Abstract
The objective of the study was to describe the subcutaneous interstitial fluid (ISF) pharmacokinetics of fluconazole in critically ill patients with sepsis. This prospective observational study was conducted at two tertiary intensive care units in Australia. Serial fluconazole concentrations were measured over 24 h in plasma and subcutaneous ISF using microdialysis. The concentrations in plasma and microdialysate were measured using a validated high-performance liquid chromatography system with electrospray mass spectrometer detector method. Noncompartmental pharmacokinetic analysis was performed. Twelve critically ill patients with sepsis were enrolled. The mean in vivo fluconazole recovery rates ± standard deviation (SD) for microdialysis were 51.4% ± 16.1% with a mean (±SD) fluconazole ISF penetration ratio of 0.52 ± 0.30 (coefficient of variation, 58%). The median free plasma area under the concentration-time curve from 0 to 24 h (AUC0–24) was significantly higher than the median ISF AUC0–24 (340.4 versus 141.1 mg · h/liter; P = 0.004). There was no statistical difference in median fluconazole ISF penetration between patients receiving and not receiving vasopressors (median, 0.28 versus 0.78; P = 0.106). Both minimum and the maximum concentrations of drug in serum (Cmax and Cmin) showed a significant correlation with the fluconazole plasma exposure (Cmax, R2 = 0.86, P < 0.0001; Cmin, R2 = 0.75, P < 0.001). Our data suggest that fluconazole was distributed variably, but incompletely, from plasma into subcutaneous interstitial fluid in this cohort of critically ill patients with sepsis. Given the variability of fluconazole interstitial fluid exposures and lack of clinically identifiable factors by which to recognize patients with reduced distribution/exposure, we suggest higher than standard doses to ensure that drug exposure is adequate at the site of infection.
INTRODUCTION
Infections and related sepsis are among the most important reasons for admission to intensive care units (ICUs). Candida sp. infections are responsible for up to 30% and 38% of fungal sepsis and septic shock episodes, respectively (1–4), and currently are the third leading cause of infections in ICUs globally (5). The attributable mortality associated with candidiasis in ICU patients is estimated to be 28.3% (6).
Most Candida infections are extravascular in origin, with interstitial fluid (ISF) of tissues and other body fluids representing the actual target site for antifungal therapy to treat the vast majority of Candida infections (7–9). While antibacterial treatment failures have been attributed to impaired target site penetration in critically ill patients with sepsis and shock (9, 10), data on antifungals are limited. However, it is highly likely that successful treatment with antifungal agents relies on the achievement of adequate concentrations at the site of infection, i.e., the ISF.
Fluconazole is a frequently used triazole antifungal agent in ICUs. In addition to delay in initiating fluconazole therapy, inadequate fluconazole dosing is an independent predictor of mortality in ICU patients with Candida infections (11). Given increasing evidence on the fluconazole exposure-response relationship (12–15), knowledge of plasma and ISF fluconazole concentrations is important to optimize exposure and hence response to fluconazole treatment in this cohort of critically ill patients.
In the present study, we investigated the pharmacokinetic (PK) profile of fluconazole in plasma and subcutaneous (SC) ISF in ICU patients with sepsis.
MATERIALS AND METHODS
This prospective observational study was conducted at two tertiary ICUs in Australia between May 2012 and January 2014. The study protocol was approved by the respective local human ethics committees (2012039 and HREC/13/QRBW/75). Prior informed written consent was obtained from either the patient or a substitute decision maker for the patient.
Study subjects.
All ICU patients were screened, and eligible patients were identified for participation in the study. Patients were eligible for inclusion if the following criteria were met: at least 18 years old, admitted to the ICU, presence of sepsis, arterial line in situ or planned insertion, indwelling urinary catheter in situ or planned insertion, ability to obtain prior informed consent from the patient or person responsible, and prescription of intravenous fluconazole. Sepsis was defined as the presence of a suspected or confirmed infection and two or more systemic inflammatory response syndrome criteria documented in the previous 24 h: a core temperature of <36.0°C or >38.0°C; a heart rate of >90 beats/minute; a respiratory rate of >20 breaths/minute, partial pressure of carbon dioxide in the arterial blood (PaCO2) of <32 mm Hg, or the patient being mechanically ventilated for an acute process; and a white cell count (WCC) of >12.0 × 109 or <4.0 × 109/liter or >10% immature bands (16). Patients who met one or both of the following criteria were excluded: (i) known or suspected allergy to triazole antifungal agents or penicillins and (ii) pregnancy.
