue (Sigma) and 20 μl of PBS (1:4 dilution), and total inflammatory cells were counted with a Neubauer hemocytometer. A further 100 μl was used for a differential cell count following cytospinning (Shandon, United Kingdom) for 5 min at 400 rpm. Slides were fixed with methanol and stained using a May-Grünwald-Giesma Quick Stain kit prior to mounting with DPX (Sigma, United Kingdom). A total of 300 cells per slide were counted by one investigator following blinding of the slides by a second investigator; unblinding took place at the end of each part of the study.
Statistical analyses.
Based on modest group sizes and the assumption of non-Gaussian data distribution, Mann-Whitney t tests were performed on all data sets using Prism 6.0 (GraphPad, USA). Eight was the arbitrary number of mice decided upon for each arm of each condition being tested; if clear differences became apparent with fewer (a minimum of six mice in each arm), the study was stopped in accordance with the ethical standards of animal research. Median data and ranges are presented. The null hypothesis was rejected if the P value was <0.05.
RESULTS
Lytic activity of bacteriophage cocktail in vitro.
All three bacteriophage cocktails were effective against PAO1 at phage dilutions from undiluted to 10−5. This result matched our expectations, given the reported activity of the individual phages against this strain (see Table S1 in the supplemental material). When tested against the clinical isolates, bacteriophage cocktail 1 was active against the 5 clinical isolates/strains tested, while bacteriophage cocktails 2 and 3 infected only 3 out of the 5 isolates/strains. The sensitivities of each clinical strain tested to each phage cocktail are shown in Table 1.
TABLE 1.
Susceptibility of five clinical strains of P. aeruginosa to three bacteriophage cocktailsa
| Clinical isolate | Phage dilution for: |
||
|---|---|---|---|
| Cocktail 1 | Cocktail 2 | Cocktail 3 | |
| PA 12B-4854 | 10−2 | No effect | No effect |
| PA 12B-4973 | 10−6 | 10−4 | 10−6 |
| PA 12B-5001 | 10−5 | 10−5 | 10−6 |
| PA 12B-5025 | 10−2 | 10−2 | 10−4 |
| PA 12B-5099 | 10−2 | No effect | No effect |
Cocktail 1 was more broadly efficacious and PA12B-4973 (the clinical strain) was most broadly sensitive and therefore was used for ongoing work.
The broad spectrum of activity of a bacteriophage cocktail has been suggested as an important characteristic to overcome the limitations of specificity associated with bacteriophages. Based on the susceptibility results obtained, bacteriophage cocktail 1 was selected for in vivo use. Similarly, as there are reports suggesting good correlation between in vitro activity and in vivo phage efficacy (36), the isolate/strain PA 12B-4973 was selected for in vivo experimentation, as the phage cocktail 1 was very efficient against this isolate/strain even at a very low concentration (10−6).
Simultaneous administration of bacteriophage and P. aeruginosa.
Two experimental conditions were tested. Initially, mice were infected with 2.5 × 106 bacteria (50 μl of 5 × 107 CFU/ml) for strain PAO1 (n = 16) or the clinical strain (n = 12), and immediately afterward, while the mice were under the same inhalational anesthetic, 20 μl of phage (n = 14) or buffer (n = 14) was administered. Samples were harvested at 48 h. BALF cultures demonstrated that all phage-treated mice and most control mice cleared Pseudomonas; 2/6 control mice infected with the clinical strain had persistent infection but with low bacterial loads (20 and 40 CFU/ml) on quantitative cultures. Systemic spread, as indicated by positive splenic cultures, was not seen in either group. However, the inflammation was significantly reduced in the phage-treated animals. The total inflammatory cell (predominantly neutrophils) numbers were lower with both bacterial strains (see Table S2 in the supplemental material and Fig. 1) as were the levels of several cytokines although this was observed only with the clinical strain (see Tables S3a and S3b in the supplemental material and Fig. 2).
FIG 1.

Differential cell counts (median/range) from BAL performed at 48 h in mice inoculated with 2.5 × 106 of a clinical strain of P. aeruginosa (Pa) and simultaneously treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer. *, P < 0.05; **, P < 0.01.
FIG 2.

Proinflammatory cytokine levels (median/range) from BAL performed at 48 h in mice inoculated with 2.5 × 106 of a clinical strain of P. aeruginosa and simultaneously treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer. *, P < 0.05.
