Abstract
Few oral antibiotics exist for the empirical treatment of extended-spectrum β-lactamase (ESBL) urinary tract infections (UTI). In this study, we sought to determine the activity of fosfomycin against ESBL-producing uropathogens from patients at 3 Veterans Affairs (VA) facilities between 2010 and 2013. Among the ESBL uropathogens, 19.9% were fosfomycin resistant. Klebsiella species were more likely than Escherichia coli to be resistant (46% versus 4%; P < 0.001). Fosfomycin remains active against a majority of the ESBL uropathogens, although resistance among Klebsiella spp. was higher than that in previous reports.
TEXT
The isolation of extended-spectrum-β-lactamase (ESBL)-producing uropathogens is increasing among both hospitalized patients and patients in the community (1, 2). Large national surveys of isolates demonstrate that many isolates of Escherichia coli, which cause the majority of urinary tract infections (UTI), are now resistant to most oral antibiotics, including fluoroquinolones, trimethoprim-sulfamethoxazole, and β-lactam agents (1, 3). Treatment options are limited in these situations, thus making empirical antibiotic choices more challenging for physicians.
Previous surveys have shown that fosfomycin, an oral phosphonic acid derivative that disrupts cell wall synthesis, is active against 85 to 100% of multidrug-resistant (MDR) uropathogens (4–7). The majority of these studies have focused on E. coli, and there is limited information about the likelihood of fosfomycin activity against the full spectrum of multidrug-resistant uropathogens (8). Knowledge of the rates of resistance can help optimize the use of fosfomycin and improve the accuracy of empirical therapy, as susceptibility results for this agent are not routinely available (9–11). The aim of the present study was to determine the prevalence of fosfomycin resistance among ESBL uropathogens collected from 2010 through 2013 and to describe patterns of coresistance with routinely tested antimicrobials.
(This study was presented in part at the Infectious Disease Society of America Clinical Meeting, 2 to 6 October 2013, San Francisco, CA.)
The Veterans Affairs (VA) Boston (MA) clinical laboratory processes bacterial cultures for 3 hospital campuses in the Boston area, including acute-care and long-term-care facilities, and also for 6 regional community clinics. All MDR Gram-negative uropathogens were collected from January 2010 to June 2013 and stored as part of standard laboratory policy. The presence of an ESBL was determined by screening and confirmation testing, as per standard CLSI guidelines (12). Additional standard susceptibility testing results for each isolate were available from the clinical microbiology database. The isolates were retrieved from the freezer and tested for resistance to fosfomycin. Fosfomycin use was limited during this time period, and none of the patients in the study cohort had received fosfomycin at the time of urine specimen collection.
Fosfomycin resistance was determined by use of disk diffusion and standard published breakpoints (fosfomycin resistant for zone size <16 mm for E. coli) (13). Duplicate isolates corresponding to the same pathogen from the same patient isolated within 2 weeks of each other were excluded. Resistance rates to each of the various antimicrobials were calculated, and P values were calculated using chi-square or Fisher's exact test.
A total of 204 MDR urine isolates were tested. Of these, 120 (58.8%) isolates were E. coli, 71 (34.8%) isolates were Klebsiella species, 5 (2.5%) isolates were Pseudomonas sp., and 8 (3.9%) isolates were other and included Acinetobacter, Serratia, Morganella, Citrobacter, Proteus, and Enterobacter species.
Overall resistance to fosfomycin was 21.6% (44/204). E. coli isolates had a significantly lower rate of resistance to fosfomycin (4.2% [5/120]) than that of Klebsiella species (46.4% [33/71]) (P < 0.01). The percentages of isolates that were fosfomycin resistant increased between the years of 2010 and 2013 from 17.0% (7/41) to 25.5% (13/51), but this increase was not statistically significant (P = 0.44).
The rates of fosfomycin resistance were similar when analyses were limited to the first uropathogen detected for each unique patient, for a total of 20.9% (34/163) compared to 21.6% of the total cohort. When stratified by species, fosfomycin resistance among unique patients was 3.5% for cultures with E. coli and 49.0% for cultures with Klebsiella spp., compared to 4.1% and 46.4%, respectively, for the whole cohort.
Among the 204 MDR uropathogens, 170 (83.3%) uropathogens were resistant to fluoroquinolones, and 130 (63.7%) uropathogens were resistant to trimethoprim-sulfamethoxazole. Nitrofurantoin was the most active oral agent on the routine susceptibility panel, with 36.0% resistance (53/147 isolates). Of the total 147 ESBL uropathogens with full susceptibility panels, 31 (21.1%) uropathogens were resistant to all oral agents, including fluoroquinolones, nitrofurantoin, and trimethoprim-sulfamethoxazole. Among these, 10 isolates (32%) were also resistant to fosfomycin; all of these uropathogens were Klebsiella species.