Drug administration.
Fluconazole initiation and dose were at the discretion of the treating physician based on the clinical requirements.
Microdialysis procedure to measure ISF concentrations and in vivo calibration.
Microdialysis was used to measure the free (or unbound) fluconazole concentration in subcutaneous-tissue ISF. The principles and details of microdialysis have been described previously (17). In this study, a microdialysis probe (CMA 60; Microdialysis AB, Stockholm, Sweden) with a molecular-mass cutoff of 20 kDa, an outer diameter of 0.6 mm, and a membrane length of 30 mm was aseptically placed in the subcutaneous tissue of the upper arms of the patients following the local injection of 0.5% lidocaine solution (<1.5 ml). The probe was then perfused with flucloxacillin (10 mg/liter; internal standard, perfusate) in 0.9% sodium chloride at a flow rate of 1.5 μl/min. Flucloxacillin was chosen as the internal standard for analytical purposes. The perfusion was initiated at least 60 min prior to the sampling time to establish equilibrium. After commencement of the fluconazole infusion, microdialysis samples were collected (the sampling frequency is described below). The in vivo recovery of fluconazole in the microdialysate was interpolated from the loss of the internal standard (flucloxacillin) across the microdialysis membrane into the subcutaneous ISF (18–22) using the following equation: percent in vivo fluconazole recovery = 100 × (Cin − mean Cout/Cin), where Cin is flucloxacillin at 10 mg/liter (perfusate) and Cout is the measured flucloxacillin concentration in the microvial (microdialysate).
Sample collection and processing.
Arterial-blood samples were collected during one dosing interval from an indwelling arterial line just before commencing the fluconazole infusion (0 h) and at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, and 24 h after commencement. Microdialysate samples were collected at 30-min intervals between 0 and 6 h, hourly between 6 and 12 h, and then at 24 h. A 24-h urine sample was collected to measure creatinine clearance. Blood samples were centrifuged at 2,000 × g for 10 min, and plasma was collected. The plasma and microdialysate samples were stored at −80°C until analysis. Sample transport was carried out by using dry ice and adhering to cold-chain procedures.
Data collection.
Demographic and clinical data were collected, including age, gender, height, weight, admission diagnosis, acute physiological and chronic health evaluation (APACHE) II score, sequential organ failure assessment (SOFA) score, receipt of mechanical ventilation, vasoactive treatment, and response to fluconazole therapy (defined as cessation of fluconazole therapy without antifungal recommencement within 14 days).
Sample analysis.
Plasma fluconazole concentrations and microdialysate fluconazole and flucloxacillin concentrations were analyzed with a high-performance liquid chromatography (HPLC) system with electrospray mass spectrometer detector (Applied Biosystems [Foster City, CA, USA] API2000; Shimadzu [Kyoto, Japan] HPLC). Briefly, for plasma samples, the method was as follows. Plasma (300 μl) was mixed with 50 μl internal standard (voriconazole; 1 mg/liter) and precipitated with 150 μl of 10% trichloroacetic acid. The samples were centrifuged at 12,000 × g for 6 min at 4°C after being thoroughly vortexed for 30 s. The analytes were separated through an Agilent Zorbax Eclipse XDB-c18 (2.1 by 150 mm; 3.5-μm particle size) by gradient elution using water containing 0.1% formic acid and methanol containing 0.1% formic acid with a total flow rate of 0.3 ml/min and detected by an electrospray positive-ionization mode of tandem mass spectrometry. The mass-to-charge ratios (m/z) in multiple-reaction monitoring were 307.3/127.0 for fluconazole and 349.9/224.0 for the internal standard. Microdialysate samples (10 μl) were assayed directly without any sample preparation under the same conditions used for the plasma samples. The calibration curve was linear over the concentration range of 0.1 to 20 mg/liter for both plasma and microdialysate samples. The intra- and interday coefficients of variation were validated to be within 10% at low, medium, and high concentrations of the calibration range. Assays were validated and conducted using criteria from the U.S. Food and Drug Administration guidance on bioanalysis (23).
PK analysis.
