Abstract
Hospitalized ill patients, at risk for invasive candidiasis, often receive multiple medications, including proton pump inhibitors (PPIs). The antifungal fluconazole perturbs the vacuolar proton ATPase. The PPI omeprazole antagonized Candida albicans growth inhibition by fluconazole. A C. albicans codon-adapted pHluorin, Ca.pHluorin, was generated to measure cytosolic pH. The fungal cytosol was acidified by omeprazole and realkalinized by coexposure to fluconazole. Vacuolar pH was alkalinized by fluconazole. Off-target effects of any medication on fungal pathogens may occur.
TEXT
Candida species are the fourth most common cause of nosocomial bloodstream infections (1). Invasive candidiasis is a disease of ill and hospitalized patients. These patients often receive a large number of medications, of which the impact on the infecting Candida cells is not usually considered.
Animals and fungi are closely related among the eukaryotes; both phyla are members of the opisthokonts. Drugs approved for various human indications, therefore, have been examined for antifungal activity (2, 3), since molecular targets are often conserved between humans and fungi. Repurposed drugs, like the estrogen receptor antagonist tamoxifen (2) and the antiarrhythmic amiodarone (4), have antifungal activity alone and in combination with the antifungal fluconazole.
Fluconazole inhibits the ergosterol biosynthetic enzyme Cyp51/Erg11, consequently disrupting membrane functions. An important mechanism of its fungistatic activity is perturbation of the vacuolar proton ATPase (V-ATPase), resulting in loss of vacuolar acidification (5). We wondered whether perturbing V-ATPase may additionally lead to cytosolic acidification, if protons generated by metabolic processes are not efficiently sequestered in the vacuole. If this were the case, the antifungal activity of fluconazole might be potentiated by other drugs which also acidify the cytosol.
Proton pump inhibitors (PPIs) sold over the counter, like omeprazole, or by prescription, like rabeprazole, are taken by millions of patients each year (6). In 2009, a PPI was prescribed at 79.4 million physician visits in the United States (7). Omeprazole has an antifungal effect and is known to inhibit the fungal plasma membrane proton ATPase Pma1 (8).
We wanted to test the idea that blocking the outflow of protons from the fungal cytosol into the extracellular space with omeprazole (8), while perturbing proton pumping from the cytosol into the vacuole with fluconazole (5), may result in toxic acidification of the cytosol and antifungal synergy. We first confirmed the inhibitory effect of omeprazole on Candida albicans (Fig. 1A).
FIG 1.
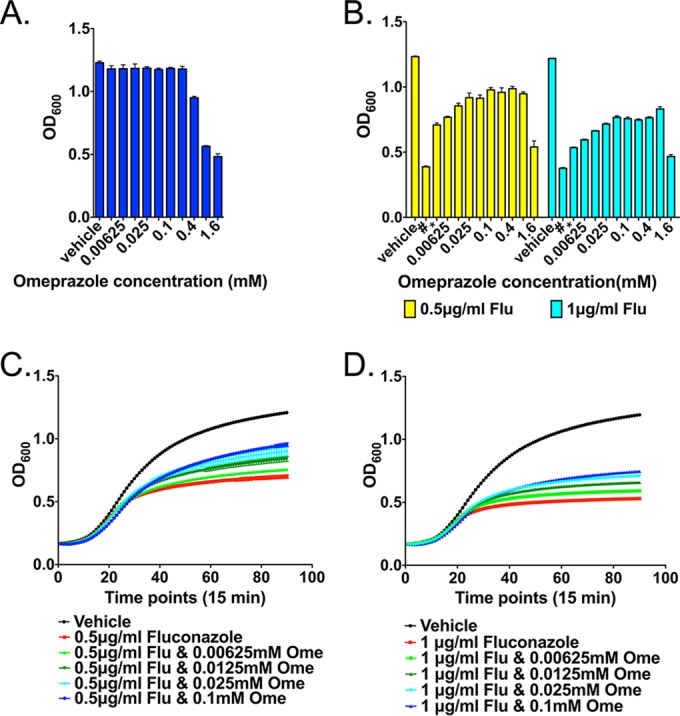
Effect of omeprazole (Ome), fluconazole (Flu), and their combination on the growth of C. albicans. (A) Effect of omeprazole on C. albicans growth. Results shown are representative of three biologically independent experiments, each comprising four technical replicates; error bars represent the standard deviation. (B) Antagonistic effect of fluconazole and omeprazole on the growth of C. albicans. Results shown are representative of three biologically independent experiments. Error bars represent standard deviations of four technical replicates. (C and D) Dose-dependent rescue of the fluconazole fungistatic effect by omeprazole, at fluconazole doses of 0.5 μg/ml (C) and 1 μg/ml (D). Error bars represent standard deviations of four technical replicates. Results shown are representative of three biologically independent experiments.