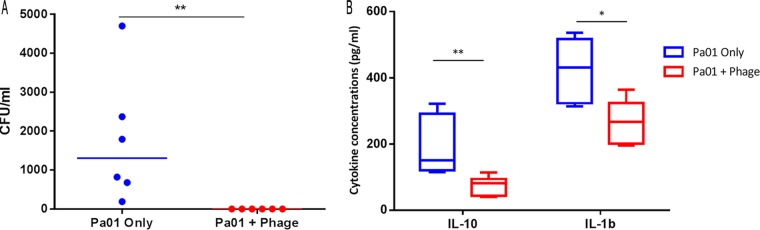
These data provided evidence for a phage effect, but the ability of control animals to clear this dose of P. aeruginosa meant that no signal on bacterial killing was demonstrated. Therefore, we next infected mice with a higher dose of PAO1 (2.5 × 107 CFU/ml) and chose an earlier (24 h) time point for sampling. Mice infected with higher inocula of the clinical strain became terminally unwell in less than 24 h, and thus, only PAO1 was used for ongoing work. Under these conditions, all control mice had detectable P. aeruginosa infection (median, 1,305 CFU/ml [range, 190 to 4,700 CFU/ml]). In contrast, no bacteria were cultured from BAL fluid from any phage-treated mice (P < 0.01) (Fig. 3A). There was no growth from the splenic cultures in either group. The interleukin-10 (IL-10) (P < 0.01) and IL-1β (P < 0.05) levels were significantly reduced in phage-treated mice compared with those in controls (Fig. 3B), but there was no difference in the levels of the five other cytokines measured or in the inflammatory cell counts (see Tables S4 and S5 in the supplemental material). Having demonstrated efficacy with simultaneous administration and recognizing how poorly this mirrored any clinical context, we went on to assess delayed and prophylactic phage administration.
FIG 3.
(A) Colony counts per milliliter from BAL performed at 24 h in mice inoculated with 2.5 × 107 of PAO1 and simultaneously treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer. If no colonies were visible to the naked eye, this is reported as 0 CFU/ml; the theoretical limit of detection was 100 CFU/ml, as 10-μl drops of BALF were cultured. (B) Proinflammatory cytokine levels (median/range) from BAL performed at 24 h in mice inoculated with 2.5 × 107 of PAO1 and simultaneously treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer. *, P < 0.05; **, P < 0.01.
Delayed administration of bacteriophage.
High-dose (2.5 × 107 CFU/ml) PAO1 was inoculated intranasally, and bacteriophage or buffer was administered 24 h later. Samples were obtained a further 24 h after this. In contrast to control mice, who all had positive BALF cultures (median, 5,950 CFU/ml [range, 40 to 194,000 CFU/ml]), complete clearance was seen in 6/7 (86%) phage-treated mice, and the median CFU/ml was significantly lower (0 CFU/ml [range, 0 to 160 CFU/ml], P < 0.01) (Fig. 4A). Two control mice had growth of P. aeruginosa from splenic cultures, indicating systemic spread of the infection. This was not seen in any of the phage-treated animals. There was a reduction in IL-10 (P < 0.05) and keratinocyte chemoattractant (KC) (P < 0.01) in phage-treated mice (Fig. 4B), but no reductions in other inflammatory cytokine levels or in cell counts (see Tables S6 and S7 in the supplemental material).
FIG 4.
(A) Colony counts per milliliter from BAL performed at 48 h in mice inoculated with 2.5 × 107 of PAO1 and treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer 24 h after the initial infection. If no colonies were visible to the naked eye, this is reported as 0 CFU/ml; the theoretical limit of detection was 100 CFU/ml, as 10-μl drops of BALF were cultured. (B) Proinflammatory cytokines (median/range) from BAL performed at 48 h in mice inoculated with 2.5 × 107 of PAO1 and treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer 24 h after the initial infection. *, P < 0.05; **, P < 0.01.
“Prophylactic” administration of bacteriophage.