Plasma and ISF PK parameters were estimated using noncompartmental methods. The apparent terminal elimination rate constant (kel) was determined from log-linear least-squares regression. The minimum and maximum concentrations of drug in serum for the dosing period (Cmin and Cmax) were the observed values. The half-life (t1/2) was calculated as ln (2)/kel. The area under the plasma concentration-time curve from 0 to 24 h (AUC0–24) was calculated using the linear trapezoidal approximation. The area under the plasma concentration-time curve from time zero to infinity (AUC0–∞) was calculated by the log-linear trapezoidal rule until the time of the last quantifiable plasma concentration and then extrapolated to infinity by using the quotient of the last measurable concentration to kel. Clearance (CL) was calculated using the following formula: dose/AUC0–∞. Protein binding of fluconazole was assumed to be 12% to determine free AUC0–24 (fAUC0–24) (24). An fAUC0–24/MIC ratio of ≥100 was chosen as the target PK/pharmacodynamic (PD) index associated with efficacy based on the EUCAST guidelines (25). The current EUCAST MIC breakpoints for fluconazole are as follows: susceptible, ≤2 mg/liter, and resistant, >4 mg/liter. The current CLSI MIC breakpoints are as follows: susceptible, ≤2 mg/liter; susceptible dose dependent, 4 mg/liter; and resistant, ≥8 mg/liter. These breakpoints apply to Candida albicans, Candida tropicalis, and Candida parapsilosis.
The estimated concentrations from microdialysis samples were corrected, using the in vivo recovery calculated for fluconazole, prior to pharmacokinetic analyses with the following equation: CISF = 100 × (Cmicrodialysate/percent recovery in vivo), where CISF is the drug concentration in ISF and Cmicrodialysate is fluconazole concentration in the microdialysate. ISF penetration ratios were calculated using the AUC0–24 for subcutaneous ISF concentrations and the fAUC0–24 in plasma (fAUC0–24 plasma) as follows: ISF penetration ratio = AUCISF/fAUC0–24 plasma. A two-sided t test was used to compare the means of concentrations in plasma and subcutaneous ISF and PK parameters. The Mann-Whitney U test was used to compare the ISF penetration between patients receiving and not receiving vasopressors. All analyses were done using GraphPad Prism 5.03 for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
Twelve patients were included in this study; six were female. All patients were treated with 400 mg fluconazole (5.1 mg/kg of body weight) administered once daily as an intravenous infusion over 60 min. All sampling occurred between days 3 and 5 after initiation of fluconazole treatment. Seven (58.3%) patients received fluconazole for the directed treatment—six (50%) patients for intra-abdominal sepsis and one (8.3%) patient for lower respiratory tract infection. Three (25%) patients received empirical fluconazole for intra-abdominal sepsis, one (8.3%) patient received empirical fluconazole for lower respiratory tract infections, and one (8.3%) patient received fluconazole to treat C. albicans in a submandibular abscess. An overview of the patient characteristics is shown in Table 1.
TABLE 1.
Patient characteristics
| Characteristica | Median value (IQR) |
|---|---|
| Age (yr) | 53 (43–67) |
| Wt (kg) | 75 (70–87) |
| Height (m) | 1.7 (1.6–1.8) |
| CrCLmeasured (ml/min) | 117.2 (74.0–143.3) |
| APACHE II score | 18 (17.7–20.5) |
| SOFA score | 3.5 (3.0–6.5) |
| Invasive ventilation [n (%)] | 9 (75) |
| Vasopressor/inotropic support [n (%)] | 5 (42) |
CrCLmeasured, measured creatinine clearance using 24-h urine collected on the sampling day. APACHE and SOFA scores were calculated using the data obtained over the preceding 24 h in relation to the sampling day. Vasopressor/inotropic support was defined as the patient having received one of the following to maintain clinician-targeted mean arterial pressure: noradrenaline, adrenaline, dopamine, vasopressin, and dobutamine.
Microbiology.
Abdominal fluid was positive for C. albicans in five patients and for Candida glabrata in one patient. Fluconazole was changed to caspofungin in the last patient after identification to the species level. Lower respiratory tract secretions were positive for C. albicans in one patient, and in one patient, the submandibular abscess aspirate was positive for C. albicans. No other Candida spp. were isolated in this patient cohort. The MIC values were reported for only two patients (0.25 and 0.6 mg/liter), both of which were below EUCAST and CLSI breakpoints for fluconazole.
Microdialysis.