To test the effect of the drug combination, overnight-grown cells were diluted to an optical density at 600 nm (OD600) of 0.1 in microtiter plates containing RPMI 1640 (Lifetech) with 2% glucose, buffered to a pH of 7.0 with 165 mM MOPS, to a pH of 5 with 50 mM MES, or to a pH of 3.0 with 50 mM sodium citrate. The medium contained 2-fold serial drug dilutions or the drug vehicle dimethyl sulfoxide. The OD600 was measured after 24 h of incubation at 35°C. Experiments involving omeprazole, which needs to be activated to the H+K+-ATPase-binding sulfenamide by low pH (8) (and whose active sulfenamide form is unstable at a pH >4), were performed at a pH of 3, after confirming fluconazole activity at a pH of 3 (see Fig. S1 in the supplemental material). Rabeprazole, which has a molecular interaction with the gastric H+K+-ATPase that differs slightly from that of omeprazole, and which is activated in a higher pH but is less stable at a neutral pH than omeprazole (9), was tested at a pH of 7, after preactivation of the compound for 1 h in the presence of 0.1 M HCl prior to addition to culture media. Cells were inoculated at an OD600 of 0.1 into RPMI, which was buffered to a pH of 3 or 7, as above, and contained 2-fold decreasing omeprazole or rabeprazole concentrations. Cultures were incubated at 35°C for 24 h and monitored for the OD600. For growth curve experiments, wild-type C. albicans was grown overnight on a yeast extract-peptone-dextrose agar medium at 30°C, and cells were diluted to an initial OD600 of 0.1 in RPMI medium 1640 (pH 3). Cells were inoculated into 96-well plates with the addition of drugs as noted and incubated at 35°C, and the OD600 was read automatically for 24 h at 15-min intervals.
Instead of synergistic or additive growth inhibition when combining fluconazole with a proton-pump inhibitor, we observed an antagonistic effect: omeprazole and its analog rabeprazole rescued cells from growth inhibition by fluconazole (Fig. 1B to D; see also Fig. S2 in the supplemental material).
