Abstract
Enterovirus 71 (EV71) (Picornaviridae family) and hepatitis C virus (HCV) (Flaviviridae family) are the causative agents of human hand, foot, and mouth disease (HFMD) and hepatitis C, resulting in a severe pandemic involving millions of infections in the Asia-Pacific region and worldwide. The great impact of EV71 and HCV on public health highlights the need to further our understanding of the biology of these two viruses and develop effective therapeutic antivirals. Here, we evaluated a total of 32 lycorine derivatives and demonstrated that 1-acetyllycorine suppressed the proliferation of multiple strains of EV71 in various cells. The results of the drug resistance analysis revealed that 1-acetyllycorine targeted a phenylalanine (F76) in EV71 2A protease (2Apro) to stabilize the conformation of a unique zinc finger. Most interestingly, the zinc binding site in EV71 2Apro is the exclusive homolog of HCV NS3 among all viruses. Further analysis revealed that 1-acetyllycorine also inhibits HCV with high efficacy, and the mutation on R118 in HCV NS3, which corresponds to F76 in EV71 2Apro, confers the resistance of HCV to 1-acetyllycorine. These results revealed a conserved mechanism of 1-acetyllycorine against EV71 and HCV through targeting viral proteases. We also documented the significant synergistic anti-EV71 and anti-HCV effects of 1-acetyllycorine with reported inhibitors, supporting potential combination therapy for the treatment of EV71 and HCV infections.
INTRODUCTION
Enterovirus 71 (EV71) is one of the major etiological agents of human hand, foot, and mouth disease (HFMD) in the Asia-Pacific region. Particularly, young children and immunodeficient populations are more susceptible to EV71 infection. EV71 infection results in severe aseptic meningitis, encephalitis, myocarditis, acute flaccid paralysis, and pulmonary edema, which lead to high fatality rates (1, 2). Chronic infection with hepatitis C virus (HCV) affects 180 million people worldwide, and 350,000 people die each year due to HCV-related complications (3). Hepatitis C not only affects the liver function but also induces liver fibrosis and cirrhosis, eventually leading to liver cancer (4).
Both EV71 and HCV are positive-sense single-stranded RNA (+ssRNA) viruses. EV71 is a member of the Enterovirus genus within the Picornaviridae family (5–7). The genome of EV71 encodes a polyprotein that is cleaved through viral proteases to generate four structural proteins (VP1 to VP4) required for viral capsid formation and seven nonstructural proteins (2Apro, 2B, 2C, 3A, 3B, 3Cpro, and 3Dpol) for viral replication (8–10). HCV is a member of the Hepacivirus genus in the Flaviviridae family. The translated polyprotein of HCV is further processed into the structural proteins, including core protein and envelope glycoproteins E1 and E2; the nonstructural proteins are processed into NS2, NS3, NS4A, NS4B, NS5A, and NS5B (11).
The successful replication of most viruses depends on the correct proteolytic processing of polyproteins. EV71 employs two viral proteases: 2Apro mediates the initial cleavage of its own N terminus and the C terminus of VP1, thereby distinguishing the structural protein precursor from the nonstructural portions, and 3Cpro accomplishes the subsequent proteolytic processing of the polyprotein (12, 13). HCV also produces two viral proteases for viral proliferation, in which NS2 is autoreleased from NS3, while NS3-NS4A is released from other individual proteins to obtain full activity. Therefore, the effective impairment of the activities of the proteases of EV71 and HCV could be a promising strategy to inhibit viral propagation. The current efforts to develop anti-EV71 agents targeting viral proteases have generated a few successes, including rupintrivir (AG7088) and NK-1.8k (targeting EV71 protease 3Cpro) (2, 14–16) and the peptide LVLQTM (targeting 2Apro) (17). Despite the increasing amount of research aimed at identifying antiviral agents against EV71, no direct-acting anti-EV71 drugs are currently available in the clinic to combat EV71 infections (7, 18, 19). The inhibitors of HCV NS3 protease have shown much progress in clinical usage. For example, HCV NS3 protease inhibitors, such as boceprevir, telaprevir, and simeprevir, were approved through the FDA for use in combination with alpha interferon (IFN-α)/ribavirin (RBV) for genotype 1 treatment (20–22).
Lycorine, an alkaloid isolated from Amaryllidaceae plants, shows diverse biological properties, including anticancer, antiplasmodial, antitrypanosomal, anti-inflammatory, analgesic, and emetic properties (23–26). Lycorine and lycorine derivatives also inhibit several virus species, including poliovirus, severe acute respiratory syndrome-associated coronavirus, herpes simplex virus 1, vaccinia virus, and bunyaviruses (27–31). Particularly, lycorine presents potent inhibitory activities against flaviviruses, including West Nile virus (WNV), dengue virus (DENV), and yellow fever virus (YFV) (32, 33). Recent results have also revealed that lycorine inhibits EV71 in an animal model and lycorine-derived phenanthridine inhibits HCV (34, 35). However, the inhibitory mechanisms of lycorine and lycorine derivatives against various viruses remain unclear. Therefore, in the present study, we conducted a systematic analysis of the inhibition by lycorine derivatives of EV71 and HCV to identify variations or conserved lycorine inhibition against various viruses.
MATERIALS AND METHODS
Viruses, cell lines, and antibody.
Human rhabdomyosarcoma (RD) cells, African green monkey kidney E6 (Vero E6) cells, and human embryonic kidney 293T cells were purchased from the American Type Culture Collection (ATCC). The Huh7.5.1 cell line was gifted by J. Zhong from the Institute Pasteur of Shanghai, China. All these cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) at 37°C in a humidified incubator with 5% CO2.
The plasmids containing a green fluorescent protein (GFP) reporter gene inserted into the EV71 strain SK-EV006 (EV71-GFP) were donated by S. Koike (Tokyo Metropolitan Institute of Medical Science) for the initial phenotype screening. A single-round reporter system, the EV71(FY)-Luc pseudotype virus system, with pseudotype EV71 containing plasmids pcDNA6-FY-capsid and pEV71-Luc-replicon lacking the P1 region, was kindly supplied by W. Li from the National Institute of Biological Sciences, China. Human EV71 strains AnHui1 (GenBank accession number GQ994988.1) and BrCr (GenBank accession number U22521) were kindly provided by B. Zhang and H. Wang from the Wuhan Institute of Virology, China.
Antibodies used for Western blot (WB) analysis were purchased from Abcam (mouse monoclonal antibody [MAb] to enterovirus 71 VP1; 10F0) and ProMab (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]).
Lycorine derivatives and reagents.
Lycorine derivatives were synthesized by our group (Table 1) (33, 36). Stock solutions at a concentration of 10 mM were prepared in distilled water with dimethyl sulfoxide (DMSO) and diluted in DMEM with 10% FBS to different concentrations before use.
TABLE 1.
Anti-EV71 effect of testing lycorine derivatives based on visible cytopathic effecta

Abbreviations: Compd, compound; CPE50, 50% CPE.
TRIzol reagent and a SuperScript III first-strand synthesis system for reverse transcription-PCR (RT-PCR) were purchased from Invitrogen. A MEGAscript transcription kit was purchased from Promega. A QuantiTect SYBR green RT-PCR kit was purchased from Qiagen. A cell viability and proliferation assay (WST-1) was purchased from Roche.
Virus titration.
For EV71-GFP virus, virus titers were determined by measuring the expression level of GFP in RD cells (15, 37). The measurement was performed by seeding 3 × 104 RD cells per well in 96-well microtiter plates. After overnight culture, EV71-GFP virus was serially diluted 10-fold with DMEM containing 10% FBS (10−1- to 10−8-fold dilutions) and added to RD cells. The plates were then incubated at 37°C in 5% CO2. The GFP expression level was monitored using an epifluorescence microscope after 2 days.
For living virus, virus titers were determined by endpoint dilution assays (EPDAs) (38, 39). Briefly, 3 × 104 RD cells were seeded per well in 96-well microtiter plates. After overnight culture, virus was serially diluted 10-fold with DMEM containing 10% FBS (10−1- to 10−8-fold dilutions) and added to the RD cells. The plates were then incubated at 37°C with 5% CO2. Virus titers were determined after 3 to 4 days by observing cytopathic effect (CPE) under the microscope. The virus titer, expressed as the 50% tissue culture infective dose (TCID50), was determined using the Reed and Muench calculator.
