Abstract
Pyrazinamide (PZA) is a key sterilizing drug in first-line tuberculosis (TB) regimens and exerts its activity entirely during the first 2 months in human infections. We recently described the reduced activity of PZA in C3HeB/FeJ mice with large caseous tubercles due to neutral pH. Here, we aimed to determine the contribution of PZA to the sterilizing activity of the first-line TB regimen in C3HeB/FeJ and BALB/c mice. Three regimens were compared (in combinations: R, rifampin; H, isoniazid; E, ethambutol; Z, pyrazinamide; with numbers indicating the treatment duration, in months): 2RHEZ/4RH, 2RHE/4RH, and 2RHEZ/4RHZ. Lung CFU counts were assessed after 0 and 2 months of treatment, and relapse rates were assessed 3 months after 3, 4.5, and 6 months of treatment. The relapse rates after 3 months of treatment were 53% and 95% in C3HeB/FeJ mice receiving 2RHEZ/1RH and 2RHE/1RH, respectively, and 67%, 100%, and 80% in BALB/c receiving 2RHEZ/1RH, 2RHE/1RH, and 2RHEZ/1RHZ, respectively. The relapse rates after 4.5 months of treatment were 32%, 20%, and 0% in C3HeB/FeJ mice receiving 2RHEZ/2.5RH, 2RHE/2.5RH, and 2RHEZ/2.5RHZ, respectively, and 0% and 67% in BALB/c receiving 2RHEZ/2.5RH and 2RHE/2.5RH, respectively. The month-6 relapse rates were 0%, 13%, and 0% in C3HeB/FeJ mice given 2RHEZ/4RH, 2RHE/4RH, and 2RHEZ/4RHZ, respectively, and 7% in BALB/c mice receiving 2RHE/4RH. The addition of PZA shortens the duration of treatment needed to prevent relapse in both mouse strains. However, while its contribution is limited to the first 2 months of treatment in BALB/c mice, continuing PZA beyond the first 2 months is beneficial in C3HeB/FeJ mice by preventing relapse among those with the highest disease burden.
INTRODUCTION
Pyrazinamide (PZA, Z in combinations) has a unique and important role in the first-line regimen used to treat tuberculosis (TB). Whether administered alone or in combination with other first-line drugs, PZA has little or no effect on viable bacterial counts in sputum over the first 2 weeks of treatment. However, when it is absent from the regimen, the subjects are significantly less likely to have their sputum cultures sterilized by 2 months of treatment (1), and the recommended treatment duration is routinely extended from 6 to 9 months in order to achieve comparable rates of cure without relapse (2). Interestingly, PZA contributes this treatment-shortening, or sterilizing, effect within the first 2 months of treatment (3). Indeed, when PZA was administered in combination with rifampin (RIF, R in combinations), isoniazid (INH, H in combinations), and streptomycin (STR, S in combinations), no difference in relapse was observed between the arms treated with PZA for 2 months (2RHSZ/RH) and the arms treated with PZA for up to 6 months (2RHSZ/RHZ) (3). The limited activity of PZA in the continuation phase of treatment has also been demonstrated in murine models of TB (4, 5). This unique time-limited contribution of PZA in the first-line regimen is attributed to its pH-dependent activity, which is diminished with time with effective treatment, due to changes in lesion pH and/or the number of viable bacilli remaining in sufficiently acidic microenvironments (1).