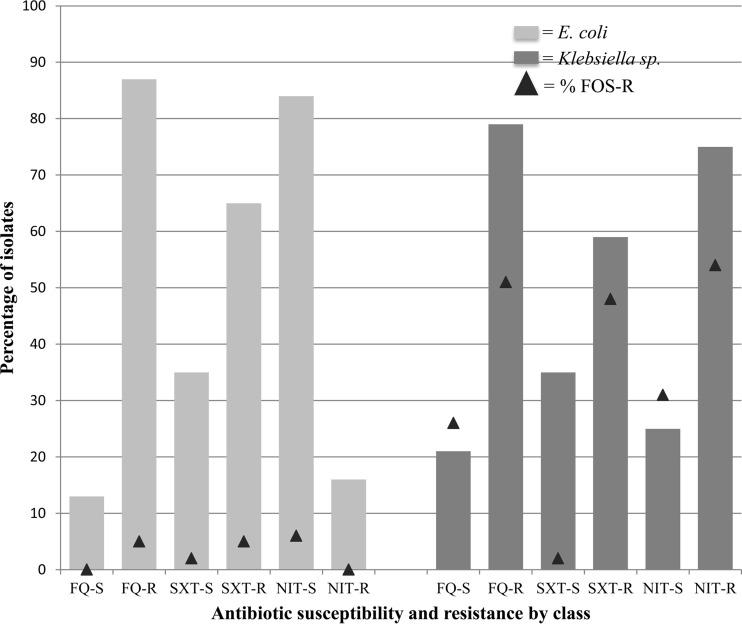
The likelihood of fosfomycin resistance among E. coli and Klebsiella uropathogens was not significantly associated with resistance to other oral agents (Fig. 1). Among fluoroquinolone- or trimethoprim-sulfamethoxazole-resistant E. coli, coresistance to fosfomycin was relatively low, at 5% for each drug. Conversely, among fluoroquinolone- or trimethoprim-sulfamethoxazole-resistant Klebsiella spp., approximately half were also resistant to fosfomycin. Fosfomycin resistance relative to nitrofurantoin activity was varied.
FIG 1.
Antibiotic coresistance patterns for ESBL uropathogens. FOS, fosfomycin; FQ, fluoroquinolones; SXT, trimethoprim-sulfamethoxazole; NIT, nitrofurantoin.
Rates of ESBL uropathogens are rising, both within health care settings and in the community. Since treatment of a UTI is usually initiated empirically, continued evaluations of the most likely active agents are critical for clinical and microbiological success. In the absence of routine testing of uropathogens to alternative agents, such as fosfomycin, knowledge of the expected activity to this agent is limited. Our study provides estimates of the activity of fosfomycin in patients with EBSL-resistant uropathogens and promotes optimal empirical therapy of these infections.
In this study of predominantly male veterans, we found an overall resistance rate of 19.9% to fosfomycin among our ESBL uropathogen collection. Previous studies have found rates of susceptibility of as high as 99.4% among E. coli (14), similar to the 96% rate of susceptibility observed in our study. However, the resistance rate of 46% among Klebsiella spp. was almost double that reported in a U.S.-based study of 95 carbapenem-resistant Enterobacteriaceae (CRE), in which 26% were resistant to fosfomycin (5). Surveys of resistance among isolates from other countries have reported rates ranging from 0 to 5% for E. coli and 7 to 25% for Klebsiella species (9, 14–18). Thus, our data suggest higher rates of resistance among Klebsiella spp. than those previously reported.
Evaluating patterns of coresistance with other routinely tested oral agents was somewhat helpful in predicting fosfomycin resistance but not statistically significant for any specific pattern. The main finding of interest was that all but 10 uropathogens were susceptible to an oral antimicrobial when fosfomycin was included. Among E. coli isolates that are susceptible to fluoroquinolones, fosfomycin susceptibility was 100%. This could be used by clinicians to avoid fluoroquinolone use for a UTI and prescribe a genitourinary-specific agent instead, in keeping with a fluoroquinolone-sparing approach advocated by guidelines for UTI treatment (3).