EV71 pseudotype virus.
The EV71 pseudotype virus [EV71(FY)-Luc] was produced to exclude virus reinfection by using the method developed as previously described (40). Briefly, the plasmids pEV71-Luc subgenomic replicon and pcDNA6-FY-capsid were extracted using the HighPure maxiplasmid kit (Tiangen). The pEV71-Luc replicon DNA was linearized by digestion with SalI restriction enzyme and was used as a template for RNA transcription. The EV71 replicon RNA transcripts were prepared in vitro using Promega MEGAscript kits. The pcDNA6-FY-capsid plasmid was transfected into 293T cells at 60 to 80% confluence. After 24 h posttransfection (hpt), EV71 subgenomic replicon RNA was then transfected using Lipofectamine 2000 (Invitrogen). EV71(FY)-Luc virus in the culture was harvested at 24 hpt. To quantify the EV71 pseudotype virus, the stocks were serially diluted 10-fold and incubated with RD cells for 24 h at 37°C. The luminescence was detected as instructed in the manufacturer's protocol for the Bright-Glo luciferase assay system (Promega). For the inhibition assay, the final concentration of EV71(FY)-Luc was diluted to give 1,000,000 relative luminescence units (RLU) per well in a 96-well plate.
Phenotype screening.
RD cells were seeded at 3 × 104 cells per well in 96-well microtiter plates. After overnight culture at 37°C in 5% CO2, RD cells were treated with a gradient of concentrations of different compounds ranging from 0.15 to 40 μM. After 2 h, RD cells were infected with EV71-GFP at a multiplicity of infection (MOI) of 1. At 24 h postinfection (hpi), the level of GFP expression was monitored using an epifluorescence microscope.
Western blot analysis.
RD cells were seeded at 6 × 104 cells per well in 6-well microtiter plates and treated with 1-acetyllycorine at concentrations ranging from 1.25 to 10 μM. RD cells were lysed in a buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, 1 mM NaVO4, 10 mM NaF, and protease inhibitors after 24 hpi, and the protein concentration in the lysates was determined with a spectrophotometer. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane (Millipore). These membranes were blocked with a 5% nonfat dry milk solution in Tris-buffered saline for 4 h, incubated with specific primary antibodies (catalog number ab36367; Abcam), and then subsequently incubated with secondary antibodies conjugated with horseradish peroxidase. Proteins were visualized by chemiluminescence using Clarity Western ECL substrate (Bio-Rad, USA).
Cytotoxicity.
The cytotoxicity was measured by WST-1 cell proliferation using a cytotoxicity assay kit according to the manufacturer's protocol (catalog no. 05015944001; Roche). Briefly, Vero, 293T, and RD cells (3 × 104 per well in 100 μl 10% FBS-DMEM) were cultured at 37°C under 5% CO2 in 96-well plates, followed by the addition of 50 μl compound solution with a concentration ranging from 5 to 100 μM. The cells were incubated at 37°C for 48 h, and then 10 μl WST-1 reagent was added to each well. The luminescent signals were read at 490 nm with a microplate reader (Bio-Rad, USA). The percentage of viable cells after treatment with different concentrations of compound was calculated as follows: percentage of inhibition = 100 × optical density at 490 nm (OD490) of treated sample/OD490 of cell control sample.
qRT-PCR.
The reduction of viral genome after treatment with compound in host cells was determined using EV71 and RD cells in a quantitative RT-PCR (qRT-PCR)-based assay according to a standard protocol. Briefly, 1.5 × 105 RD cells were seeded in each well of 24-well tissue culture plates and were allowed to attach overnight in complete culture medium. The medium was then replaced with medium containing EV71 Fuyang virus at an MOI of 1 and serially diluted compounds in the presence of 10% FBS and 0.5% DMSO. After the cells were treated for 24 h, total cellular RNA was isolated by TRIzol reagent using a standard protocol. A quantitative RT-PCR assay (primer sequences, GAPDH, forward primer 5′-CCCACTCCTCCACCTTTGACG-3′ and reverse primer 5′-CACCACCCTGTTGCTGTAGCCA-3′; EV71 5′ untranscribed region [UTR] forward primer 5′-TGAATGCGGCTAATCCCAACT-3′ and reverse primer 5′-AAGAAACACGGACACCCAAAG-3′) was performed using a QuantiTect SYBR green RT-PCR kit (Qiagen, CA) according to the manufacturer's protocol. EV71 and GAPDH transcript levels were determined by the threshold cycle (ΔΔCT) method (41). The percentage of inhibition was calculated as follows: percentage of inhibition = [1 − (average of compound-treated cells)/(average of control cells)] × 100. Each data point represents the average from three replicates in cell culture.
Time-of-addition assay.
The time-of-addition effect was examined for 1-acetyllycorine, NITD008, and ALD (15). RD cells (3 × 104 cells per well in 100 μl of 10% FBS-DMEM) were cultured overnight at 37°C under 5% CO2 in 96-well plates. Subsequently, the cells were treated with 2 μM 1-acetyllycorine, 2 μM NITD008, and 1 pM ALD either concurrently with 100 TCID50 of EV71(FY)-Luc or at intervals of −6, −4, −2, 0, 2, 4, 6, 8, and 10 hpi. RD cells in another 96-well plate were treated with 2 μM 1-acetyllycorine, 2 μM NITD008, and 1 pM ALD and transfected with EV71(FY)-Luc replicon RNA after 4 h. After incubation at 37°C for 24 h, antiviral activity was determined by measurement of the reduction of luciferase activity.
Inhibitory effect on different cells.
In order to eliminate the differences between cells, the 50% effective concentrations (EC50s) for RD, Vero, and 293T cells were determined. RD (1.5 × 105 per well), Vero (1 × 105 per well), and 293T (1.5 × 105 per well) cells were seeded in 24-well plates and cultured at 37°C in 5% CO2 overnight. Cells were treated with a gradient of dilutions of 1-acetyllycorine ranging in concentration from 0.02 to 10 μM. EV71 (strain Fuyang) was added to 24-well plates at an MOI of 1. The RNA was detected after 24 hpi, and the EC50s on different cells were calculated by the use of GraphPad Prism software.
Selection of drug-resistant virus.
RD cells were seeded at 6 × 105 cells per well in 6-well plates. The following day, the medium was removed and replaced with DMEM containing 10% FBS and 4 μM 1-acetyllycorine, while 0.5% DMSO was used as a control. After 2 h, EV71 (strain Fuyang) was used to infect RD, Vero, and 293T cells at an MOI of 0.1 in complete medium. The initial high concentration of inhibitor, around 10-fold of the best EC50, ensured the quick emergence of resistant virus. After 3 days, the virus (termed passage 1 virus [P1]) was harvested when RD cells reached 70 to 90% confluence. A fresh 6-well plate was prepared and replaced with 3 μM 1-acetyllycorine. RD cells were infected with P1 EV71 at an MOI of 0.1, and virus of passage 2 was harvested. After two rounds of passage 2, the concentration of 1-acetyllycorine was decreased to 2 μM for another two rounds of passage. These four rounds of passage at concentrations of 3 μM and 2 μM helped the resistant virus to propagate in reasonable titers. Further, the concentration of inhibitor was increased to 4 μM for five rounds of passage to remove any remaining nonresistant virus.
Identification of drug resistance-conferring mutations.
For analysis of EV71 resistance-conferring mutations, cellular RNA was extracted after nine rounds of culture with the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. For reverse transcription-PCR, first-strand cDNA was synthesized using a gene-specific primer (5′-ACCCCCACCAGTCACATTCACG-3′) and the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen) according to the manufacturer's instructions. The protein-coding region of the EV71 genome was amplified by PCR.
The RT-PCR products of resistant EV71 or control EV71 were ligated into the pEASY-Blunt simple cloning vector (Transgen). Multiple individual bacterial colonies were isolated, and the purified plasmid DNA was sequenced and analyzed by using GeneRunner software.
Sensitivity of EV71 WT and F76L mutant virus to 1-acetyllycorine.