The time-limited pH-dependent sterilizing activity of PZA in the first-line regimen (1) makes it a useful test drug for qualifying new preclinical models for use in TB drug development. The C3HeB/FeJ mouse has attracted attention recently as a promising murine model because the mice develop caseous necrosis, and even cavities, that other commonly used murine models do not (6, 7). Unlike the nonnecrotic cellular lung lesions found in commonly used mouse strains, like BALB/c, in which the vast majority of infecting bacilli are found intracellularly, C3HeB/FeJ mice harbor abundant extracellular bacilli in the caseous center of necrotic lesions and intracellular bacilli in nonnecrotic lesions and the cellular cuff surrounding necrotic lesions (8, 9). In mice developing large liquefying caseous lung lesions, the bacterial burden is typically at least an order of magnitude higher, and the vast majority of the bacilli are extracellular. However, due to differences in the rate and extent of development of such caseous lesions in C3HeB/FeJ mice, significant heterogeneity in the presence, size, and degree of liquefaction of such lesions is often observed between mice and between lesions within the same mouse at the initiation of treatment (9). We previously demonstrated that PZA monotherapy for 4 to 8 weeks has little or no effect on the overall size of the viable bacterial population in the lungs of C3HeB/FeJ mice with large caseous tubercles, but we demonstrated a bactericidal effect in other C3HeB/FeJ mice without large caseous lesions, which was similar to that in BALB/c mice in which the infecting bacilli are virtually all intracellular (9, 10). This lack of demonstrable bactericidal activity of PZA monotherapy in C3HeB/FeJ mice with large caseous lesions was attributed to the near-neutral pH of the caseum, which presumably abrogates PZA activity against the great majority of infecting bacilli residing extracellularly in the caseum (9, 10). The limited activity of PZA monotherapy in C3HeB/FeJ mice with large caseous lesions raises important questions about whether the activity of PZA in C3HeB/FeJ mice is representative of its sterilizing activity in the first-line regimen against human TB. We recently postulated that in the context of the first-line regimen, PZA exerts its treatment-shortening effect against intracellular bacilli persisting in acidified phagosomal compartments rather than against extracellular bacilli in the liquefied caseum (10). To answer the question of whether PZA retains its treatment-shortening effects in the first-line regimen in C3HeB/FeJ mice with heterogeneous lung lesions, we compared the sterilizing activities of 3 regimens with differing durations of PZA (2RHE/4RH, 2RHEZ/4RH, and 2RHEZ/4RHZ) (E, ethambutol in combinations) in C3HeB/FeJ and BALB/c mice.
(Portions of the results of this study have been presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy, abstract A-004, 17 to 21 September 2015, San Diego, CA [11]).
MATERIALS AND METHODS
Mycobacterial strain.
Mycobacterium tuberculosis strain H37Rv was used as a frozen stock prepared from a log-phase culture in Middlebrook 7H9 broth after mouse passage, and it was diluted in 7H9 broth before infection (12).
Drugs.
RIF, INH, and ethambutol (ETH) were purchased from Sigma-Aldrich (St. Louis, MO). PZA was purchased from Acros Organics (Thermo Fisher Scientific, NJ). All drugs were prepared weekly, dissolved in distilled water only, and stored at 4°C.
Aerosol infection with M. tuberculosis.
All animal procedures were approved by the Animal Care and Use Committee of Johns Hopkins University. Six- to 7-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA, USA) and C3HeB/FeJ mice (Jackson Laboratory, Bar Harbor, ME, USA) were used. Each mouse was infected via aerosol using the inhalation exposure system (Glas-Col, Terre Haute, IN, USA) with 30-fold and 70-fold dilutions of a frozen stock whose titers of M. tuberculosis H37Rv had been determined for BALB/c and C3HeB/FeJ mice, respectively. One day after infection, 2 to 3 mice from each aerosol run were humanely killed to determine the number of bacteria implanted in the lungs.
Study design.
The experimental scheme is detailed in Table 1. Three regimens were tested: the control arm (2RHEZ/4RH), and the same regimen without PZA (2RHE/4RH) or with PZA throughout the course of treatment (2RHEZ/4RHZ). Drugs were administered orally by gavage for 5 days/week. RIF was given at least 60 min before the other drugs to avoid a known drug-drug interaction (13). The doses were 10 mg/kg of body weight of RIF, 10 mg/kg of INH, 100 mg/kg of ETH, and 150 mg/kg of PZA (14).
TABLE 1.
Experimental design
| Treatment regimen by mouse strain | No. of mice intended to be killed at time pointa: |
|||||
|---|---|---|---|---|---|---|
| W-6 | D0 | M2 | M3 + 3 | M4.5 + 3 | M6 + 3 | |
| C3HeB/FeJ | ||||||
| 2RHE/4RH | 6 | 10 | 10 | 20 | 30 | 30 |
| 2RHEZ/4RH | 10 | 20 | 30 | 30 | ||
| 2RHEZ/4RHZ | 30 | 30 | ||||
| Total (n = 256) | 6 | 10 | 20 | 40 | 90 | 90 |
| BALB/c | ||||||
| 2RHE/4RH | 3 | 5 | 5 | 15 | 15 | 15 |
| 2RHEZ/4RH | 5 | 15 | 15 | |||
| 2RHEZ/4RHZ | 15 | |||||
| Total (n = 108) | 3 | 5 | 10 | 45 | 30 | 15 |
W-6, time of M. tuberculosis infection; D0, start of treatment; M2, 2 months after the start of treatment; M3 + 3, relapse assessment 3 months after 3 months of treatment; M4.5 + 3, relapse assessment 3 months after 4.5 months of treatment; M6 + 3, relapse assessment 3 months after 6 months of treatment.