The mean in vivo fluconazole recovery rate for microdialysis ± standard deviation (SD) was 51.4% ± 16.1%. The fluconazole ISF penetration was 0.52 ± 0.30 (coefficient of variation, 58%). The median (interquartile range [IQR]) fluconazole ISF penetrations in patients receiving and not receiving vasopressors were 0.28 (0.17 to 0.57) and 0.70 (0.48 to 0.94), respectively (P = 0.106). The ISF PK parameters are presented in Table 2.
TABLE 2.
Plasma and ISF pharmacokinetics of fluconazole in critically ill patientsa
| Parameterb | Median value (IQR) |
|
|---|---|---|
| Plasma | ISF | |
| Cmax (mg/liter) | 20.3 (17.2–26.4) | 9.8 (6.3–16.0) |
| Tmax (h) | 1.4 (1–1.4) | 3.8 (1.2–4.8) |
| Cmin (mg/liter) | 11.6 (8.1–16.4) | 3.5 (1.4–6.7) |
| t1/2 (h) | 33.7 (24.7–74.5) | |
| fAUC0–24 (mg · h/liter) | 340.4 (263.5–523.7) | 141.1 (68.2–267.0) |
| CLobs (liters/h) | 0.5 (0.2–0.6) | |
| Vss (liters) | 20.4 (15.5–26.7) | |
Pharmacokinetics after receiving a 400-mg dose.
Cmax, maximum concentration observed; Tmax, time to reach Cmax; Cmin, minimum concentration observed; CLobs, observed clearance; Vss, steady-state volume of distribution.
Fluconazole PK.
The concentration-time profile of fluconazole in plasma and ISF of subcutaneous soft tissue in critically ill patients with sepsis is shown in Fig. 1. The free median plasma AUC0–24 was significantly higher than the median ISF AUC0–24 (340.4 versus 141.1 mg · h/liter; P = 0.004) (Fig. 2). Both Cmax and Cmin showed significant correlation with the fluconazole plasma exposure (Cmax, R2 = 0.86, P < 0.001; Cmin, R2 = 0.75, P < 0.001). Of the patients with Candida sp. isolates, those that responded to treatment did not seem to have a fluconazole exposure different from that of the patients who did not respond to the treatment; the respective median (IQR) plasma exposures were 366 mg · h/liter (257.4 to 485.7 mg · h/liter) and 454 mg · h/liter (391.9 to 516.1 mg · h/liter), and the respective median (IQR) ISF exposures were 96.6 mg · h/liter (65.2 to 179.4 mg · h/liter) and 352.1 mg · h/liter (254.6 to 449.6 mg · h/liter).
FIG 1.
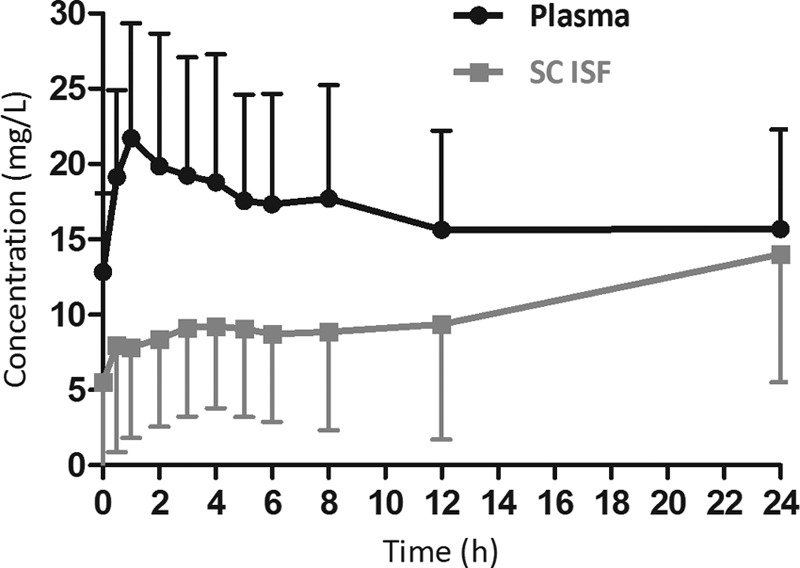
Free median (interquartile range [error bars]) plasma and SC ISF fluconazole concentration-time profile in critically ill patients (pharmacokinetic profile after receiving a 400-mg dose).
FIG 2.