The recombinant full-length plasmid of EV71 (strain Fuyang) with a T7 promoter, a HindIII site before the 5′ UTR, and an XbaI site in the poly(A) sequence after the 3′ end was ligated into pcDNA3.1. The F76L amino acid substitution was mutated by a Fast mutagenesis system kit (Transgen) with forward primer 5′-CACTACCCAGTCAGTTTATCAAAACCTAGCCTGATC-3′ and reverse primer 5′-TAAACTGACTGGGTAGTGTTTTCTCCTCGAGTTACAGTA-3′. The full-length RNAs of wild-type (WT) EV71 and F76L mutant virus were transcribed from recombinant plasmid linearized with XbaI in vitro using a T7 RiboMAX large-scale RNA production kit (Promega). RD cells were electroporated with 10 μg of WT or mutant genome RNA. The WT and mutant viruses were harvested at 24 h posttransfection. RD cells were seeded in two 96-well plates and cultured at 37°C for 24 h. The WT and mutant virus titers were determined by EPDA as described above. For testing the inhibitory activity of NK-1.8k between the WT and F76L virus, 500 μl of RD cells was seeded in two 24-well plates and incubated for 24 h. Cells were treated with serial dilutions of 1-acetyllycorine and infected with WT or F76L virus. After 24 hpi, RNAs were extracted and quantified by qRT-PCR.
HCV and inhibition assay.
The HCV assay was performed using a previously described method with some modifications (42). A humanized Renilla luciferase reporter gene was introduced into the C terminus of the plasmid pJFH-1 (a gift from Apath, L.L.C.) between amino acids 399 and 400 of NS5A in the JFH-1 genome. The resulting plasmid phRluc-JFH-1 was digested with XbaI and used as a template for RNA transcription. The transcripts were prepared in vitro using the Ambion MEGAscript kit; subsequently, 10 μg of RNA was mixed with 400 μl Huh7.5.1 cells (1 × 107 cells/ml). Following electroporation, Huh7.5.1 cells containing virus transcripts were seeded onto a 10-cm dish. After the cells were cultured for 10 days and passaged every 3 days, the supernatant was collected and filtered to obtain the HCV(JFH-1)-Rluc virus stock solution.
The antiviral activity and cytotoxicity of 1-acetyllycorine were determined using HCV(JFH-1)-Rluc-infected Huh7.5.1 cells. A total of 1.5 × 105 Huh7.5.1 cells were seeded onto 96-well tissue culture plates and grown in DMEM supplemented with 10% FBS at 37°C in a 5% CO2 humidified incubator. WT or R118L-mutated HCV(JFH-1)-Rluc viruses were subsequently used to infect Huh7.5.1 cells in the presence of serially diluted 1-acetyllycorine. After 48 h, the luciferase activity in the cells was determined using the Renilla-Glo luciferase reagent (Promega) and a Glomax plate reader (Promega).
Drug-drug interactions of 1-acetyllycorine with other inhibitors of EV71 and HCV replication.
For EV71, RD cells were seeded at 3 × 104 cells per well onto 96-well microtiter plates. After culturing overnight at 37°C, a different final concentration of 1-acetyllycorine was added to each row of the 96-well plate. Simultaneously, a different final concentration of NITD008 was added to each column of the plate. The RD cells were infected with EV71(FY)-Luc virus after treatment with the two inhibitors and incubated for 24 h. The combination of 1-acetyllycorine and ALD was examined using the same method, with the adjustment of the ALD concentrations to 0.0000, 0.0005, 0.0019, 0.0078, 0.0312, 0.1250, 0.50000, and 2.0000 μM. The antiviral activities were determined after measuring the reduction of luciferase activity in the cells.
Huh7.5.1 cells were seeded at 3 × 104 per well onto 96-well microtiter plates. After culturing overnight at 37°C, a different final concentration of 1-acetyllycorine was added to each row of the 96-well plate. Simultaneously, a different final concentration of sofosbuvir was added to each column of the plate. Huh7.5.1 cells were infected with the HCV(JFH-1)-Rluc virus line after treatment with the two inhibitors and incubation for 48 h. The combination of 1-acetyllycorine and ribavirin (RBV) was examined using the same method with RBV concentrations adjusted to 0.00000, 0.78125, 1.56250, 3.12500, 6.25000, 12.50000, 25.0000, and 50.0000 μM. Antiviral activities were determined after measuring the reduction of luciferase activity in the cells.
To determine the drug-drug interactions, differential surface plots at the 95% confidence level (CI) were generated using MacSynergy II according to the Bliss independence model (43). The combinatorial effect was determined after subtracting theoretical additive values from the experimental values (43, 44). In the three-dimensional differential surface plot, the peaks above or below a theoretical additive plane represent synergy or antagonism, respectively (43, 44). The data sets were interpreted using the following scale: volumes of synergy or antagonism at values of <25 mm2% were insignificant, those at 25 to 50 mm2% were minor but significant, those at 50 to 100 mm2% were moderate and likely important in vivo, and those at >100 mm2% are strong and likely important in vivo.
RESULTS
The lycorine derivative inhibits EV71 proliferation.
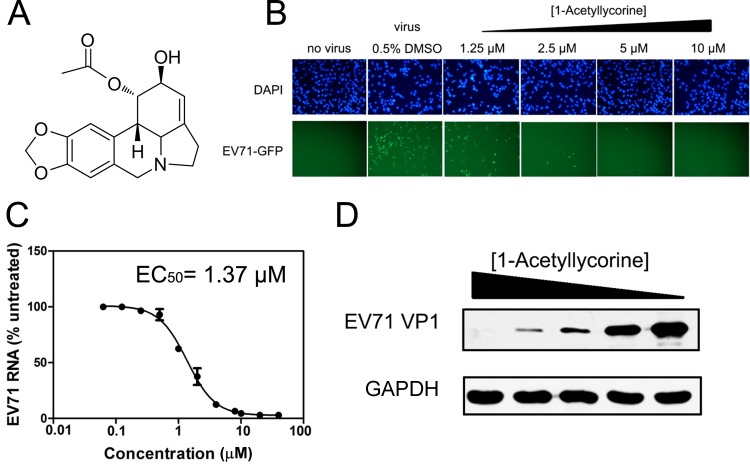
The initial aim of the resent study was to verify the inhibitory effect of lycorine derivatives on enterovirus. Rapid phenotype screening was performed using a total of 26 previously synthesized lycorine derivatives (33, 36) to assay the inhibitory activity of these molecules against EV71-GFP (Table 1). Most of the tested compounds did not exhibit an anti-EV71 effect at a concentration of 40 μM, with two significant exceptions: treatment with compounds 18 (1-acetyllycorine) (Fig. 1A) and 25 obviously reduced GFP expression at a concentration of 2.5 μM. Compound 25 displayed clear cytotoxicity at 20 μM, but the cytotoxicity of 1-acetyllycorine was not observed until 100 μM.
FIG 1.
1-Acetyllycorine suppressed EV71 proliferation. (A) Structural conformation of 1-acetyllycorine. (B) Concentration-dependent reduction of EV71 proliferation following treatment with 1-acetyllycorine. RD cells were infected with EV71-GFP at an MOI of 1, and the level of GFP expression was monitored using an epifluorescence microscope at 24 hpi (bottom panel). DAPI was used to visualize the nuclear staining (top panel) in the host cells. (C) Inhibitory effect of 1-acetyllycorine on EV71 RNA synthesis measured through qRT-PCR. The results of each point were presented as the average results from three independent experiments, and the error bars represent the standard errors of the means (n = 3). (D) Dose-dependent reduction of EV71 VP1 expression. The concentration of 1-acetyllycorine in lanes 1 to 5 was 10, 5, 2.5, 1.25, and 0 μM, respectively (top panel). The expression level of GAPDH did not vary (bottom panel).
Moreover, 1-acetyllycorine inhibited EV71-GFP in a concentration-dependent manner (Fig. 1B). The anti-EV71 activity of 1-acetyllycorine was further examined after infection of RD cells with WT EV71 (strain SK-EV006). The results of qRT-PCR measurement revealed that 1-acetyllycorine reduced EV71 RNA in the infected cells in a concentration-dependent manner, with an EC50 of 1.37 μM (Fig. 1C). Furthermore, the expression level of VP1 protein in EV71 capsid, but not glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a host housekeeping gene, in the infected cells, was diminished after treatment with 1-acetyllycorine (Fig. 1D). These results revealed a specific inhibitory effect of 1-acetyllycorine against EV71.
1-Acetyllycorine suppressed the propagation of multiple EV71 strains.