Assessment of treatment efficacy and relapse.
Treatment efficacy was assessed on the basis of lung CFU counts at the completion of 2 months of treatment (M2) and the proportion of mice relapsing with lung CFU detected upon sacrifice 3 months after completing 3, 4.5, and 6 months of treatment (M3 + 3, M4.5 + 3, and M6 + 3, respectively). Serial dilutions of whole-lung homogenates were plated on selective Middlebrook 7H11 agar plates enriched with 10% oleic acid-albumin-dextrose-catalase (OADC) (Becton Dickinson, Franklin Lakes, NJ). The plates were incubated for 6 to 8 weeks at 37°C before determining the final CFU counts. Relapse was assessed by plating the entire lung homogenate.
Assessment of drug resistance.
The selection of mutants resistant to RIF or INH was assessed in all mice at M2 and M3 + 3. Quantitative cultures were performed with 0.5 ml (20%) of the entire lung homogenate plated directly on 7H11 agar supplemented with either 1 mg/liter RIF or 0.2 mg/liter INH. A subset of mice relapsing at M4.5 + 3 with CFU counts of >1 log10 lung CFU among C3HeB/FeJ mice and >3.5 log10 lung CFU in BALB/c mice and all mice relapsing at M6 + 3 had the susceptibilities of the isolates determined indirectly. In this case, colonies isolated initially on drug-free plates were scraped together and suspended by bead beating (0.5-mm sterile beads) in 2.5 ml of phosphate-buffered saline (PBS). Quantitative cultures were performed with 0.5 ml of supernatant from this suspension on selective 7H11 agar with 10% OADC supplemented with either 1 mg/liter RIF or 0.2 mg/liter INH. In all cases in which colonies were isolated on RIF-containing and INH-containing plates, the isolates from each type of drug-containing plate were replated using similar methods on 7H11 agar containing both 1 mg/liter RIF and 0.2 mg/liter INH to exclude multidrug resistance.
Data analysis.
Lung CFU counts (x) were log transformed as log10 (x + 1) before analysis. Group mean CFU counts were compared using nonparametric tests (Mann-Whitney or Kruskal-Wallis, as appropriate) with Prism version 6 (GraphPad Software, San Diego, CA) and Dunn's posttest to adjust for multiple comparisons because of the nonnormal distribution of CFU in the C3HeB/FeJ mice. Relapse rates were compared using a test of proportions with Stata 13.1 (StataCorp, College Station, TX).
RESULTS
Mortality.
Among the 256 C3HeB/FeJ mice infected for the study, 18 mice died before the end of the study: 8 died before the start of treatment due to overwhelming infection, 4 died during treatment (3 of which died during the first 2 weeks of treatment) and were sick before the start of treatment, and 6 died after the end of treatment (4 by drowning accident and 2 from unknown causes). The four drowned mice received 2RHEZ/2.5RHZ and died 13 days after the end of treatment. The two other mice died 13 and 71 days after completing treatment with 2RHEZ/4RHZ. Lung CFU counts were available from 5 out of 6 mice that died after treatment completion and did not yield any CFU, leaving open the possibility that these mice were cured by the treatment.
Among the 108 BALB/c mice infected for the study, only 2 mice died: 1 mouse died before the start of treatment due to overwhelming infection, and 1 mouse died 94 days after the start of treatment from a non-TB cause.
All mice that died before the prespecified endpoint for efficacy assessment were excluded from the analysis of relapse proportion.
BALB/c mice.