Bacteriophage or buffer was instilled 48 h prior to intranasal infection with high-dose (2.5 × 107 CFU/ml) PAO1. Samples were obtained 24 h after the bacterial infection. Two control mice died in this 24-h period. Of those surviving, all had persistent and high levels of bacteria in BALF (median, 1.8 × 106 CFU/ml [range, 1,140 to 1.64 × 1010 CFU/ml]). In contrast, 5/7 (71%) phage-pretreated mice had successfully cleared the infection, and those that did not had only low levels of bacteria detected (median, 0 CFU/ml [range, 0 to 20 CFU/ml], P < 0.01) (Fig. 5A). Four of five (80%) surviving control mice had positive splenic cultures, indicating systemic spread. This was not seen in any of the phage-treated mice (n = 7).
FIG 5.
(A) Colony counts per milliliter from BAL performed at 24 h in mice inoculated with 2.5 × 107 of PAO1 and treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer prophylactically, 48 h prior to infection. If no colonies were visible to the naked eye, this is reported as 0 CFU/ml; the theoretical limit of detection was 100 CFU/ml, as 10-μl drops of BALF were cultured. (B) KC (median/range) from BAL performed at 24 h in mice inoculated with 2.5 × 107 of PAO1 and treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer prophylactically, 48 h prior to infection. **, P < 0.01.
KC (Fig. 5B) (P < 0.01) and total and differential cell counts (Fig. 6) in BALF of the mice pretreated with phage were significantly reduced compared with those in controls (see Table S8 in the supplemental material and Fig. 6), although there was no difference in other cytokine levels (see Table S8).
FIG 6.

Differential cell counts (median/range) from BAL performed at 24 h in mice inoculated with 2.5 × 107 of PAO1 and treated with 20 μl of bacteriophage cocktail (containing 1.24 × 109 PFU) or SM buffer prophylactically, 48 h prior to infection. *, P < 0.05.
DISCUSSION
We have shown that delivery of selected bacteriophage cocktails during, before, or after lung infection with P. aeruginosa has a significant impact on local bacterial burdens, systemic spread of infection, and lung inflammatory responses.
We first confirmed the expected activity of three bacteriophage cocktails in vitro against the laboratory strain PAO1 and demonstrated the activity of the three cocktails against some but not all of the clinical isolates of P. aeruginosa taken from patients with CF. The ability of a phage to form plaques on a lawn of the target bacteria is seen as the basic requirement for phage therapy. Furthermore, a correlation between bacteriophage activity in vitro and subsequent success in vivo has been reported before (36). This study supports the importance of this correlation, although care should be taken not to assume that this is the only property required for efficacy (37). Subsequently, bacteriophage reduced the infective burden and inflammatory responses in a murine infection model when used at an initial theoretical multiplicity of infection (MOI) of ∼100. At lower bacterial doses, no difference in the infective burden was demonstrated, as mice were capable of spontaneous clearance, but there was a significant reduction in neutrophils. At higher infective doses, the objective of achieving persistent infection was achieved, but only in control mice; all phage-treated mice retained the ability to clear their lungs of infection. Similarly, in experiments where phage or buffer was administered postinfection, there were significantly lower CFU/ml in BALF of phage-treated mice than controls, although no difference was seen in inflammatory cells. Finally, the efficacy of prophylactic phage was also demonstrated: all treated mice survived a high dose of inoculum and had significantly lower CFU/ml and neutrophils in BALF than controls.
In keeping with the observation that BALB/c mice are inherently resistant to P. aeruginosa infection (38), most mice in this study were able to clear a low dose of intranasally administered P. aeruginosa with no evidence of systemic spread even in the absence of phage treatment. However, such mice demonstrated neutrophilic inflammation at 48 h in response to the two strains of P. aeruginosa administered. This inflammatory response was significantly reduced when bacteriophage was administered simultaneously. This finding is significant because, although inflammation and infection may be dissociated in CF (39, 40), the role of neutrophils in mediating tissue injury is clear, and therefore, treatments that reduce their number may be of benefit (41). However, as trials of leukotriene B4 receptor antagonists have demonstrated, this paradigm may be oversimplistic (42).