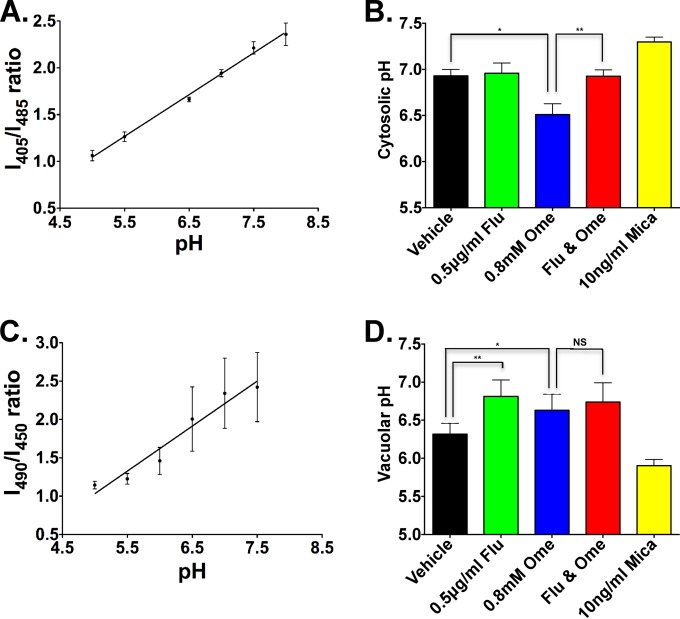
To directly examine the effect of these drugs on cytosolic pH, we adapted a ratiometric pHluorin protein (10) to C. albicans codon usage. The synthesized gene (GenScript, Piscataway, NJ) was cloned into plasmid pJK1027 (11) behind the strong ACT1 promoter and integrated at the ACT1 locus in strain JKC915 (12) to create JKC1559, the strain used in this study. The cytosolic pH of cells exposed to fluconazole, omeprazole, and their combination was determined, as described in reference 13, by measuring the fluorescence emission at 508 nm with excitation at 405 nm and 485 nm and calculating the pH using a calibration curve (Fig. 2A and B). To generate the calibration curves, pHluorin-expressing, permeabilized cells were equilibrated in 6 buffers of increasing pH (pH 5 to 8). Then, 20 μl of cell suspension was added to 2 ml of calibration buffer and incubated at 30°C for 60 min before obtaining measurements of emission intensity at 508 nm during excitation at 405 nm and at 485 nm. Three biological replicates, each comprising 3 technical replicates, were obtained for each condition. All measurements were graphed and statistically analyzed using Prism software (GraphPad software, San Diego, CA), applying Student's two-tailed t test to calculate the P values.
FIG 2.
Effect of omeprazole (Ome), fluconazole (Flu), and their combination on the pH in intracellular compartments of C. albicans. (A and B) Cytosolic pH of cells exposed to vehicle alone, fluconazole, omeprazole, and their combination. (A) Calibration curve showing the measured ratio of fluorescence intensity at 405 nm to intensity at 485 nm (I405∕I485) of pHluorin-expressing, permeabilized cells, equilibrated in buffers of increasing pH. (B) Cytosolic pH of prototrophic C. albicans cells exposed to the indicated drugs. The cytosolic pH measurement was performed as described in reference 13 and calculated according to the calibration curve. Cells treated with 10 ng/ml of micafungin were used as a control. Data are reported as mean ± standard deviation (SD) of three biologically independent experiments with three technical replicates each. Statistical comparisons were performed with Student's two-tailed t tests (paired). *, P = 0.0025; **, P = 0.0016; ***, P = 0.0021. (C and D) The effect of omeprazole and fluconazole on the vacuolar pH. (C) Calibration curve showing measured ratio of fluorescence intensity at 490 nm to intensity at 450 nm (I490∕I450) versus pH of cells preincubated with BCECF, subsequently permeabilized and equilibrated in buffers of increasing pH. (D) Vacuolar pH of prototrophic C. albicans cells exposed to the indicated drugs. The vacuolar pH measurement was performed as described in reference 13 and calculated according to the calibration curve. Cells treated with 10 ng/ml of micafungin were used as a control. Data are reported as mean ± standard error of the mean (SEM), and statistical comparisons were performed with Student's two-tailed t tests. Data are reported as mean ± SD from three biologically independent experiments with three technical replicates each. *, P = 0.0175; **, P = 0.0109; NS, P = nonsignificant.
The cytosolic pH of omeprazole-treated cells dropped, consistent with the concept that omeprazole inhibits Pma1 (Fig. 2B). Contrary to our prediction, the cytosol of fluconazole-treated cells was not acidified (Fig. 2B). Adding fluconazole to omeprazole normalized the cytosolic pH (Fig. 2B), antagonizing the effect of omeprazole. Low concentrations of the β-1,3 d-glucan synthase inhibitor micafungin, as a control for general fungal cell damage, alkalinized cytosolic pH (Fig. 2B), confirming the specificity of the effect of each drug.