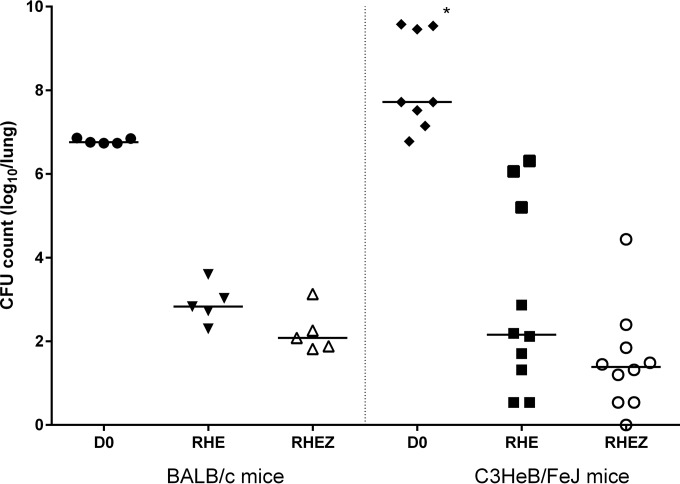
Mean (standard deviation [SD]) lung CFU counts after aerosol infection and at the start of treatment (D0) were 2.44 (0.08) log10 CFU/lung and 6.79 (0.06) log10 CFU/lung, respectively. After 2 months of treatment, the mean (SD) lung CFU counts were 2.23 (0.53) log10 CFU/lung in the control arm (RHEZ) and 2.9 (0.47) log10 CFU/lung in the arm without PZA (RHE) (Fig. 1).
FIG 1.
Lung CFU counts at start of treatment (D0) and after 2 months of treatment in BALB/c (left) and C3HeB/FeJ mice (right). The horizontal bars indicate the median CFU counts. *, these 3 mice were euthanized 3 days before the actual D0 time point due to their moribund status.
The proportions of BALB/c mice relapsing after treatment with 2RHEZ/4RH and 2RHE/4RH are displayed in Fig. 2A. The addition of PZA to the regimen for the first 2 months significantly reduced the proportion of mice relapsing after 3 months (from 100% to 67%, P = 0.014) and after 4.5 months (from 67% to 0%, P < 0.001) of treatment. Only a single relapse was observed in the 2RHE/4RH group after 6 months of treatment. The lung CFU counts at the time of relapse assessment are presented in Fig. 2B. After the completion of 3 and 4.5 months of treatment, the mean CFU counts were significantly lower among mice receiving PZA for the first 2 months (2.21 [1.02] and 0 log10 CFU/lung, respectively) than those in mice that did not receive PZA (3.59 [0.37] log10 CFU/lung and 1.9 [1.59] log10 CFU/lung, respectively; P < 0.001). The relapse proportions and mean CFU counts observed after 3 and 4.5 months of the 2RHZE/4RH regimen were as low as or lower than those observed after 4.5 and 6 months, respectively, of the 2RHE/4RH regimen, indicating that the addition of PZA for the first 2 months reduced the treatment duration necessary to produce similar outcomes by at least 1.5 months (or 25% of the 6-month duration of RHE/RH needed to cure nearly all mice); this was a proportional reduction similar to the 33% reduction in treatment duration (i.e., from 9 to 6 months) that is recommended when PZA is added to rifampin-containing regimens to treat TB patients (2).
FIG 2.
(A and C) Relapse rates 3 months after treatment for 3, 4.5, and 6 months in BALB/c (A) and C3HeB/FeJ mice (C). NS, not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (relapse rates were compared using test of proportions). (B and D) Lung CFU counts at time of relapse in BALB/c (B) and C3HeB/FeJ (D) mice; the relapse rates (%) are located above the data points in each column. The horizontal bars indicate the median CFU counts (group mean CFU counts were compared using nonparametric tests [Mann-Whitney or Kruskal-Wallis, as appropriate]).
Extending the duration of PZA treatment from 2 months to 3 months did not significantly alter either the proportion of mice relapsing after 3 months of treatment (80%) or the mean lung log10 CFU count among relapsing mice (2.48 log10 CFU). Although the effect of longer durations of PZA was not examined in BALB/c mice, the absence of relapses among mice receiving 4.5 months of the control regimen would have negated any opportunity to assess the potential superiority of longer PZA durations.
C3HeB/FeJ mice.
The mean (SD) lung CFU counts after aerosol infection and at start of treatment (D0) were 2.07 (0.04) log10 CFU/lung and 7.38 (0.41) log10 CFU/lung, respectively. After 2 months of treatment, the mean (SD) lung CFU counts were 1.52 (1.24) log10 CFU/lung in the control arm (RHEZ) and 2.89 (2.19) log10 CFU/lung in the arm without PZA (RHE). However, the CFU counts in C3HeB/FeJ mice showed a bimodal distribution, a result previously shown to be attributable to higher CFU counts among mice with large caseous lung lesions (Fig. 1) (9). In a comparison of the median lung CFU counts after 2 months of treatment, the magnitude of the effect of PZA was similar between BALB/c and C3HeB/FeJ mice. Among those C3HeB/FeJ mice in the subset with lower CFU counts, the mean lung CFU count after treatment with either regimen was lower than that observed in BALB/c mice, despite similar CFU counts at treatment initiation (after 2RHEZ, 1.2 [0.74] log10 CFU/lung versus 2.23 [0.53] log10 CFU/lung for C3HeB/FeJ versus BALB/c mice, respectively; after 2RHE, 1.61 [0.87] log10 CFU/lung versus 2.9 [0.47] log10 CFU/lung, respectively), although the difference was not statistically significant.