In addition, reduced levels of BALF IL-10, IL-6, tumor necrosis factor alpha (TNF-α), and IL-12p70 were demonstrated in phage-treated mice infected with the clinical strain of P. aeruginosa, with a trend toward reduced KC. TNF-α plays a key role in the acute-phase response, promoting recruitment of neutrophils to sites of infection (43, 44), and is also one of the physiological stimuli for IL-6 production, along with bacterial endotoxin (45). IL-12p70 is the biologically active form of IL-12, which is important in Th1 immune responses to bacteria and viruses (46), while KC is a major neutrophil chemoattractant (47). The reduction in the neutrophil counts and cytokine levels in BALF of phage-treated mice 48 h following infection with a clinical P. aeruginosa strain suggests that bacteriophage complements the inherent resistance of these mice to P. aeruginosa, hastening clearance and thereby diminishing the inflammatory response. That there was no significant reduction in cytokine levels in phage-treated mice infected with PAO1 most likely reflects a difference in virulence between the two strains of bacteria, as differences became apparent when the inoculum of PAO1 was increased.
When the dose of nasally instilled PAO1 was increased 10-fold, and BAL was performed earlier at 24 h, control mice had significant numbers of P. aeruginosa present in the BALF, whereas all phage-treated mice had completely cleared the infection. Lower levels of inflammation (IL-1β and IL-10 and a trend in IL-6) were also observed.
In addition to the coadministration experiments, we demonstrated efficacy when phage was administered either after bacterial infection, mimicking a clinical “treatment” scenario, or beforehand, as “prophylaxis.” Both resulted in significant impacts on bacterial loads and inflammatory responses, which suggests potential clinical utility. The prophylaxis experiments also indicate that phage is relatively stable in the murine lung (for at least 24 h). This raises a concern that carryover phage might be present in the plating of BALF from infected animals, which has the potential to reduce CFU counts ex vivo. The way in which samples were processed was designed to minimize the risk of phage-bacterium interactions in vitro, but it was not possible to demonstrated that no carryover phage was present in cultured BALF. This question has been addressed previously; studies using bioluminescent strains to monitor phage efficacy in real time (23, 48) demonstrated that phage activity clearly occurs in the lungs and is not the result of ex vivo culturing only. This issue is analogous to culturing BALF or sputum samples from patients already taking antibiotics. The fact that bacteria do not grow in vitro leads to the conclusion that infection is not present; it is not possible to be sure if this is because of efficacy in vivo or an in vitro effect after samples are collected. Molecular assay testing to address this issue may be applied to future experimental models.
What we have not done in this set of experiments is model chronic infection with mucoid or biofilm modes of growth. Transgenic CF mice in general do not recapitulate the lung disease characteristic of human CF, and most investigators have resorted to the use of artificial means of establishing chronic infection, such as agar beads. While this model is potentially useful for studying host responses, we decided against it for the testing of a topically applied therapeutic agent, penetration of which might have been adversely affected by the presence of the agar. We may in the future be able to study such mechanisms in alternative animal models, such as the beta epithelial sodium channel (β-ENaC)-overexpressing mouse or the CF pig or ferret. Data from other fields suggesting that bacteriophages are effective against biofilm-growing organisms (20, 49–51) provide encouraging support for this approach.
While all mice infected with P. aeruginosa and simultaneously treated with bacteriophage cleared infection (Fig. 3A), colonies were still present in BALF of some mice that received delayed or prophylactic dosing of phage (Fig. 4A and 5A), albeit in far lower quantities than in untreated mice. This is most likely indicative of incomplete clearance due to higher bacterial loads in mice where phage treatment was delayed and/or because BAL was performed at an earlier time point (24 h rather than 48 h), but the possibility that the recovered P. aeruginosa had evolved phage resistance cannot be discounted. The recovered colonies were not retested in vitro for phage susceptibility, but this will be done in future experiments, as the question of whether sensitive bacterial strains become resistant to bacteriophage over time is key to clinical application.
Although the majority of the data support a reduction and benefit in the general inflammatory response when bacteriophages are used, different conditions led to variable changes in specific soluble inflammatory markers. The levels of five cytokines were lower in phage-treated mice infected with clinical P. aeruginosa, whereas no phage-related differences were seen with PAO1 at the same inoculum; given the severity of illness noted in the mice infected with higher doses of the clinical strain, this might be attributed to differences in the virulence of the P. aeruginosa strains. At higher inocula of PAO1, IL-10 and IL-1β were lower in phage-treated animals following simultaneous administration, IL-10 and KC were lower when phage was given 24 h postinfection, and only KC was lower with prophylactic phage administration. Difficulties in standardization of animals, exacerbating inherent biological variability under each condition, may have contributed to this; although all mice were adult female BALB/c, their exact ages and weights could not be matched, which may have affected their responses. There may also have been underpowering for some of these effects due to our attempts to limit the numbers of animals used in the experiments.