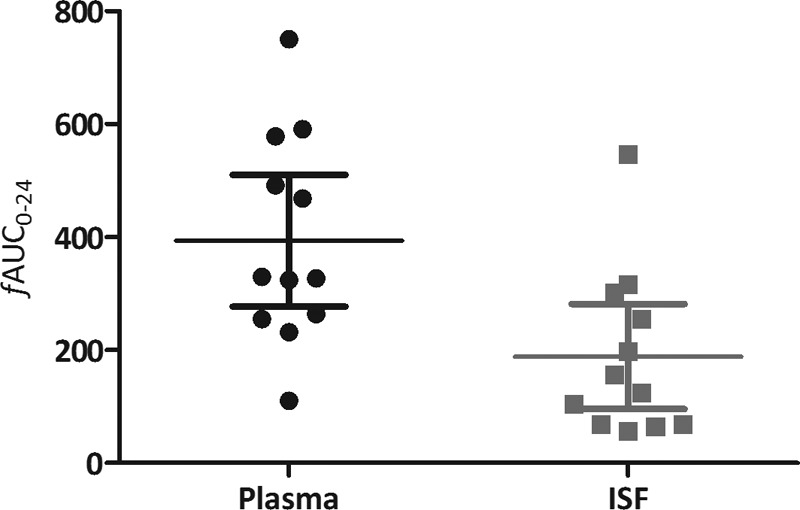
Comparison of the free area under the concentration-time curve from 0 to 24 h in plasma and subcutaneous interstitial fluid (means ± SD in plasma and interstitial fluid were 393.3 ± 183.7 and 188.6 ± 146.6, respectively; P = 0.006).
PK/PD.
Fluconazole PK/PD target attainment in both plasma and ISF at EUCAST and CLSI breakpoints for C. albicans is shown in Fig. 3. Both patients with reported MICs in this cohort attained the PK/PD target (fAUC0–24/MIC ≥ 100) in both plasma and ISF. However, only one patient responded to the treatment as defined above.
FIG 3.

Percentages of patients who achieved the pharmacokinetic/pharmacodynamic target of an fAUC0–24/MIC of ≥100 in plasma and subcutaneous interstitial fluid at breakpoints (n = 12).
DISCUSSION
Our data suggest that fluconazole was distributed incompletely from plasma into subcutaneous ISF in this cohort of critically ill patients with sepsis, with a mean penetration ratio ± SD of 0.52 ± 0.30. Peak concentrations in ISF were reached at 3.8 h compared to 1.4 h in plasma, suggesting delayed distribution into subcutaneous ISF. The variability in the observed fluconazole ISF penetration among patients was high. This was evident from the coefficient of variation (58%) observed among the patients. A large proportion of patients in the cohort would have achieved an fAUC0–24/MIC of ≥100 in plasma at EUCAST and CLSI breakpoints (Fig. 3). However, a majority of the patients would require higher doses in order to achieve an fAUC0–24/MIC of ≥100 in ISF (Fig. 3).
Sepsis- and septic-shock-related hemodynamic changes lead to tissue hypoperfusion, which results in alterations in antimicrobial pharmacokinetics (9, 26). During the initial stages of sepsis-related blood flow changes, hypoperfusion of vital organs occurs, while peripheral tissues still receive high blood flow as a result of an increased cardiac workload and peripheral vasodilation, whereas peripheral tissue hypoperfusion occurs during the later stages of sepsis-related blood flow changes as a consequence of a physiological effort to increase blood perfusion to vital organs (27). Thus, using physiologically based PK simulations, Sasongko et al. (24) have shown that fluconazole uptake into subcutaneous ISF is dependent on blood flow to the subcutaneous tissue in healthy volunteers. Moreover, significant sustained compromised microcirculation in peripheral tissues was observed even after restoration of hemodynamics in sepsis patients (28, 29). Although not statistically significant, our results suggest that use of vasopressors, which result in peripheral vasoconstriction, may have an influence on peripheral subcutaneous ISF penetration of fluconazole.
Mauric et al. (30) investigated fluconazole penetration into skeletal muscle of rats with severe sepsis using microdialysis and showed that free plasma fluconazole concentrations were superimposable with ISF concentrations in skeletal muscle. This finding is in contrast to the observed penetration in our study. However, the lipopolysaccharide-induced-sepsis rat model used by Mauric et al. does not mimic all forms of inflammation observed in humans. Moreover, inflammation and sampling were limited in duration to 6 h. It is possible that these differences—inflammatory changes and a shorter inflammatory period—might explain the incomplete distribution observed in our study.