As in BALB/c mice, the addition of PZA for the first 2 months of treatment significantly reduced the proportion of mice relapsing after 3 months of treatment (from 95% to 53%, P = 0.002) and after 6 months of treatment (from 13% to 0%, P < 0.05) (Fig. 2C). Although the addition of PZA for the first 2 months did not reduce the proportion of mice relapsing after 4.5 months of treatment (20% versus 32%, P = 0.29), it did significantly reduce the mean CFU count among the relapsing mice at this time point (i.e., from 2.8 to 0.6 log10 CFU/lung; P < 0.05) (Fig. 2D). Thus, the benefit of adding PZA for the first 2 months of treatment was evident by at least 1 outcome measure at each relapse time point.
In contrast to the results in BALB/c mice, the administration of PZA beyond the first 2 months improved the sterilizing activity of the regimen in C3HeB/FeJ mice. Although the difference between the proportions of C3HeB/FeJ mice relapsing after 2RHEZ/2.5RH and 2RHE/2.5RH was not statistically significant, treatment with 2RHEZ/2.5RHZ resulted in no relapses, a proportion significantly lower than that produced by 2RHE/2.5RH (P = 0.02) or 2RHEZ/2.5RH (P = 0.003). After 6 months of treatment, no relapses were observed among mice treated with PZA for 2 or 6 months, a result superior to that with treatment with 2RHE/4RH (13% versus 0%, P < 0.05).
Selection of drug-resistant mutants.
After 2 months of treatment, no RIF-resistant mutants were isolated, and INH-resistant mutants were isolated in only three C3HeB/FeJ mice treated with RHE at a proportion of <0.05% of the total CFU count, which could not be discriminated from the background proportion of spontaneous resistance.
After 3 months of treatment, although 37 BALB/c mice relapsed among the 3 treatment groups, none of them harbored resistant mutants. Conversely, among C3HeB/FeJ mice treated with 2RHE/1RH, 6 of 19 relapsing mice harbored RIF-resistant mutants, 2 of which had RIF-resistant mutants comprising 0.5 and 1.5% of the total CFU count. In the remaining 4 mice, RIF-resistant mutants comprised 0.015 to 0.06% of the total CFU count. In addition, 3 mice relapsing after 2RHE/1RH treatment harbored INH-resistant mutants at a proportion of <0.05%. Only 1 of the 6 mice harboring RIF-resistant mutations also had resistant colonies growing on INH-containing plates, but it did not appear to harbor any multidrug-resistant (MDR) clones. In C3HeB/FeJ mice treated with 2RHEZ/1RH, only 2 of 10 relapsing mice harbored RIF-resistant mutants at a proportion of ∼0.01%. The same mice also harbored INH-resistant mutants (all at a proportion of ≤0.05%). However, none of them harbored MDR colonies. The difference in the proportion of mice harboring RIF-resistant mutants between the two treatment groups was not statistically significant (P = 0.24), but the numerically higher frequency of mice harboring RIF-resistant mutants and the higher proportion of RIF-resistant CFU among the total CFU in 2 mice treated without PZA suggest that the addition of PZA may have reduced the selective amplification of RIF-resistant mutants.
No further enrichment of RIF- or INH-resistant mutants was evident among relapsing mice after the completion of 4.5 or 6 months of treatment.
DISCUSSION
This study provides new findings for the emerging pathologically distinct C3HeB/FeJ mouse model and for traditional mouse models of TB chemotherapy, such as the BALB/c mouse model. The C3HeB/FeJ mouse strain has attracted recent interest as an economical and tractable animal model that develops caseous lesions and therefore overcomes some of the principal drawbacks of traditional murine models and larger animal models (15). The qualification of this new model as a new tool for use in TB drug development will require evidence that it yields results consistent with observed clinical outcomes. To that end, our demonstration of the treatment-shortening effect of adding PZA for the first 2 months of treatment in combination with RIF and INH in C3HeB/FeJ mice is consistent with the clinical experience and, as such, provides supportive evidence for the utility of this mouse strain.