Reductions in IL-10 in phage-treated animals were seen across several of the conditions tested. This finding initially seemed counterintuitive, as IL-10 inhibits the production of proinflammatory cytokines (including IL-1β, IL-6, IL-12, and TNF-α) by T cells, thereby downregulating the acute immune response (52); there were close correlations of IL-10 with IL-1β, IL-6, and TNF-α (r2 = 0.734 to 0.787) but not with IL-12p70 (r2 = 0.368) in this study. However, recent evidence suggests that the IL-10 response is related to the severity of a preceding proinflammatory response (52), which is subsequently downregulated by IL-10 to prevent ongoing inflammation. Hence, high levels are associated with protracted infection, and blockade of IL-10 may in fact promote clearance of bacteria (53). If this is the case, and there remains no consensus in the literature due to the complexity of the IL-10 signaling (52), then reduced IL-1β, IL-6, and TNF-α in experiments with the clinical strain, reduced IL-1β and a trend toward reduced IL-6 (P = 0.06) when the inoculum of PAO1 was increased with simultaneous dosing of phage, and a trend toward reduced IL-1β and IL-6 with later dosing of phage might account for the reduced “anti-inflammatory” IL-10 in this study; as there was less initial inflammation in phage-treated mice, less IL-10 was detected. Further support for this theory is the fact that IL-10, of all the cytokines measured in this study, correlated most strongly with the absolute neutrophil count across each of the conditions tested (r2 = 0.503).
From a translational perspective, there were three key findings from this study. First, no evidence of murine toxicity following rapid lysis of P. aeruginosa by bacteriophage was seen, suggesting that this approach may be safe in a human clinical trial. Second, a beneficial effect of phage treatment once infection was established provides support for bacteriophage as a therapy. Third, and perhaps most encouraging, administration prior to infection is efficacious (both aiding clearance once infection is encountered and reducing neutrophilic inflammation), raising the possibility of prophylaxis, perhaps only at times of increased infection risk, for example, during viral infection, which has been linked to acquisition of P. aeruginosa. United Kingdom Registry data (54) currently demonstrate a window of opportunity in childhood and early adolescence, before the majority of patients have become chronically infected with P. aeruginosa, for such a prophylactic approach. Clearly, further work is needed to establish the longevity of phage in the non-bacterium-infected host, the frequency with which this would have to be administered, and potential host responses (either inflammatory or immune) associated with acute administration or long-term use. It will also be crucial to assess the development of phage resistance in any persisting bacteria. Recent studies have demonstrated proof of concept for prophylactic phage therapy in humans, particularly for gastrointestinal infections (55); regular dosing from a young age of anti-P. aeruginosa bacteriophage cocktails, selected with knowledge of local strains and sensitivities, is therefore an attractive strategy by which to attempt to reduce the incidence of infection and burden of long-term morbidity and mortality associated with chronic infection.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01426-15.
REFERENCES
- 1.World Health Organization. 2013. Antimicrobial resistance. http://www.who.int/mediacentre/factsheets/fs194/en/.