To test whether omeprazole antagonized alkalinization of the fungal vacuole caused by fluconazole (5), we measured the vacuolar pH of cells exposed to these drugs (Fig. 2C and D), using BCECF-AM (2′7′-bis-[2-carboxyethyl]-5-[and 6]-carboxyfluorescein acetoxymethyl ester; Lifetech), a pH-sensitive fluorophore that accumulates in the yeast vacuole, as in reference 13. To generate calibration curves (Fig. 2C), cells preincubated with BCECF were permeabilized and equilibrated in 6 buffers of increasing pH. Then, 20 μl of cell suspension was added to 2 ml of calibration buffer (pH 5 to 8) and incubated at 30°C for 60 min, as described in reference 13. Replicates and calculations were performed as for the cytosolic pH. Fluconazole-exposed cells lost vacuolar acidification, as expected (5) (Fig. 2D). Contrary to our expectation, the vacuolar pH of omeprazole-treated cells, and of cells treated with both drugs, also showed a trend toward alkalinization (Fig. 2D). Exposure to a low concentration of micafungin strongly acidified the vacuole, confirming the specific effect of fluconazole and omeprazole on the vacuolar pH. We did not identify the mechanism by which omeprazole antagonizes the fungistatic effect of fluconazole.
We examined omeprazole concentrations in the range of 10 μM, as they occur in human plasma during omeprazole treatment with standard doses, e.g., for gastric reflux disease (14), for the interaction studies with fluconazole. At these concentrations, omeprazole alone had no effect on C. albicans growth (Fig. 1A) but did antagonize the fungistatic effect of fluconazole (Fig. 1B to D). Only at 40-fold-higher concentrations did omeprazole alone exert an inhibitory effect on C. albicans (Fig. 1A). In clinical practice, such high concentrations are unlikely to occur, so we do not expect omeprazole alone to have utility as an antifungal agent. We tested rabeprazole, which is activated at slightly higher pH than omeprazole, at a pH of 7. We found that, under these conditions, its concentrations antagonizing fluconazole were ∼32-fold higher than standard plasma levels during rabeprazole therapy (15). The pH of spaces occupied by C. albicans during invasive disease is likely to range from neutral to acidic, since inflammatory foci like abscesses can reach proton concentrations to a pH of 5.5 (16). Reproducibility of antagonism between fluconazole and a second PPI, albeit in higher-than-clinical concentrations at a neutral pH, supports the possibility of clinical antagonism of this drug class with fluconazole. Confirmation of this possibility would require in vivo studies.
Homeostatic mechanisms of cytosolic and vacuolar pH, which include cation and anion pumps and intracellular protein buffers (17), are central to the function of multiple critical enzymatic processes (18). The net effect of these multiple homeostatic mechanisms apparently needs to be experimentally determined for each pharmacological perturbation, with respect to fungal growth and to pH in important fungal cellular compartments. With the C. albicans-adapted pHluorin, we have generated a molecular tool to facilitate such experiments.
In conclusion, critical assessment of all drugs used in treating ill patients is necessary, not only because of potential drug interactions in the patient. Apparently, in patients with invasive fungal disease, drugs active on human targets may also elicit unexpected and undesirable effects on the infecting fungus. Given the very widespread usage of PPIs (6), it may not be possible to confirm this effect in a clinical study, since a sufficiently large patient group not prescribed a PPI may be difficult to recruit. Nevertheless, our results underscore the importance of critically assessing patient needs for long-term medications and of avoiding unnecessary medication use (6). In addition, they underscore the importance of testing not only human pharmacokinetics (19) but also pharmacodynamics toward the fungus of drug combinations for invasive fungal infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank Patricia Kane, Karlett Parra and Rajini Rao for technical tips on intracellular pH measurements. We thank Susan Lindquist and Luke Whitesell for use of, and training with, the Fluorolog spectrofluorometer. We thank Paula Watnick and Robert Husson for helpful comments on the manuscript.