Our study is limited in generalizability, as the majority of isolates were from white male veterans. In addition, fosfomycin testing by disk diffusion for Klebsiella spp. is not standard clinical microbiology practice, and fosfomycin is currently not FDA approved for the treatment of Klebsiella species infections. However, previous studies have evaluated fosfomycin for Klebsiella using similar diffusion cutoff values. Our findings suggest that fosfomycin is active against the majority of multidrug-resistant uropathogens at our institution.
ACKNOWLEDGMENTS
This material is the result of work supported in part with resources and the use of facilities at the VA Boston Healthcare System, West Roxbury, Massachusetts.
We declare no conflicts of interest.
REFERENCES
- 1.Li B, Sun JY, Liu QZ, Han LZ, Huang XH, Ni YX. 2011. High prevalence of CTX-M β-lactamases in faecal Escherichia coli strains from healthy humans in Fuzhou, China. Scand J Infect Dis 43:170–174. doi: 10.3109/00365548.2010.538856. [DOI] [PubMed] [Google Scholar]
- 2.Gibold L, Robin F, Tan RN, Delmas J, Bonnet R. 2014. Four-year epidemiological study of extended-spectrum β-lactamase-producing Enterobacteriaceae in a French teaching hospital. Clin Microbiol Infect 20:O20–O26. doi: 10.1111/1469-0691.12321. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Trautner B. 2012. In the clinic. Urinary tract infection. Ann Intern Med 156:ITC3-1–ITC3-15. [DOI] [PubMed] [Google Scholar]
- 4.Bonkat G, Muller G, Braissant O, Frei R, Tschudin-Suter S, Rieken M, Wyler S, Gasser TC, Bachmann A, Widmer AF. 2013. Increasing prevalence of ciprofloxacin resistance in extended-spectrum-β-lactamase-producing Escherichia coli urinary isolates. World J Urol 31:1427–1432. doi: 10.1007/s00345-013-1031-5. [DOI] [PubMed] [Google Scholar]
- 5.Pogue JM, Marchaim D, Abreu-Lanfranco O, Sunkara B, Mynatt RP, Zhao JJ, Bheemreddy S, Hayakawa K, Martin ET, Dhar S, Kaye KS, Lephart PR. 2013. Fosfomycin activity versus carbapenem-resistant Enterobacteriaceae and vancomycin-resistant Enterococcus, Detroit, 2008–10. J Antibiot (Tokyo) 66:625–627. doi: 10.1038/ja.2013.56. [DOI] [PubMed] [Google Scholar]
- 6.Neuner EA, Sekeres J, Hall GS, van Duin D. 2012. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 56:5744–5748. doi: 10.1128/AAC.00402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier S, Weber R, Zbinden R, Ruef C, Hasse B. 2011. Extended-spectrum β-lactamase-producing Gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection 39:333–340. doi: 10.1007/s15010-011-0132-6. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Avial C, Rodriguez-Avial I, Hernandez E, Picazo JJ. 2013. Increasing prevalence of fosfomycin resistance in extended-spectrum-beta-lactamase-producing Escherichia coli urinary isolates (2005–2009–2011). Rev Esp Quimioter 26:43–46. (In Spanish.) [PubMed] [Google Scholar]
- 9.Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, Kapaskelis A, Nikita D, Michalopoulos A. 2010. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents 35:194–199. doi: 10.1016/j.ijantimicag.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 11.Cai Y, Wang R, Liang B, Bai N, Liu Y. 2011. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 55:1162–1172. doi: 10.1128/AAC.01402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. CLSI document M100-19 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.de Cueto M, Lopez L, Hernandez JR, Morillo C, Pascual A. 2006. In vitro activity of fosfomycin against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: comparison of susceptibility testing procedures. Antimicrob Agents Chemother 50:368–370. doi: 10.1128/AAC.50.1.368-370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waiwarawooth J, Jutiworakul K, Joraka W. 2006. The prevalence and susceptibility patterns of ESBL-producing Klebsiella pneumonia and Escherichia coli in Chonburi Hospital. J Infect Dis Antimicrob Agents 23:57–65. [Google Scholar]
- 16.Abdullah FEMA, Ishrad M, Rauf H, Afzal N, Rasheed A. 2013. Current efficacy of antibiotics against Klebsiella isolates from urine samples–a multi-centric experience in Karachi. Pak J Pharm Sci 26:11–15. [PubMed] [Google Scholar]
- 17.Tharavichitkul P, Khantawa B, Bousoung V, Boonchoo M. 2005. Activity of fosfomycin against extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli in Maharaj Nakorn Chiang Mai Hospital. J Infect Dis Antimicrob Agents 22:121–126. [Google Scholar]
- 18.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]