Because enteroviruses from different genotypes share high conservation, we speculated that 1-acetyllycorine might exhibit inhibition on multiple EV71 strains. To verify this hypothesis, RD cells were infected with three different virus strains, including strains BrCr (genotype A), SK-EV006 (genotype B), and Fuyang (genotype C), in the presence of 1-acetyllycorine, and the remaining levels of EV71 RNA inside host cells were measured using the qRT-PCR method.
The results showed that 1-acetyllycorine inhibited the viruses of strains BrCr, SK-EV006, and Fuyang with EC50s of 0.50, 1.13, and 0.47 μM, respectively, suggesting that 1-acetyllycorine indeed exhibits similar inhibition through a conserved mechanism of different EV71 strains.
Quantification of the anti-EV71 effect of 1-acetyllycorine.
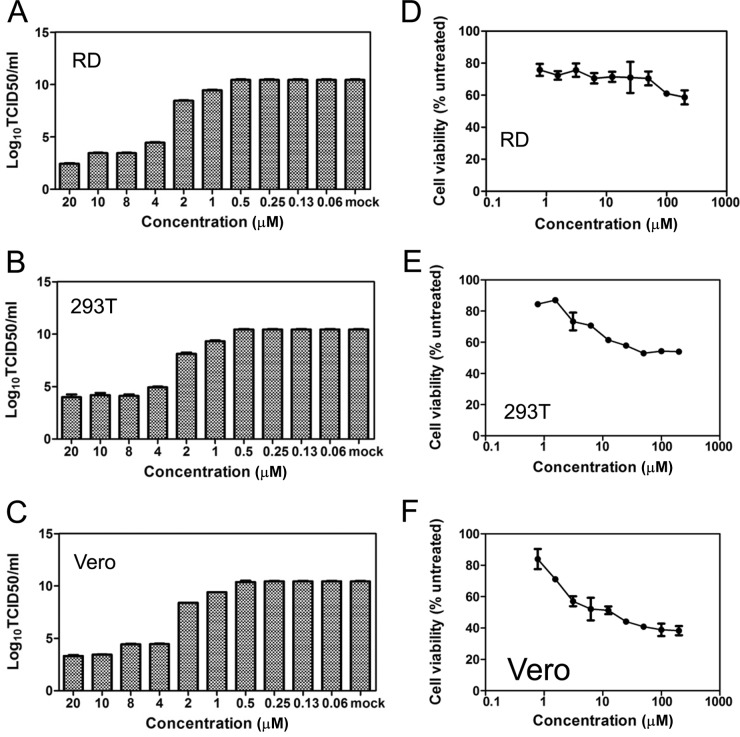
To further quantify the anti-EV71 activity of 1-acetyllycorine, the inhibition of 1-acetyllycorine was quantified in RD, 293T, and Vero cells infected with WT EV71 (strain Fuyang) (Fig. 2). The EC50s for 1-acetyllycorine inhibition of WT EV71 (strain Fuyang) infection in RD, 293T, and Vero cells were 0.45, 1.83, and 0.47 μM, respectively.
FIG 2.
Quantification of the anti-EV71 effect of 1-acetyllycorine in different cell lines. (A to C) RD, 293T, and Vero cells were treated with 1-acetyllycorine and infected with EV71 (strain Fuyang). EV71 RNA levels were measured using qRT-PCR at 24 hpi. The virus titers in the supernatants were determined at 72 hpi. (D to F) Cytotoxicity in different cell lines. RD, 293T, and Vero cells were incubated with 1-acetyllycorine for 24 h, and the cell viability was measured using a WST-1-based assay. The results of each point are presented as the average results from three independent experiments, and the error bars represent the standard errors of the means (n = 3).
We also measured the reduction of virus titers after treatment with 1-acetyllycorine in EV71-infected RD, 293T, and Vero cells. Consistently, the results indicated that the virus titers in the supernatants were also attenuated after treatment with 1-acetyllycorine (Fig. 2A, B, and C).
Cytotoxicity of 1-acetyllycorine was also tested in RD, 293T, and Vero cells. The results revealed 50% cytotoxic concentration (CC50) values of more than 200 μM for 1-acetyllycorine on each cell line, though cytotoxicity was clearly seen at the concentration of 50 μM (Fig. 2D, E, and F). Taken together, these results indicate that the anti-EV71 activity of 1-acetyllycorine showed a high to satisfactory selectivity index (SI = CC50/EC50) value (up to 450 in RD cells).
1-Acetyllycorine downregulated the replication stage of EV71.
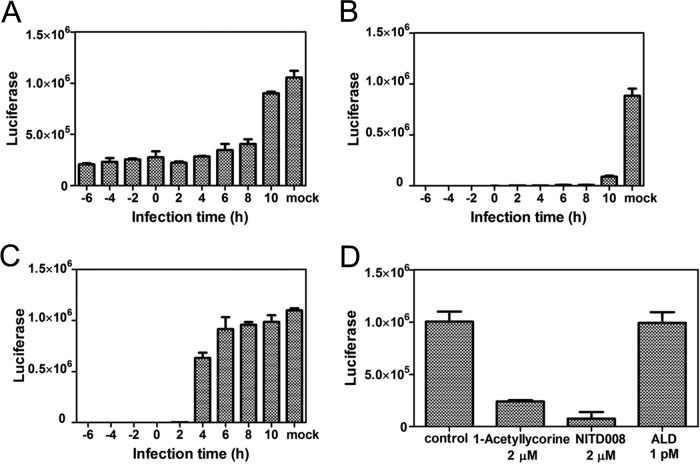
To determine which step of the EV71 life cycle was affected after treatment with 1-acetyllycorine, we infected RD cells with an EV71(FY)-Luc pseudotype virus, excluding the influence of virus reinfection, and subsequently treated the cells with 1-acetyllycorine, NITD008 (a nucleoside analog viral replication inhibitor), and ALD (an entry inhibitor) at different times. The results showed that treatment with 1-acetyllycorine at −6 hpi to 10 hpi displayed the strongest antiviral effects (Fig. 3A), consistent with the inhibition effect of the polymerase inhibitor NITD008 (Fig. 3B). In contrast, the antiviral effect of ALD was dramatically decreased from 2 hpi (Fig. 3C).
FIG 3.
Inhibition by 1-acetyllycorine, NITD008, and ALD of EV71 with different addition times. (A to C) Inhibition of EV71(FY)-Luc pseudotype virus infection of RD cells through addition of 1-acetyllycorine (2 μM) (A), NITD008 (2 μM) (B), and ALD (1 pM) (C) at various times. (D) EV71 subgenomic replicon RNA lacking the P1 region was transfected into RD cells with or without treatment with 1-acetyllycorine (2 μM), NITD008 (2 μM), and ALD (1 pM). The luciferase levels were quantified at 24 hpi. The results of each point are presented as the average results from three independent experiments, and the error bars represent the standard errors of the means (n = 3).
Next, we transfected EV71 subgenomic replicon RNA lacking the P1 region into RD cells treated with or without 1-acetyllycorine, NITD008, and ALD. The results showed that 1-acetyllycorine inhibited EV71 replication similarly to NITD008 but distinctly different from ALD (Fig. 3D). The EC50s of 1-acetyllycorine and NITD008 against EV71 replicon were further determined as 0.62 μM and 0.21 μM, respectively, but ALD did not present obvious inhibition. Because this transfection excluded viral entry, these results indicated that 1-acetyllycorine inhibited EV71 proliferation through the downregulation of virus replication.
Drug resistance to 1-acetyllycorine was mapped to EV71 2Apro.
The viruses resistant to 1-acetyllycorine were selected through the continuous culturing of WT EV71 for 10 rounds in the presence of a gradually changed concentration of 1-acetyllycorine. Three independent selections (SelA, SelB, and SelC) were performed to examine the reproducibility of the observed resistance.
Sequence analyses of the entire genome of multiresistant viruses revealed an identical T-to-A mutation at nucleotide position 3556 of the whole EV71 genome compared with three WT viruses. This mutation translated into the single-amino-acid replacement of a phenylalanine residue with a leucine residue at position 76 within the 2Apro polypeptide (residue numbers correspond to the residues in the sequence of the 2Apro protein). The EC50s of 1-acetyllycorine against the three resistant selections SelA, SelB, and SelC are 8.10, 6.22, and 7.98 μM, respectively.