- 2.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. 2002. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 3.Langton Hewer SC, Smyth AR. 2009. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev 4:CD004197. [DOI] [PubMed] [Google Scholar]
- 4.Poole K. 2001. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J Mol Microbiol Biotechnol 3:255–264. [PubMed] [Google Scholar]
- 5.Livermore DM. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 6.Rao S, Grigg J. 2006. New insights into pulmonary inflammation in cystic fibrosis. Arch Dis Child 91:786–788. doi: 10.1136/adc.2004.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downey DG, Bell SC, Elborn JS. 2009. Neutrophils in cystic fibrosis. Thorax 64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 8.Ordoñez CL, Henig NR, Mayer-Hamblett N, Accurso FJ, Burns JL, Chmiel JF, Daines CL, Gibson RL, McNamara S, Retsch-Bogart GZ, Zeitlin PL, Aitken ML. 2003. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med 168:1471–1475. doi: 10.1164/rccm.200306-731OC. [DOI] [PubMed] [Google Scholar]
- 9.O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 10.Nordmann P, Naas T, Fortineau N, Poirel L. 2007. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol 10:436–440. doi: 10.1016/j.mib.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Bradbury J. 2004. “My enemy's enemy is my friend.” Using phages to fight bacteria. Lancet 363:624–625. doi: 10.1016/S0140-6736(04)15629-8. [DOI] [PubMed] [Google Scholar]
- 12.Fruciano DE, Bourne S. 2007. Phage as an antimicrobial agent: d‘Herelle's heretical theories and their role in the decline of phage prophylaxis in the West. Can J Infect Dis Med Microbiol 18:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Łobocka M, Szybalski WT. 2012. Advances in virus research. Bacteriophages, part A. Preface. Adv Virus Res 82:xiii–xv. [DOI] [PubMed] [Google Scholar]
- 14.Fleming A. 1929. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol 10:226–236. [Google Scholar]
- 15.Kutateladze M, Adamia R. 2008. Phage therapy experience at the Eliava Institute. Med Mal Infect 38:426–430. doi: 10.1016/j.medmal.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Parisien A, Allain B, Zhang J, Mandeville R, Lan CQ. 2008. Novel alternatives to antibiotics: bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J Appl Microbiol 104:1–13. doi: 10.1111/j.1365-2672.2007.03498.x. [DOI] [PubMed] [Google Scholar]
- 17.Thiel K. 2004. Old dogma, new tricks—21st century phage therapy. Nat Biotechnol 22:31–36. doi: 10.1038/nbt0104-31. [DOI] [PubMed] [Google Scholar]
- 18.Sillankorva SM, Oliveira H, Azeredo J. 2012. Bacteriophages and their role in food safety. Int J Microbiol 2012:863945. doi: 10.1155/2012/863945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer GG. 2005. Bacteriophage control of foodborne bacteria. J Food Prot 68:1102–1111. [DOI] [PubMed] [Google Scholar]
- 20.Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. 2010. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother 54:397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meitert E, Petrovici M, Sima F, Costache G, Savulian C. 1987. Investigation on the therapeutical efficiency of some adapted bacteriophages in experimental infection with Pseudomonas aeruginosa. Arch Roum Pathol Exp Microbiol 46:17–26. [PubMed] [Google Scholar]
- 22.Wang J, Hu B, Xu M, Yan Q, Liu S, Zhu X, Sun Z, Reed E, Ding L, Gong J, Li QQ, Hu J. 2006. Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa. Int J Mol Med 17:309–317. [PubMed] [Google Scholar]
- 23.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L. 2010. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis 201:1096–1104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 24.Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L. 2011. Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS One 6:e16963. doi: 10.1371/journal.pone.0016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craig A, Mai J, Cai S, Jeyaseelan S. 2009. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones AM, Martin L, Bright-Thomas RJ, Dodd ME, McDowell A, Moffitt KL, Elborn JS, Webb AK. 2003. Inflammatory markers in cystic fibrosis patients with transmissible Pseudomonas aeruginosa. Eur Respir J 22:503–506. doi: 10.1183/09031936.03.00004503. [DOI] [PubMed] [Google Scholar]
- 27.Golshahi L, Seed KD, Dennis JJ, Finlay WH. 2008. Toward modern inhalational bacteriophage therapy: nebulization of bacteriophages of Burkholderia cepacia complex. J Aerosol Med Pulm Drug Deliv 21:351–360. doi: 10.1089/jamp.2008.0701. [DOI] [PubMed] [Google Scholar]
- 28.Wright A, Hawkins CH, Anggard EE, Harper DR. 2009. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 29.Fortuna W, Miedzybrodzki R, Weber-Dabrowska B, Gorski A. 2008. Bacteriophage therapy in children: facts and prospects. Med Sci Monit 14:RA126−RA132. [PubMed] [Google Scholar]
- 30.Kutter ESA. 2004. Bacteriophages: biology and applications. CRC Press, Boca Raton, FL. [Google Scholar]