Another important observation from this study was the heterogeneous distribution of fluconazole into subcutaneous ISF (Fig. 2). This could be a result of differences in individual PK, as well as the estimation of microdialysis probe recovery. It is generally recommended that the recovery rates be >20% to minimize the variability associated with sample handling and analysis (31). The mean in vivo recovery rate achieved in this study was 51.4%. Furthermore, the mean fluconazole recovery rates achieved in the current study were similar to those observed in healthy volunteers (57%) (24) and in an in vivo study using a C. albicans-infected rat model (50%) (32). Moreover, the observed in vivo recovery rate variability in this study was similar to those attained in other studies (coefficient of variation, 31% versus 28 to 37%) (24, 32, 33). Given the heterogeneous distribution and lack of readily identifiable clinical factors by which to recognize patients with reduced distribution into ISF, it is evident that care should be taken when adjusting doses based only on the concentration in plasma. In plasma, as previously reported in a cohort of critically ill patients (34), variability in the observed mean fluconazole fAUC0–24 and the Cmax and Cmin was higher than in surgical ICU patients (35) and healthy volunteers (36). The underlying causes of this variability could be 2-fold—physiological changes that occur during sepsis and patient-related factors, like weight and renal function. Thus, a recent study (34) has shown that a standard one-dose-fits-all approach, i.e., 400 mg without taking patient weight into account, would result in subtherapeutic fluconazole exposure in critically ill patients. This variability in plasma exposures might have also contributed to the incomplete distribution to ISF concentrations described above.
For fluconazole, an fAUC0–24/MIC of ≥100 has been shown to be the predictive PK/PD target associated with clinical success (25). A predictive PK/PD target in ISF has yet to be identified for fluconazole. In general, the percentage of patients who attained the PK/PD target decreased with increasing MICs in both plasma and ISF. Considering that similar PK/PD targets may apply to ISF, the percentage of patients attaining the PK/PD target was further reduced in ISF (Fig. 3). However, a different PK/PD target for ISF is highly possible. In patients infected with a susceptible Candida sp. with a MIC below the breakpoint, the PK/PD target in plasma would be attained in the majority of the patients with standard doses. However, higher than standard doses would be required to achieve similar targets in ISF. In patients infected with less-susceptible Candida spp., a lower exposure is highly likely to be a problem in both plasma and ISF. If the MIC is high or unknown, we recommend measuring fluconazole exposure or using higher than standard doses to ensure that the exposure is adequate.
This study had some limitations. We did not measure the unbound concentrations of fluconazole in plasma and used a published protein binding value (12%) to estimate free plasma fluconazole concentrations. Given the low protein binding of fluconazole, the difference between the measured and estimated fluconazole concentrations is unlikely to be significant (37). We used the breakpoint MICs determined by EUCAST and CLSI to estimate fluconazole PK/PD targets because of the lack of actual MIC data for 10 of 12 patients. We measured the fluconazole ISF concentration in the SC tissue of the upper arm in this study. The PK in this peripheral site may be different from that in other sites of infection, e.g., intra-abdominal or epithelial lining fluid, for the above-mentioned reasons. Considering the ethical constraints for critically ill patients, we chose to use SC tissue of the upper arm for this study. Therefore, the results should interpreted cautiously, taking patient status and the site of infection into consideration.
In summary, fluconazole was distributed incompletely from plasma into subcutaneous ISF, with substantial variability between patients. In this cohort of critically ill patients, based on the PK/PD target, the observed exposures obtained in plasma should be sufficient to effectively treat susceptible Candida spp. Given the heterogeneous distribution and lack of clinically identifiable factors by which to recognize patients with reduced distribution/exposure, we recommend using higher than standard doses to ensure that the exposure is adequate at the site of infection. Decreased ISF penetration is one of the possible causes of treatment failure, and further studies are warranted.
ACKNOWLEDGMENTS
We acknowledge ICU medical and, especially, nursing staff at both sites for assisting in patient identification, recruitment, and sample collection and registrars/senior registrars of the units for assisting with microdialysis probe insertion.