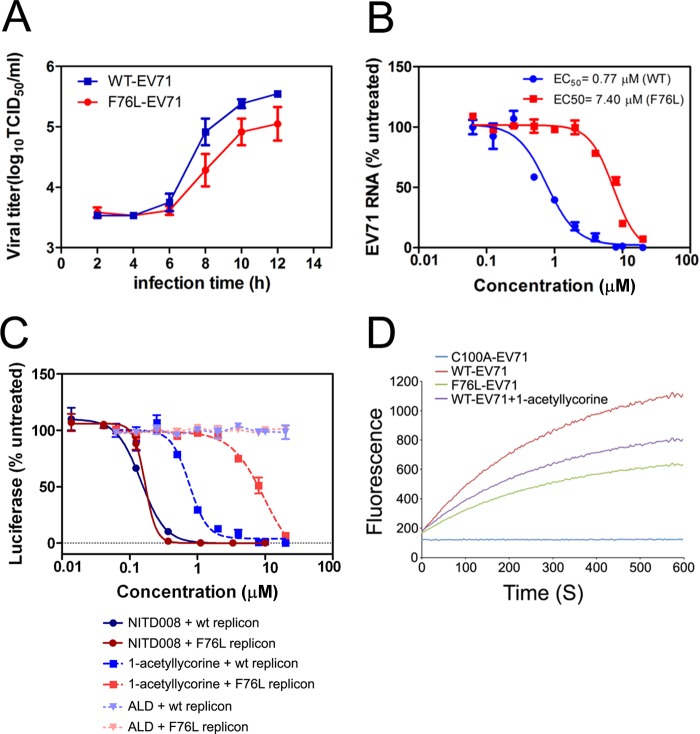
To further validate the contribution of the F76L substitution to the fitness and drug resistance of EV71, we engineered a mutated virus harboring an F76L mutation in 2Apro (EV71-F76L). We first examined the effect of this mutation on viral replication in the absence of 1-acetyllycorine. The results revealed an apparent decrease in the titer of the F76L-mutated virus compared with the WT virus, suggesting that the fitness of the F76L mutated virus is inferior to that of the WT virus (Fig. 4A). Moreover, because we measured the virus titers within 12 hpi to exclude the impact of virus reinfection, the growth curve also indicated that F76L affects the virus replication cycle from the early stage.
FIG 4.
Drug sensitivity of 1-acetyllycorine on EV71 WT and F76L virus. (A) Growth curves of EV71 WT and F76L viruses. (B) EC50s of 1-acetyllycorine against EV71 WT and F76L viruses were measured in parallel using qRT-PCR. (C) EC50s of 1-acetyllycorine, NITD008, and ALD against EV71 WT and F76L subgenomic replicons were measured in parallel by measuring luciferase activity. Each point represents the average results from three independent experiments, and the error bars represent the standard errors of the means (n = 3). (D) Enzymatic activities of EV71 WT 2Apro with or without 1-acetyllycorine, F76L, and C100A-mutated 2Apro. Each reaction was performed by using 25 μM protein and 20 μM substrate.
Moreover, a parallel comparison showed that 1-acetyllycorine inhibited WT EV71 with an EC50 of 0.77 μM but with a significantly increased EC50 of 7.4 μM (10-fold higher than that on WT EV71) against EV71-F76L, suggesting that the F76L mutation on EV71 2Apro conferred resistance to 1-acetyllycorine (Fig. 4B). Furthermore, 1-acetyllycorine inhibited WT and F76L EV71 subgenomic replicon activities with EC50s of 0.62 μM and 8.13 μM, respectively. Meanwhile, NITD008 inhibited WT and F76L EV71 subgenomic replicon activities with EC50s of 0.21 μM and 0.17 μM, which is similar to previously reported results (38), but ALD did not show observable inhibition of WT or mutated replicon activities (Fig. 4C). Collectively, these results demonstrated that the F76L change on EV71 2Apro attenuates the fitness of virus growth and facilitates escape from 1-acetyllycorine inhibition.
We also examined the impact of F76L mutation on the enzymatic activity of EV71 2Apro according to the previously reported experimental procedure (45). The results showed that WT EV71 2Apro displayed high enzymatic activity, whereas the catalytic defective C100A mutation completely diminished this protease activity (Fig. 4D). F76L-mutated 2Apro presented only a modest effect on 2Apro protease activity. Moreover, the treatment of 1-acetylycorine (2 μM) showed only minor attenuation of 2Apro activity. Compared with the high inhibition by 1-acetyllycorine of EV71, 1-acetyllycorine affects EV71 propagation not only through affecting the correct processing of polyprotein after protein translation but also through other replication steps.
Structural homologies of EV71 2Apro and HCV NS3-NS4A proteases.
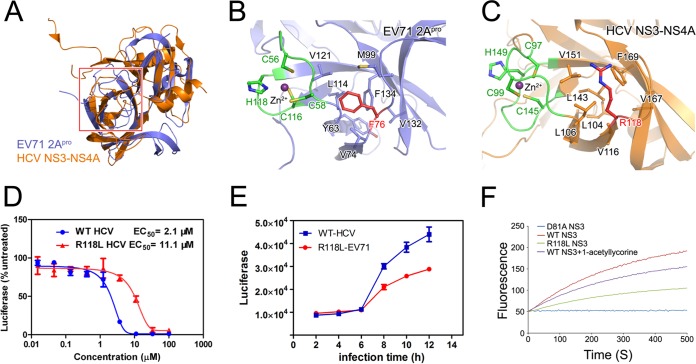
Structural analysis revealed that the 2Apro of all picornaviruses possesses a chymotrypsin-like fold and features a CCCH zinc finger outside the catalytic reaction center (45–47). Although the precise biological function of the zinc finger remains unclear, this site might be an essential structural element for the stabilization of the overall fold of 2Apro (46) and the removing of the bound zinc ion obviously attenuated the protease activity of 2Apro (46). The mutation site in EV71 2Apro, F76, which confers resistance to 1-acetyllycorine, is located at the back side of the zinc finger region. The aromatic side chain of F76 packs with a cluster of the hydrophobic core formed by the side chains of residues Y63, V74, M99, L114, V121, V132, and F134, thereby stabilizing the conformation of the zinc finger for the full activity of 2Apro (Fig. 5B).
FIG 5.
Conserved mechanism of 1-acetyllycorine against EV71 and HCV. (A) Structural comparison of EV71 2Apro and HCV NS3 proteases. The structures of EV71 2Apro and HCV NS3 protease were aligned and presented as cartoon diagrams in blue and gold, respectively. (B and C) Unique zinc finger region in EV71 2Apro and HCV NS3 protease. Key residues were displayed in stick mode with different colors. (D) Inhibitory activity of 1-acetyllycorine on WT or R118L-mutated HCV(JFH-1)-Rluc viruses. (E) Growth curves of HCV WT and R118L-mutated viruses. Each point represents the average results from three independent experiments, and the error bars represent the standard errors of the means (n = 3). (F) Enzymatic activities of HCV WT NS3/4A with or without 1-acetyllycorine and R118L- and D81A-mutated NS3/4A. Each reaction was performed by using 15 μM protein and 10 μM substrate.
Interestingly, another known example of a zinc binding site in a chymotrypsin-related viral protease is the NS3 serine protease of hepatitis C virus, in which the zinc ion is also involved in maintenance of the structure (46). Furthermore, the overall structure of the core domain and the zinc binding site in the HCV NS3 protease presents high structural homology with picornavirus 2Apro, with a root mean square deviation (RMSD) of 2.3 Å (Fig. 5A), and the removal of the zinc ion affects the activities of both proteases. Notably, residue R118 of HCV NS3 is located in a corresponding site of F76 of EV71 2Apro and similarly stacks with the hydrophobic core comprising residues L104, L106, V116, L143, V151, V167, and F169 for the correct conformation of the NS3 protease (Fig. 5C). We therefore hypothesized that 1-acetyllycorine inhibits HCV through a conserved mechanism for inhibition of EV71.
1-Acetyllycorine inhibits HCV with high potency, and the R118L mutation on HCV NS3 protease confers the resistance.