Moreover, when considered in the context of our recent studies of PZA monotherapy in C3HeB/FeJ and BALB/c mice, the current results indicate how C3HeB/FeJ mice may add value to the use of traditional mouse strains, like BALB/c mice, stemming from the lesion heterogeneity observed. For example, we recently demonstrated that there is little or no measurable effect of PZA monotherapy for up to 8 weeks on the total bacterial population in the lungs of C3HeB/FeJ mice with large caseous lesions (9). This surprising result, which was in stark contrast to the clear-cut bactericidal activity and dose-response effect of PZA in BALB/c mice and in C3HeB/FeJ mice without large caseous lesions, appears to be due to the near-neutral pH of the liquefying caseum, which prevents the bactericidal effect of PZA (9, 10). For a drug like PZA, which is known to have such important sterilizing activity in the treatment of human TB, to have no measurable effect in some C3HeB/FeJ mice, critical questions are raised about the potential utility of this mouse strain. However, it was noted that even among mice with large caseous lesions in which there was little or no effect on the total bacillary population, PZA monotherapy selectively amplified a PZA-resistant subpopulation, indicating that the drug indeed exerted an effect, most likely against the smaller population of bacilli residing inside activated macrophages in cellular lesions and in the cellular cuffs of granulomas (10). We therefore hypothesized that PZA exerts its sterilizing effect against this same population when used as part of combination therapy. The present results showing that PZA reduced the proportion of mice relapsing after 6 months of treatment, even if it was stopped after the first 2 months (i.e., a point at which PZA monotherapy had little or no measurable effect on the total population in large caseous lesions in the preceding experiments), supports this hypothesis and provides additional evidence of the lesion-dependent activity of PZA that has not been obtained in traditional mouse models.
That the benefit of adding PZA for the first 2 months in C3HeB/FeJ mice was evident in only one of the two outcome measures (i.e., the proportion of mice relapsing and mean CFU count at relapse) after the completion of 3 and 4.5 months of treatment may also be a function of the presence or absence of large caseous lesions causing a bimodal distribution of the bacterial burden. Whereas the uniform lesions among BALB/c mice lead to normally distributed bacterial burdens and monophasic declines in the proportion of mice relapsing over time on treatment, the decline in relapse proportions in C3HeB/FeJ mice occurred in a more stepwise pattern, in which a relative plateau between downward steps occurred between 3 and 4.5 months of 2RHZE/4RH, because the subgroup of mice with less severe disease had been largely cured by 3 months, whereas those with more severe disease would not be cured by 4.5 months of treatment but would still experience a significant decline in lung CFU counts. This finding illustrates the potential importance of measuring CFU counts at the time of relapse assessment in addition to the proportion of mice relapsing in studies using C3HeB/FeJ mice.
Remarkably, although RIF and PZA have been available for testing in animal models for 50 years, this study is the first, to our knowledge, to directly compare the contribution of 2 initial months of PZA to that of longer durations of PZA in combination with RIF and INH. Previous studies in intravenous infection models in mice demonstrated that (i) adding PZA for the first 2 months of a 6-month RIF+INH regimen reduced the number of relapses (4), (ii) adding PZA for the entire duration of a 3.5-month STR+RIF+INH-containing regimen reduced the rate of relapse (16), (iii) adding PZA during only the last 3 months of a 6-month RIF+INH regimen did not reduce the rate of relapse (4), and (iv) PZA monotherapy after 2 months of RIF+INH+PZA treatment has only bacteriostatic activity (5), but we have not directly compared regimens with different PZA durations for which there are clinical data.