- 31.Finney M. 2001. Pulsed-field gel electrophoresis. Curr Protoc Mol Biol Chapter 2:Unit 2.5B. doi: 10.1002/0471142727.mb0205bs51. [DOI] [PubMed] [Google Scholar]
- 32.Adams MH. 1959. Bacteriophages. Interscience Publishers Inc., New York, NY. [Google Scholar]
- 33.Holloway BW. 1955. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol 13:572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- 34.Klockgether J, Munder A, Neugebauer J, Davenport CF, Stanke F, Larbig KD, Heeb S, Schöck U, Pohl TM, Wiehlmann L, Tümmler B. 2010. Genome diversity of Pseudomonas aeruginosa PAO1 laboratory strains. J Bacteriol 192:1113–1121. doi: 10.1128/JB.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles AA, Misra SS, Irwin JO. 1938. The estimation of the bactericidal power of the blood. J Hyg (Lond) 38:732–749. doi: 10.1017/S002217240001158X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry M, Lavigne R, Debarbieux L. 2013. Predicting in vivo efficacy of therapeutic bacteriophages used to treat pulmonary infections. Antimicrob Agents Chemother 57:5961–5968. doi: 10.1128/AAC.01596-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bull JJ, Gill JJ. 2014. The habits of highly effective phages: population dynamics as a framework for identifying therapeutic phages. Front Microbiol 5:618. doi: 10.3389/fmicb.2014.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morissette C, Skamene E, Gervais F. 1995. Endobronchial inflammation following Pseudomonas aeruginosa infection in resistant and susceptible strains of mice. Infect Immun 63:1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. 1995. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 151:1075–1082. doi: 10.1164/ajrccm.151.4.7697234. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, Smith A, Martin TR, Ramsey BW. 2001. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol 32:356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 41.Segel GB, Halterman MW, Lichtman MA. 2011. The paradox of the neutrophil's role in tissue injury. J Leukoc Biol 89:359–372. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konstan MW, Doring G, Heltshe SL, Lands LC, Hilliard KA, Koker P, Bhattacharya S, Staab A, Hamilton A. 2014. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J Cyst Fibros 13:148–155. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Furth R, van Zwet TL, Buisman AM, van Dissel JT. 1994. Anti-tumor necrosis factor antibodies inhibit the influx of granulocytes and monocytes into an inflammatory exudate and enhance the growth of Listeria monocytogenes in various organs. J Infect Dis 170:234–237. doi: 10.1093/infdis/170.1.234. [DOI] [PubMed] [Google Scholar]
- 44.Staugas RE, Harvey DP, Ferrante A, Nandoskar M, Allison AC. 1992. Induction of tumor necrosis factor (TNF) and interleukin-1 (IL-1) by Pseudomonas aeruginosa and exotoxin A-induced suppression of lymphoproliferation and TNF, lymphotoxin, gamma interferon, and IL-1 production in human leukocytes. Infect Immun 60:3162–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hedges S, Svensson M, Svanborg C. 1992. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun 60:1295–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. 2003. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev 14:361–368. doi: 10.1016/S1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 47.Rovai LE, Herschman HR, Smith JB. 1998. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol 64:494–502. [DOI] [PubMed] [Google Scholar]
- 48.Alemayehu D, Casey PG, McAuliffe O, Guinane CM, Martin JG, Shanahan F, Coffey A, Ross RP, Hill C. 2012. Bacteriophages φMR299-2 and φNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. mBio 3:e00029-12. doi: 10.1128/mBio.00029-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu TK, Collins JJ. 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A 104:11197–11202. doi: 10.1073/pnas.0704624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes KA, Sutherland IW, Jones MV. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144(Pt 11):3039−3047. doi: 10.1099/00221287-144-11-3039. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Hu Z. 2013. Combined treatment of Pseudomonas aeruginosa biofilms with bacteriophages and chlorine. Biotechnol Bioeng 110:286–295. doi: 10.1002/bit.24630. [DOI] [PubMed] [Google Scholar]
- 52.Couper KN, Blount DG, Riley EM. 2008. IL-10: the master regulator of immunity to infection. J Immunol 180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 53.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. 2009. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol 9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cystic Fibrosis Trust. 2013. Annual UK CF registry data report 2013, p 25–26. http://www.cysticfibrosis.org.uk/media/598466/annual-data-report-2013-jul14.pdf.
- 55.Sulakvelidze A, Alavidze Z, Morris JG Jr. 2001. Bacteriophage therapy. Antimicrob Agents Chemother 45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.