To verify the proposed hypothesis, we measured the inhibitory effect of 1-acetyllycorine on HCV. The constructed HCV(JFH-1)-Rluc virus was used to infect host cells, followed by treatment with a gradient concentration of 1-acetyllycorine. The inhibition assay revealed that 1-acetyllycorine effectively inhibited the growth of HCV(JFH-1)-Rluc virus, with an EC50 of 2.1 μM (Fig. 5D).
To verify whether 1-acetyllycorine shares a conserved inhibitory mechanism on EV71 and HCV, we constructed a mutated HCV(JFH-1)-Rluc virus harboring a replacement of R118 with a leucine residue in the NS3 protease. The results showed that 1-acetyllycorine inhibited R118L-mutated HCV(JFH-1)-Rluc virus with an EC50 of 11.1 μM, 5-fold over that of 1-acetyllycorine inhibition on WT HCV(JFH-1)-Rluc virus (Fig. 5D). This result supported the hypothesis that 1-acetyllycorine inhibited HCV replication through the inhibition of molecular folding and thereby the function of NS3 protease. Furthermore, R118L also reduced the fitness of HCV, which is similar to the effect of the F76L mutation in EV71 (Fig. 5E). Similarly to what appeared in EV71 experiments, R118L displayed modest attenuation of HCV NS3/4A protease activity and 1-acetyllycorine presented a minor effect on this activity in a reported enzymatic assay (48) (Fig. 5F). These results indicated that 1-acetyllycorine affects HCV proliferation not only through affecting the correct processing of polyprotein after protein translation but also through other replication steps, which is similar to the findings for EV71.
Combinatorial effects of 1-acetyllycorine and other inhibitors of EV71 and HCV.
Combination therapies with multiple drugs with different mechanisms of action have been demonstrated as effective approaches to treat virus infections (18). Because the inhibition of each step of the virus life cycle affects viral proliferation, we reasoned that the combination of different inhibitors of EV71 of HCV with 1-acetyllycorine could offer benefits over each single monotherapy. To examine this hypothesis, we used NITD008 as the polymerase inhibitor and ALD as an entry inhibitor of EV71 and sofosbuvir (PSI-7977) (49) and RBV (50) against HCV and measured the inhibition by these drugs in combination with 1-acetyllycorine.
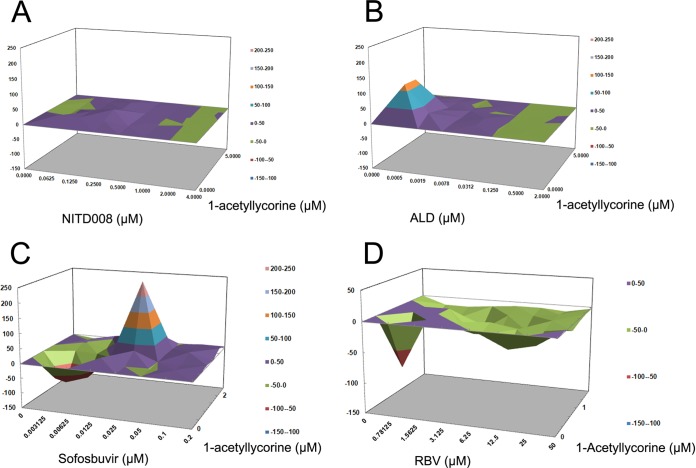
RD cells were infected with EV71(FY)-Luc and treated with various concentrations of 1-acetyllycorine and NITD008 or 1-acetyllycorine and ALD, either alone or in combination. The data were further analyzed using a mathematic model, MacSynergy II, to determine whether the effect of the combinations was synergistic, additive, or antagonistic (43). The results revealed that the combination of 1-acetyllycorine and NITD008 showed a slightly increased effect over that of the theoretical additive effects, indicating that the combination had a largely additive effect (Fig. 6A). In contrast, 1-acetyllycorine–ALD had antiviral effects that were significantly more potent than the theoretical additive effects, suggesting that this combination was indeed synergistic (Fig. 6B). No evidence of antiviral antagonism was observed with any of the tested doses. According to the criteria of Prichard et al. (43), the combination of 1-acetyllycorine and ALD is strong and will likely be important in vivo. We also measured the inhibitory effects of ALD and NITD008 against F76L EV71 mutated virus and found EC50s consistent with those against WT EV71 virus. This is consistent with the working target that was identified by drug resistance.
FIG 6.
Drug-drug interactions of 1-acetyllycorine with other inhibitors of EV71 and HCV proliferation. (A and B) Differential surface plots at the 95% confidence level (CI) were calculated and generated using MacSynergy II for the drug-drug interactions for the combinations of 1-acetyllycorine and NITD008 (A) and 1-acetyllycorine and ALD (B) targeting EV71. The drug-drug interactions for the combinations of 1-acetyllycorine and sofosbuvir (C) and 1-acetyllycorine and RBV (D) on HCV were also evaluated.
Combinations of lycorine with sofosbuvir and RBV have also been documented. Huh7.5.1 cells were infected with HCV(JFH-1)-Rluc virus and treated with various concentrations of 1-acetyllycorine and sofosbuvir, or 1-acetyllycorine and RBV, either alone or in combination for 48 h. The results revealed that the combination of 1-acetyllycorine and sofosbuvir had significantly synergistic antiviral effects (Fig. 6C). In contrast, the combination of 1-acetyllycorine and RBV presented clear antagonistic effects compared with the theoretical model (Fig. 6D). Similarly, the combination of 1-acetyllycorine and sofosbuvir will also be likely important in vivo. Additionally, sofosbuvir inhibited WT and R118L HCV with EC50s of 0.12 μM and 0.18 μM, and RBV inhibited WT and R118L HCV with EC50s of 17.0 μM and 15.8 μM, suggesting again that the mutation at the R118 position of NS3 is specific to 1-acetyllycorine resistance.
DISCUSSION
The correct function of viral proteases ensures the efficient proliferation of viruses. EV71 encodes 2Apro and 3Cpro and HCV encodes NS2 and NS3 for the proteolysis of viral polyproteins into individual functional proteins (12, 13). EV71 2Apro and 3Cpro and HCV NS3 are all chymotrypsin-like proteases with high structural similarities in the core catalytic domain. Specifically, both EV71 2Apro and HCV NS3 proteases contain a CCCH-type zinc finger for the stabilization of the overall conformation and the precise function of both proteins, which is a unique feature among all viruses (45–47). In the present study, we examined several lycorine derivatives against EV71 and HCV and observed that 1-acetyllycorine restrained the proliferation of EV71 and HCV. Interestingly, the mutations for drug resistance to 1-acetyllycorine on both EV71 and HCV are located at a conserved position in EV71 2Apro (F76) and HCV NS3 (R118). Although the primary sequences of EV71 2Apro and HCV NS3 proteases have limited homology, the environments of F76 in EV71 2Apro and R118 in HCV NS3 are highly similar (Fig. 5B and C). The side chains of F76 and R118 pack with the hydrophobic center to constitute a firm base to stabilize the conformation of the essential zinc finger. Notably, F76 of EV71 2Apro and R118 in HCV NS3 protease are strictly conserved in various enteroviruses and different HCV genotypes, suggesting that the unique zinc fingers of enterovirus 2Apro and HCV NS3 protease have an identical function and share a conserved inhibition mechanism through 1-acetyllycorine.
Besides the essential function in processing the viral proteins, enterovirus 2Apro has been reported to have multiple biological functions. For example, poliovirus 2Apro was reported to shut off the host's cap-dependent protein production by cleaving the elongation factors eIF4GI/II for the synthesis of viral proteins (51), interfering with the nuclear traffic (52, 53), and hijacking the splicing and transcription machinery (54). Moreover, coxsackievirus B3 (CVB3) 2Apro was revealed to cleave cytoskeletal protein dystrophin, resulting in dilated cardiomyopathy (55). In our in vitro enzymatic assays, 1-acetyllycorine displayed only minor attenuations of EV71 2Apro against the substrate spanning the peptides in EV71 polypeptide. Interestingly, previous studies have revealed that lycorine blocks the protein synthesis either for eukaryotic cells or for virus (56–58). All these observations indicate that 1-acetyllycorine not only affects the process of viral polyprotein but also employs multiple inhibitory mechanisms to inhibit the proliferation of either EV71 or HCV. One point that should be noted is that the change of viral titers of EV71 with the treatment of 1-acetyllycorine is from 109/ml to 105/ml. Because virus in the titer of 105/ml would probably still be lethal to the patient, an assay in an animal model is warranted to verify the clinical potential of 1-acetyllycorine against EV71.