Two published clinical trials compared the administration of PZA for the first 2 months to PZA for the entire duration of RIF+INH-containing regimens (3). In these studies, patients randomized to receive either 2RHSZ/2RH or 2RHSZ/2RHZ experienced relatively high rates of relapse (i.e., 8 to 11% in the 2RHSZ/2RH arm versus 11 to 16% in the 2RHSZ/2RHZ arm), whereas those receiving 6-month regimens experienced few relapses (i.e., 2% in the 2RHSZ/4RH arm versus 0% in the 2RHSZ/4RHZ arm). Our results demonstrating no benefit to prolonging the duration of treatment beyond 2 months in BALB/c mice are clearly consistent with these trial results. Our results in C3HeB/FeJ mice are not as straightforward to interpret. Mice receiving 2RHEZ/2.5RH relapsed at a significantly higher rate than mice receiving 2RHEZ/2.5RHZ (of which none relapsed). Although this result appears to be inconsistent with the clinical trial results, a number of important caveats must be considered. First, 5 of the 9 relapsing mice had ≤3 CFU detected, and all but one relapsing mouse had <60 CFU detected at the relapse assessment 3 months after the completion of treatment. Thus, the majority of the mice that relapsed were on the cusp of achieving cure with just 2 months of PZA and presumably would not have relapsed if only a modest extension of PZA treatment had been given. Furthermore, mice were treated 5 days per week with PZA doses selected to produce mean plasma area under the concentration-time curve from 0 h to infinity (AUC0–∞) values of 425.6 mg · h/liter, similar to those of the 20- to 30-mg/kg human dose that is recommended today, whereas the doses of PZA used in the clinical trials were >30 mg/kg, which produce average AUC0–∞ values of 662 to 760 mg · h/liter (17). The higher cumulative PZA exposures in the clinical trials may be expected to more rapidly eradicate the PZA-susceptible subpopulation (10, 18). Finally, the clinical trial regimens included STR rather than ETH. There is some evidence that the sterilizing effect of RIF is greater in the presence of STR than in the presence of ethambutol (19), which might reduce the contribution of PZA. Considering the small sample sizes of the clinical trials (between 40 and 80 per arm with ≥12 months follow-up), the lower dose of PZA used today, and the use of ETH in place of STR, we believe the available data are insufficient to conclude whether extending PZA beyond 2 months in the current first-line regimen would be beneficial, as observed in C3HeB/FeJ mice. While any benefit of prolonging PZA administration would have to be weighed against any increased risk of hepatotoxicity, these results in C3HeB/FeJ mice support new clinical trial protocols that are under development to better define the optimal dose and duration of PZA.
Our study has several limitations. First, we studied only one dose of each drug in the first-line regimen. At the population level, TB patients experience a much greater range of drug exposures than the exposures produced by these doses in mice, especially for RIF and INH. The relative contribution of PZA to the activity of the regimen is likely to be influenced by RIF and INH exposures (20). This is an especially important issue given the recent interest in using higher RIF doses but also because some patients receiving currently recommended doses have very low exposures to these agents, and a previous study in BALB/c mice indicated that PZA adds sterilizing activity beyond the first 2 months of treatment in regimens lacking RIF and INH (21). Studies are under way to better characterize the effect of different RIF exposures on the contribution of PZA. Second, our attempts to quantify the proportion of RIF- and INH-resistant subpopulations at different points during treatment by testing relapse isolates may have been confounded by the reduced fitness of resistant mutants, causing them to decline in numbers relative to the wild-type parent strain during the follow-up period after treatment completion and, in the case of the M4.5 + 3 and M6 + 3 time points, the additional round of growth on culture plates before the resistant proportion was determined.
In conclusion, this study provides the first demonstration that PZA contributes sterilizing activity to the first-line regimen in C3HeB/FeJ mice, a strain considered to be an emerging tool for TB drug development, and it even may help when administered for a longer duration. Together with prior studies indicating the poor activity of PZA in large caseous lesions (9, 22), this study demonstrates the potential value of this heterogeneous disease model for identifying more precisely the lesion microenvironments in which certain drugs exert their activity and contribute to the efficacy of combination therapy. Strategies to increase the proportion of C3HeB/FeJ mice with large caseous lesions are under investigation. If this aspect of human TB that is absent in BALB/c mice can be more uniformly produced in C3HeB/FeJ mice, the two strains may be best employed as complementary models in order to better define the relationships between lesion type and drug efficacy and enable a more refined composition of drug combinations and selection of drug dosing to optimize regimen efficacy for the variety of lesions and microenvironments encountered in human TB. Doing so may further improve the predictive value of preclinical models for clinical trial design.
ACKNOWLEDGMENTS
This work was supported by the Bill and Melinda Gates Foundation under grant OPP1037174 entitled “Qualification of C3HeB/FeJ mice for experimental chemotherapy of tuberculosis” and by the NIH (grant R01-AI111992).
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
We declare no conflicts of interest.
J.-P.L. and E.N. designed the study and wrote the manuscript, and J.-P.L. and F.B. performed the experiments.