This work was supported by a Royal Brisbane and Women's Hospital Research Foundation grant and by the Intensive Care Foundation (http://www.intensivecarefoundation.org.au). Jason A. Roberts is funded, in part, by a National Health and Medical Research Council Research Fellowship (APP1048652).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Eggimann P, Garbino J, Pittet D. 2003. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 3:685–702. doi: 10.1016/S1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Ebbers J, Geurtz L, Stefanik D, Major Y, Edmond MB, Wenzel RP, Seifert H. 2014. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents 43:78–81. doi: 10.1016/j.ijantimicag.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. 2006. Inflammatory response and clinical course of adult patients with nosocomial bloodstream infections caused by Candida spp. Clin Microbiol Infect 12:170–177. doi: 10.1111/j.1469-0691.2005.01318.x. [DOI] [PubMed] [Google Scholar]
- 4.Guery BP, Arendrup MC, Auzinger G, Azoulay E, Borges Sa M, Johnson EM, Muller E, Putensen C, Rotstein C, Sganga G, Venditti M, Zaragoza Crespo R, Kullberg BJ. 2009. Management of invasive candidiasis and candidemia in adult non-neutropenic intensive care unit patients. Part I. Epidemiology and diagnosis. Intensive Care Med 35:55–62. doi: 10.1007/s00134-008-1338-7. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC II Group of Investigators . 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 6.Hassan I, Powell G, Sidhu M, Hart WM, Denning DW. 2009. Excess mortality, length of stay and cost attributable to candidaemia. J Infect 59:360–365. doi: 10.1016/j.jinf.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Müller M, dela Peña A, Derendorf H. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob Agents Chemother 48:1441–1453. doi: 10.1128/AAC.48.5.1441-1453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felton T, Troke PF, Hope WW. 2014. Tissue penetration of antifungal agents. Clin Microbiol Rev 27:68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felton TW, Hope WW, Roberts JA. 2014. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis 79:441–447. doi: 10.1016/j.diagmicrobio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Joukhadar C, Frossard M, Mayer BX, Brunner M, Klein N, Siostrzonek P, Eichler HG, Muller M. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit Care Med 29:385–391. doi: 10.1097/00003246-200102000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Labelle AJ, Micek ST, Roubinian N, Kollef MH. 2008. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 36:2967–2972. doi: 10.1097/CCM.0b013e31818b3477. [DOI] [PubMed] [Google Scholar]
- 12.Arendrup MC, Cuenca-Estrella M, Donnelly JP, Lass-Florl C, Rodriguez-Tudela JL. 2009. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob Agents Chemother 53:2704–2705. doi: 10.1128/AAC.01411-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddley JW, Patel M, Bhavnani SM, Moser SA, Andes DR. 2008. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob Agents Chemother 52:3022–3028. doi: 10.1128/AAC.00116-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Tudela JL, Almirante B, Rodriguez-Pardo D, Laguna F, Donnelly JP, Mouton JW, Pahissa A, Cuenca-Estrella M. 2007. Correlation of the MIC and dose/MIC ratio of fluconazole to the therapeutic response of patients with mucosal candidiasis and candidemia. Antimicrob Agents Chemother 51:3599–3604. doi: 10.1128/AAC.00296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinnollareddy M, Peake SL, Roberts MS, Playford EG, Lipman J, Roberts JA. 2011. Pharmacokinetic evaluation of fluconazole in critically ill patients. Expert Opin Drug Metab Toxicol 7:1431–1440. doi: 10.1517/17425255.2011.615309. [DOI] [PubMed] [Google Scholar]
- 16.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 17.de la Pena A, Liu P, Derendorf H. 2000. Microdialysis in peripheral tissues. Adv Drug Deliv Rev 45:189–216. doi: 10.1016/S0169-409X(00)00106-X. [DOI] [PubMed] [Google Scholar]
- 18.Roberts JA, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Piperacillin penetration into tissue of critically ill patients with sepsis–bolus versus continuous administration? Crit Care Med 37:926–933. doi: 10.1097/CCM.0b013e3181968e44. [DOI] [PubMed] [Google Scholar]