Interestingly, several previous studies have documented the inhibition activity of lycorine derivatives against flaviviruses. The first study showed that 1.2 μM lycorine potently reduced the viral titers of WNV, DENV, and YFV by 102- to 104-fold (32). Additional studies demonstrated that a V9M substitution in the viral 2K peptide, spanning the endoplasmic reticulum membrane between NS4A and NS4B proteins, confers WNV resistance to lycorine through the enhancement of viral RNA replication (32). Moreover, the inhibition of lycorine derivatives on DENV was similar to that of WNV (33). Although HCV, WNV, and DENV all belong to the Flaviviridae family and NS3 proteases of these three viruses present high structural conservation, the zinc finger exclusively exists in HCV NS3 proteases but not other flavivirus-encoded proteases. Therefore, differences in the working mechanisms of lycorine derivatives for the inhibition of various flaviviruses were not surprising. A recent study showed the inhibition of HCV through lycorine-derived phenanthridine via the downregulation of host Hsc70 expression (34), suggesting that different lycorine derivatives suppress HCV proliferation via various mechanisms.
We observed a significant synergistic anti-EV71 effect of 1-acetyllycorine with ALD (an entry inhibitor) but not NITD008 (a polymerase inhibitor). We also observed the clear synergistic anti-HCV activity of 1-acetyllycorine with sofosbuvir in contrast with the obvious antagonist effect of 1-acetyllycorine with RBV. These results support the potential use of 1-acetyllycorine combination therapy with other inhibitors for the treatment of EV71 and HCV infections, with consideration for potential drug-drug antagonism with current clinically used drugs.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grants 81322023, 31370733, 31170678, 31100208, 31000332, and 21202087), the National Basic Research Program of China (973 program) (grants 2013CB911100 and 2014CB542800), and Tsinghua University Initiative Scientific Research Program (2009THZ01).
REFERENCES
- 1.Yang Y, Wang H, Gong E, Du J, Zhao X, McNutt MA, Wang S, Zhong Y, Gao Z, Zheng J. 2009. Neuropathology in 2 cases of fatal enterovirus type 71 infection from a recent epidemic in the People's Republic of China: a histopathologic, immunohistochemical, and reverse transcription polymerase chain reaction study. Hum Pathol 40:1288–1295. doi: 10.1016/j.humpath.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, Mao N, Xu S, Zhu S, Cui A, Zhang Y, Yan D, Li Q, Dong X, Zhang J, Zhao Y, Wan J, Feng Z, Sun J, Wang S, Li D, Xu W. 2010. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J 7:94. doi: 10.1186/1743-422X-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pol S, Vallet-Pichard A, Corouge M, Mallet VO. 2012. Hepatitis C: epidemiology, diagnosis, natural history and therapy. Contrib Nephrol 176:1–9. doi: 10.1159/000332374. [DOI] [PubMed] [Google Scholar]
- 4.Lavanchy D. 2009. The global burden of hepatitis C. Liver Int 29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Wang Y, Shan C, Sun Y, Xu P, Zhou H, Yang C, Shi PY, Rao Z, Zhang B, Lou Z. 2013. Crystal structure of enterovirus 71 RNA-dependent RNA polymerase complexed with its protein primer VPg: implication for a trans mechanism of VPg uridylylation. J Virol 87:5755–5768. doi: 10.1128/JVI.02733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Wang Y, Shan C, Chen C, Xu P, Song M, Zhou H, Yang C, Xu W, Shi PY, Zhang B, Lou Z. 2012. Enterovirus 71 VPg uridylation uses a two-molecular mechanism of 3D polymerase. J Virol 86:13662–13671. doi: 10.1128/JVI.01712-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Sun Y, Guo Y, Lou Z. 2013. Structural perspective on the formation of ribonucleoprotein complex in negative-sense single-stranded RNA viruses. Trends Microbiol 21:475–484. doi: 10.1016/j.tim.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 8.McMinn PC. 2002. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 9.Racaniello VR. 2001. Picornaviridae: the viruses and their replication, p 685–722. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 10.Wu Y, Lou Z, Miao Y, Yu Y, Dong H, Peng W, Bartlam M, Li X, Rao Z. 2010. Structures of EV71 RNA-dependent RNA polymerase in complex with substrate and analogue provide a drug target against the hand-foot-and-mouth disease pandemic in China. Protein Cell 1:491–500. doi: 10.1007/s13238-010-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moradpour D, Brass V, Gosert R, Wolk B, Blum HE. 2002. Hepatitis C: molecular virology and antiviral targets. Trends Mol Med 8:476–482. doi: 10.1016/S1471-4914(02)02395-X. [DOI] [PubMed] [Google Scholar]
- 12.Toyoda H, Nicklin MJ, Murray MG, Anderson CW, Dunn JJ, Studier FW, Wimmer E. 1986. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell 45:761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- 13.Ryan MD, Flint M. 1997. Virus-encoded proteinases of the picornavirus super-group. J Gen Virol 78:699–723. doi: 10.1099/0022-1317-78-4-699. [DOI] [PubMed] [Google Scholar]
- 14.Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, Patick AK, Matthews DA, Lee CA, Ford CE, Burke BJ, Rejto PA, Hendrickson TF, Tuntland T, Brown EL, Meador JW III, Ferre RA, Harr JE, Kosa MB, Worland ST. 1999. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as l-glutamine replacements. J Med Chem 42:1213–1224. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Yang B, Zhai Y, Yin Z, Sun Y, Rao Z. 2015. Peptidyl aldehyde NK-1.8k suppresses enterovirus 71 and enterovirus 68 infection by targeting protease 3C. Antimicrob Agents Chemother 59:2636–2646. doi: 10.1128/AAC.00049-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhai Y, Zhao X, Cui Z, Wang M, Wang Y, Li L, Sun Q, Yang X, Zeng D, Liu Y, Sun Y, Lou Z, Shang L, Yin Z. 16 November 2015. Cyanohydrin as an anchoring group for potent and selective inhibitors of enterovirus 71 3C protease. J Med Chem doi: 10.1021/acs.jmedchem.5b01013. [DOI] [PubMed] [Google Scholar]
- 17.Falah N, Montserret R, Lelogeais V, Schuffenecker I, Lina B, Cortay JC, Violot S. 2012. Blocking human enterovirus 71 replication by targeting viral 2A protease. J Antimicrob Chemother 67:2865–2869. doi: 10.1093/jac/dks304. [DOI] [PubMed] [Google Scholar]
- 18.Lou Z, Sun Y, Rao Z. 2014. Current progress in antiviral strategies. Trends Pharmacol Sci 35:86–102. doi: 10.1016/j.tips.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Guo Y, Lou Z. 2012. A versatile building block: the structures and functions of negative-sense single-stranded RNA virus nucleocapsid proteins. Protein Cell 3:893–902. doi: 10.1007/s13238-012-2087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S. 2011. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 21.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R. 2011. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieterich D, Rockstroh JK, Orkin C, Gutierrez F, Klein MB, Reynes J, Shukla U, Jenkins A, Lenz O, Ouwerkerk-Mahadevan S, Peeters M, De La Rosa G, Tambuyzer L, Jessner W. 2014. Simeprevir (TMC435) with pegylated interferon/ribavirin in patients coinfected with HCV genotype 1 and HIV-1: a phase 3 study. Clin Infect Dis 59:1579–1587. doi: 10.1093/cid/ciu675. [DOI] [PubMed] [Google Scholar]
- 23.Lamoral-Theys D, Decaestecker C, Mathieu V, Dubois J, Kornienko A, Kiss R, Evidente A, Pottier L. 2010. Lycorine and its derivatives for anticancer drug design. Mini Rev Med Chem 10:41–50. doi: 10.2174/138955710791112604. [DOI] [PubMed] [Google Scholar]