REFERENCES
- 1.Zhang Y, Mitchison D. 2003. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 7:6–21. [PubMed] [Google Scholar]
- 2.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O'Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA, American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America . 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 3.Fox W. 1981. Whither short-course chemotherapy? Br J Dis Chest 75:331–357. doi: 10.1016/0007-0971(81)90022-X. [DOI] [PubMed] [Google Scholar]
- 4.Grosset J, Truffot C, Fermanian J, Lecoeur H. 1982. Sterilizing activity of the main drugs on the mouse experimental tuberculosis (author's transl). Pathol Biol (Paris) 30:444–448. (In French.) [PubMed] [Google Scholar]
- 5.Lalande V, Truffot-Pernot C, Paccaly-Moulin A, Grosset J, Ji B. 1993. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob Agents Chemother 37:407–413. doi: 10.1128/AAC.37.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis SL, Nuermberger EL, Um PK, Vidal C, Jedynak B, Pomper MG, Bishai WR, Jain SK. 2009. Noninvasive pulmonary [18F]-2-fluoro-deoxy-d-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob Agents Chemother 53:4879–4884. doi: 10.1128/AAC.00789-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driver ER, Ryan GJ, Hoff DR, Irwin SM, Basaraba RJ, Kramnik I, Lenaerts AJ. 2012. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:3181–3195. doi: 10.1128/AAC.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irwin SM, Driver E, Lyon E, Schrupp C, Ryan G, Gonzalez-Juarrero M, Basaraba RJ, Nuermberger EL, Lenaerts AJ. 2015. Presence of multiple lesion types with vastly different microenvironments in C3HeB/FeJ mice following aerosol infection with Mycobacterium tuberculosis. Dis Model Mech 8:591–602. doi: 10.1242/dmm.019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanoix JP, Lenaerts AJ, Nuermberger EL. 2015. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis Model Mech 8:603–610. doi: 10.1242/dmm.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanoix J-P, Ioerger T, Ormond A, Kaya F, Sacchettini J, Dartois V, Nuermberger E. 16 November 2015. Selective inactivity of pyrazinamide against tuberculosis in C3HeB/FeJ mice is best explained by neutral pH of caseum. Antimicrob Agents Chemother doi: 10.1128/AAC.01370-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanoix J-P, Betoudji F, Nuermberger E. 2015. Prolonging pyrazinamide for more than two months in the standard regimen helps cure C3Heb/FeJ mice in a chronic model of tuberculosis, abstr A-004. Intersci Conf Antimicrob Agents Chemother, 17 to 21 September 2015, San Diego, CA. [Google Scholar]
- 12.Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, Karakousis PC, Grosset JH, Nuermberger EL. 2012. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother 56:4331–4340. doi: 10.1128/AAC.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 36:548–551. doi: 10.1128/AAC.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida D, Nuermberger E, Tasneen R, Rosenthal I, Tyagi S, Williams K, Peloquin C, Grosset J. 2009. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother 53:4178–4184. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. 2015. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis 211(Suppl 3):S83–S95. [DOI] [PubMed] [Google Scholar]
- 16.Toyohara M. 1991. Experimental study on the relapse of tuberculosis after the termination of antituberculous chemotherapy using immunodeficient nude mice. Microbiol Immunol 35:825–830. doi: 10.1111/j.1348-0421.1991.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 17.Donald PR, Maritz JS, Diacon AH. 2012. Pyrazinamide pharmacokinetics and efficacy in adults and children. Tuberculosis (Edinb) 92:1–8. doi: 10.1016/j.tube.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Gumbo T, Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 53:3197–3204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jindani A, Doré CJ, Mitchison DA. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med 167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 20.Chigutsa E, Pasipanodya JG, Visser ME, van Helden PD, Smith PJ, Sirgel FA, Gumbo T, McIlleron H. 2015. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 59:38–45. doi: 10.1128/AAC.03931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad Z, Tyagi S, Minkowski A, Peloquin CA, Grosset JH, Nuermberger EL. 2013. Contribution of moxifloxacin or levofloxacin in second-line regimens with or without continuation of pyrazinamide in murine tuberculosis. Am J Respir Crit Care Med 188:97–102. doi: 10.1164/rccm.201212-2328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin SM, Gruppo V, Brooks E, Gilliland J, Scherman M, Reichlen MJ, Leistikow R, Kramnik I, Nuermberger EL, Voskuil MI, Lenaerts AJ. 2014. Limited activity of clofazimine as a single drug in a mouse model of tuberculosis exhibiting caseous necrotic granulomas. Antimicrob Agents Chemother 58:4026–4034. doi: 10.1128/AAC.02565-14. [DOI] [PMC free article] [PubMed] [Google Scholar]