This work was supported by NIAID R01AI095305 and has not been previously presented.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02043-15.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Butts A, Koselny K, Chabrier-Rosello Y, Semighini CP, Brown JC, Wang X, Annadurai S, DiDone L, Tabroff J, Childers WE Jr, Abou-Gharbia M, Wellington M, Cardenas ME, Madhani HD, Heitman J, Krysan DJ. 2014. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio 5:e00765–00713. doi: 10.1128/mBio.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, Wright GD. 2011. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol 7:499. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamarra S, Rocha EM, Zhang YQ, Park S, Rao R, Perlin DS. 2010. Mechanism of the synergistic effect of amiodarone and fluconazole in Candida albicans. Antimicrob Agents Chemother 54:1753–1761. doi: 10.1128/AAC.01728-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YQ, Gamarra S, Garcia-Effron G, Park S, Perlin DS, Rao R. 2010. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog 6:e1000939. doi: 10.1371/journal.ppat.1000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidelbaugh JJ, Goldberg KL, Inadomi JM. 2009. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk. Am J Gastroenterol 104 (Suppl 2):S27–S32. doi: 10.1038/ajg.2009.49. [DOI] [PubMed] [Google Scholar]
- 7.Gawron AJ, Feinglass J, Pandolfino JE, Tan BK, Bove MJ, Shintani-Smith S. 2015. Brand name and generic proton pump inhibitor prescriptions in the United States: insights from the national ambulatory medical care survey (2006 to 2010). Gastroenterol Res Pract 2015:689531. doi: 10.1155/2015/689531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monk BC, Mason AB, Abramochkin G, Haber JE, Seto-Young D, Perlin DS. 1995. The yeast plasma membrane proton pumping ATPase is a viable antifungal target. I: effects of the cysteine-modifying reagent omeprazole. Biochim Biophys Acta 1239:81–90. [DOI] [PubMed] [Google Scholar]
- 9.Shin JM, Kim N. 2013. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil 19:25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miesenböck G, De Angelis DA, Rothman JE. 1998. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 11.Patenaude C, Zhang Y, Cormack B, Köhler J, Rao R. 2013. Essential role for vacuolar acidification in Candida albicans virulence. J Biol Chem 288:26256–26264. doi: 10.1074/jbc.M113.494815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen J, Cowen LE, Griffin AM, Chan L, Köhler JR. 2008. The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation. Proc Natl Acad Sci U S A 105:20918–20923. doi: 10.1073/pnas.0809147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diakov TT, Tarsio M, Kane PM. 2013. Measurement of vacuolar and cytosolic pH in vivo in yeast cell suspensions. J Vis Exp 74:e50261. doi: 10.3791/50261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen JB, Lundborg P, Baak LC, Greve J, Ohman M, Stover C, Rohss K, Lamers CB. 1988. Effect of single and repeated intravenous doses of omeprazole on pentagastrin stimulated gastric acid secretion and pharmacokinetics in man. Gut 29:75–80. doi: 10.1136/gut.29.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Román M, Ochoa D, Sanchez-Rojas SD, Talegon M, Prieto-Perez R, Rivas A, Abad-Santos F, Cabaleiro T. 2014. Evaluation of the relationship between polymorphisms in CYP2C19 and the pharmacokinetics of omeprazole, pantoprazole, and rabeprazole. Pharmacogenomics 15:1893–1901. doi: 10.2217/pgs.14.141. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Bae DJ, Kim MJ, Piao ML, Kim IS. 2012. Extracellular low pH modulates phosphatidylserine-dependent phagocytosis in macrophages by increasing stabilin-1 expression. J Biol Chem 287:11261–11271. doi: 10.1074/jbc.M111.310953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey JR, Grinstein S, Orlowski J. 2010. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Muñoz GA, Kane P. 2008. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J Biol Chem 283:20309–20319. doi: 10.1074/jbc.M710470200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niece KL, Boyd NK, Akers KS. 2015. In vitro study of the variable effects of proton pump inhibitors on voriconazole. Antimicrob Agents Chemother 59:5548–5554. doi: 10.1128/AAC.00884-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.