- 24.Cedron JC, Gutierrez D, Flores N, Ravelo AG, Estevez-Braun A. 2010. Synthesis and antiplasmodial activity of lycorine derivatives. Bioorg Med Chem 18:4694–4701. doi: 10.1016/j.bmc.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Toriizuka Y, Kinoshita E, Kogure N, Kitajima M, Ishiyama A, Otoguro K, Yamada H, Omura S, Takayama H. 2008. New lycorine-type alkaloid from Lycoris traubii and evaluation of antitrypanosomal and antimalarial activities of lycorine derivatives. Bioorg Med Chem 16:10182–10189. doi: 10.1016/j.bmc.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 26.Kretzing S, Abraham G, Seiwert B, Ungemach FR, Krugel U, Regenthal R. 2011. Dose-dependent emetic effects of the amaryllidaceous alkaloid lycorine in beagle dogs. Toxicon 57:117–124. doi: 10.1016/j.toxicon.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Hwang YC, Chu JJ, Yang PL, Chen W, Yates MV. 2008. Rapid identification of inhibitors that interfere with poliovirus replication using a cell-based assay. Antiviral Res 77:232–236. doi: 10.1016/j.antiviral.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li SY, Chen C, Zhang HQ, Guo HY, Wang H, Wang L, Zhang X, Hua SN, Yu J, Xiao PG, Li RS, Tan X. 2005. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res 67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Wang Q, Pan X, Fernandez de Castro I, Sun Y, Guo Y, Tao X, Risco C, Sui SF, Lou Z. 2013. Bunyamwera virus possesses a distinct nucleocapsid protein to facilitate genome encapsidation. Proc Natl Acad Sci U S A 110:9048–9053. doi: 10.1073/pnas.1222552110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabrielsen B, Monath TP, Huggins JW, Kefauver DF, Pettit GR, Groszek G, Hollingshead M, Kirsi JJ, Shannon WM, Schubert EM, DaRe J, Ugarkar B, Ussery MA, Phelan MJ. 1992. Antiviral (RNA) activity of selected Amaryllidaceae isoquinoline constituents and synthesis of related substances. J Nat Prod 55:1569–1581. doi: 10.1021/np50089a003. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Wang W, Sun Y, Ma C, Wang X, Wang X, Liu P, Shen S, Li B, Lin J, Deng F, Wang H, Lou Z. 11 November 2015. Crystal structure of the core region of hantavirus nucleocapsid protein reveals the mechanism for ribonucleoprotein complex formation. J Virol doi: 10.1128/JVI.02523-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou G, Puig-Basagoiti F, Zhang B, Qing M, Chen L, Pankiewicz KW, Felczak K, Yuan Z, Shi PY. 2009. A single-amino acid substitution in West Nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology 384:242–252. doi: 10.1016/j.virol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang P, Li LF, Wang QY, Shang LQ, Shi PY, Yin Z. 2014. Anti-dengue-virus activity and structure-activity relationship studies of lycorine derivatives. ChemMedChem 9:1522–1533. doi: 10.1002/cmdc.201300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen D, Cai J, Yin J, Jiang J, Jing C, Zhu Y, Cheng J, Di Y, Zhang Y, Cao M, Li S, Peng Z, Hao X. 2015. Lycorine-derived phenanthridine downregulators of host Hsc70 as potential hepatitis C virus inhibitors. Future Med Chem 7:561–570. doi: 10.4155/fmc.15.14. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Yang Y, Xu Y, Ma C, Qin C, Zhang L. 2011. Lycorine reduces mortality of human enterovirus 71-infected mice by inhibiting virus replication. Virol J 8:483. doi: 10.1186/1743-422X-8-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Yuan HH, Zhang X, Li YP, Shang LQ, Yin Z. 2014. Novel lycorine derivatives as anticancer agents: synthesis and in vitro biological evaluation. Molecules 19:2469–2480. doi: 10.3390/molecules19022469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao L, Zhu S, Wang Y, Lou Z, Sun Y. 22 August 2015. A comprehensive procedure for antiviral inhibitor discovery using EV71 as an example. Biophys Rep doi: 10.1007/s41048-015-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang L, Wang Y, Qing J, Shu B, Cao L, Lou Z, Gong P, Sun Y, Yin Z. 2014. An adenosine nucleoside analogue NITD008 inhibits EV71 proliferation. Antiviral Res 112:47–58. doi: 10.1016/j.antiviral.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Qing J, Wang Y, Sun Y, Huang J, Yan W, Wang J, Su D, Ni C, Li J, Rao Z, Liu L, Lou Z. 2014. Cyclophilin A associates with enterovirus-71 virus capsid and plays an essential role in viral infection as an uncoating regulator. PLoS Pathog 10:e1004422. doi: 10.1371/journal.ppat.1004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Qing J, Sun Y, Rao Z. 2014. Suramin inhibits EV71 infection. Antiviral Res 103:1–6. doi: 10.1016/j.antiviral.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 42.Cui HK, Qing J, Guo Y, Wang YJ, Cui LJ, He TH, Zhang L, Liu L. 2013. Stapled peptide-based membrane fusion inhibitors of hepatitis C virus. Bioorg Med Chem 21:3547–3554. doi: 10.1016/j.bmc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Prichard MN, Shipman C Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res 14:181–205. doi: 10.1016/0166-3542(90)90001-N. [DOI] [PubMed] [Google Scholar]
- 44.Prichard MN, Shipman C Jr. 1996. Analysis of combinations of antiviral drugs and design of effective multidrug therapies. Antivir Ther 1:9–20. [PubMed] [Google Scholar]
- 45.Cai Q, Yameen M, Liu W, Gao Z, Li Y, Peng X, Cai Y, Wu C, Zheng Q, Li J, Lin T. 2013. Conformational plasticity of the 2A proteinase from enterovirus 71. J Virol 87:7348–7356. doi: 10.1128/JVI.03541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen JF, Cherney MM, Liebig HD, Skern T, Kuechler E, James MN. 1999. The structure of the 2A proteinase from a common cold virus: a proteinase responsible for the shut-off of host-cell protein synthesis. EMBO J 18:5463–5475. doi: 10.1093/emboj/18.20.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mu Z, Wang B, Zhang X, Gao X, Qin B, Zhao Z, Cui S. 2013. Crystal structure of 2A proteinase from hand, foot and mouth disease virus. J Mol Biol 425:4530–4543. doi: 10.1016/j.jmb.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Romano KP, Ali A, Aydin C, Soumana D, Ozen A, Deveau LM, Silver C, Cao H, Newton A, Petropoulos CJ, Huang W, Schiffer CA. 2012. The molecular basis of drug resistance against hepatitis C virus NS3/4A protease inhibitors. PLoS Pathog 8:e1002832. doi: 10.1371/journal.ppat.1002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawitz E, Gane EJ. 2013. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 369:678–679. doi: 10.1056/NEJMc1307641. [DOI] [PubMed] [Google Scholar]
- 50.Strader DB, Wright T, Thomas DL, Seeff LB. 2004. Diagnosis, management, and treatment of hepatitis C. Hepatology 39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 51.Gradi A, Svitkin YV, Imataka H, Sonenberg N. 1998. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc Natl Acad Sci U S A 95:11089–11094. doi: 10.1073/pnas.95.19.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gustin KE, Sarnow P. 2001. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J 20:240–249. doi: 10.1093/emboj/20.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park N, Katikaneni P, Skern T, Gustin KE. 2008. Differential targeting of nuclear pore complex proteins in poliovirus-infected cells. J Virol 82:1647–1655. doi: 10.1128/JVI.01670-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almstead LL, Sarnow P. 2007. Inhibition of U snRNP assembly by a virus-encoded proteinase. Genes Dev 21:1086–1097. doi: 10.1101/gad.1535607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, Knowlton KU. 1999. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med 5:320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 56.Jimenez A, Santos A, Alonso G, Vazquez D. 1976. Inhibitors of protein synthesis in eukaryotic cells. Comparative effects of some amaryllidaceae alkaloids. Biochim Biophys Acta 425:342–348. [DOI] [PubMed] [Google Scholar]
- 57.Vrijsen R, Everaert L, Van Hoof LM, Vlietinck AJ, Vanden Berghe DA, Boeye A. 1987. The poliovirus-induced shut-off of cellular protein synthesis persists in the presence of 3-methylquercetin, a flavonoid which blocks viral protein and RNA synthesis. Antiviral Res 7:35–42. doi: 10.1016/0166-3542(87)90037-4. [DOI] [PubMed] [Google Scholar]
- 58.Vrijsen R, Vanden Berghe DA, Vlietinck AJ, Boeye A. 1986. Lycorine: a eukaryotic termination inhibitor? J Biol Chem 261:505–507. [PubMed] [Google Scholar]