- 19.Varghese JM, Jarrett P, Boots RJ, Kirkpatrick CM, Lipman J, Roberts JA. 2014. Pharmacokinetics of piperacillin and tazobactam in plasma and subcutaneous interstitial fluid in critically ill patients receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 43:343–348. doi: 10.1016/j.ijantimicag.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 64:142–150. doi: 10.1093/jac/dkp139. [DOI] [PubMed] [Google Scholar]
- 21.Douglas A, Udy AA, Wallis SC, Jarrett P, Stuart J, Lassig-Smith M, Deans R, Roberts MS, Taraporewalla K, Jenkins J, Medley G, Lipman J, Roberts JA. 2011. Plasma and tissue pharmacokinetics of cefazolin in patients undergoing elective and semielective abdominal aortic aneurysm open repair surgery. Antimicrob Agents Chemother 55:5238–5242. doi: 10.1128/AAC.05033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts JA, Udy AA, Jarrett P, Wallis SC, Hope WW, Sharma R, Kirkpatrick CM, Kruger PS, Roberts MS, Lipman J. 2015. Plasma and target-site subcutaneous tissue population pharmacokinetics and dosing simulations of cefazolin in post-trauma critically ill patients. J Antimicrob Chemother 70:1495–1502. doi: 10.1093/jac/dku564. [DOI] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. September 2013. Guidance for industry—bioanalytical method validation, draft guidance. Food and Drug Administration, Washington, DC: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf. [Google Scholar]
- 24.Sasongko L, Williams KM, Day RO, McLachlan AJ. 2003. Human subcutaneous tissue distribution of fluconazole: comparison of microdialysis and suction blister techniques. Br J Clin Pharmacol 56:551–561. doi: 10.1046/j.1365-2125.2003.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EUCAST. 2008. EUCAST technical note on fluconazole. Clin Microbiol Infect 14:193–195. doi: 10.1111/j.1469-0691.2007.01899.x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL. 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulldemolins M, Roberts JA, Lipman J, Rello J. 2011. Antibiotic dosing in multiple organ dysfunction syndrome. Chest 139:1210–1220. doi: 10.1378/chest.10-2371. [DOI] [PubMed] [Google Scholar]
- 28.De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL. 2006. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 34:403–408. doi: 10.1097/01.CCM.0000198107.61493.5A. [DOI] [PubMed] [Google Scholar]
- 29.Verdant C, De Backer D. 2005. How monitoring of the microcirculation may help us at the bedside. Curr Opin Crit Care 11:240–244. doi: 10.1097/01.ccx.0000158849.94225.11. [DOI] [PubMed] [Google Scholar]
- 30.Mauric O, Thallinger C, Kugler SA, Joukhadar SM, Kovar FM, Konz KH, Graninger W, Joukhadar C. 2011. The ability of fluconazole to penetrate into ventilated, healthy and inflamed lung tissue in a model of severe sepsis in rats. Pharmacology 87:130–134. doi: 10.1159/000323738. [DOI] [PubMed] [Google Scholar]
- 31.Muller M. 2002. Science, medicine, and the future: microdialysis. BMJ 324:588–591. doi: 10.1136/bmj.324.7337.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azeredo FJ, de Araújo BV, Haas SE, Torres B, Pigatto M, de Andrade C, Dalla Costa T. 2012. Comparison of fluconazole renal penetration levels in healthy and Candida albicans-infected Wistar rats. Antimicrob Agents Chemother 56:5852–5857. doi: 10.1128/AAC.01323-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathy F-X, Ntivunwa D, Verbeeck RK, Preat V. 2005. Fluconazole distribution in rat dermis following intravenous and topical application: a microdialysis study. J Pharm Sci 94:770–780. doi: 10.1002/jps.20290. [DOI] [PubMed] [Google Scholar]
- 34.Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Dimopoulos G. 2015. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients study. Crit Care 19:33. doi: 10.1186/s13054-015-0758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buijk SLCE, Gyssens IC, Mouton JW, Verbrugh HA, Touw DJ, Bruining HA. 2001. Pharmacokinetics of sequential intravenous and enteral fluconazole in critically ill surgical patients with invasive mycoses and compromised gastro-intestinal function. Intensive Care Med 27:115–121. doi: 10.1007/s001340000771. [DOI] [PubMed] [Google Scholar]
- 36.Sobue S, Tan K, Layton G, Eve M, Sanderson JB. 2004. Pharmacokinetics of fosfluconazole and fluconazole following multiple intravenous administration of fosfluconazole in healthy male volunteers. Br J Clin Pharmacol 58:20–25. doi: 10.1111/j.1365-2125.2004.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, Roberts JA. 2013. Protein binding of beta-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 57:6165–6170. doi: 10.1128/AAC.00951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


