Abstract
A marine-derived compound, abalone hemocyanin, from Haliotis rubra was shown to have a unique mechanism of antiviral activity against herpes simplex virus 1 (HSV-1) infections. In vitro assays demonstrated the dose-dependent and inhibitory effect of purified hemocyanin against HSV-1 infection in Vero cells with a 50% effective dose (ED50) of 40 to 50 nM and no significant toxicity. In addition, hemocyanin specifically inhibited viral attachment and entry by binding selectively to the viral surface glycoproteins gD, gB, and gC, probably by mimicking their receptors. However, hemocyanin had no effect on postentry events and did not block infection by binding to cellular receptors for HSV. By the use of different mutants of gD and gB and a competitive heparin binding assay, both protein charge and conformation were shown to be the driving forces of the interaction between hemocyanin and viral glycoproteins. These findings also suggested that hemocyanin may have different motifs for binding to each of the viral glycoproteins B and D. The dimer subunit of hemocyanin with a 10-fold-smaller molecular mass exhibited similar binding to viral surface glycoproteins, showing that the observed inhibition did not require the entire multimer. Therefore, a small hemocyanin analogue could serve as a new antiviral candidate for HSV infections.
INTRODUCTION
The predominant antiviral therapies for herpes simplex virus (HSV) infections are nucleoside analogue inhibitors, such as acyclovir, its prodrug valacyclovir, famciclovir (a prodrug of penciclovir), and the second line of drugs for resistant virus, foscarnet and cidofovir. These drugs are all inhibitors of viral DNA polymerase (1). The variety is limited despite the fact that HSV has more than 80 genes that are required for its functionality and, therefore, could potentially be targeted by multiple types of inhibitors (2). Thus, improving the efficacy of current HSV treatment relies on the discovery of new antiviral compounds targeting various functions of the virus, preferably earlier stages of the HSV viral life cycle such as viral attachment and entry.
Viral attachment and entry are regulated by surface glycoproteins gC, gB, gD, and gH-gL (3, 4). Attachment is a two-step process involving the primary interaction of gC and/or gB with heparan sulfate proteoglycans (HSPG), followed by the secondary gD-mediated binding to its receptors, such as herpesvirus entry mediator (HVEM), nectin-1, or 3-O-sulfated heparan sulfate. This interaction triggers the activation of gH-gL and gB, which leads to the fusion of viral envelope and plasma membrane of the host cell either at the surface or in the endosomes (3, 4). Many steps in the entry process of HSV remain unclear; however, it is known that gD determines HSV tropism and that the conformational changes in gD upon binding to its receptors are critical in triggering an activation cascade (5, 6).
Viral attachment or entry could be inhibited by mimicking cellular receptors that are involved in these events. For example, heparin interacts with HSPG binding domains in gB and gC, thereby inhibiting viral attachment to cells and, subsequently, infection (7). This interaction is electrostatically driven and occurs between negatively charged carboxyl and sulfate groups on heparin and the lysine-rich domains in gB and gC, known as the PK region (8). The inhibitory effect of heparin is, however, limited to viral binding (9).
Attempts to find novel inhibitors of HSV binding and entry have directed many researchers toward natural products such as small molecules, including phenols, polyphenols, flavonoids, sugar-containing compounds, and peptides (10). Recently, it was shown that hemolymph from molluscs has antiviral activity against herpes simplex virus 1 (HSV-1) (11–15). The hemolymph contains hemocyanin as its major constituent (16). However, endogenous antimicrobial peptides are also present or synthesized and secreted into hemolymph upon microbial challenge (11, 17–19). Several studies reported that the antiviral activity of hemolymph against HSV-1 is related to hemocyanin (20–22). However, peptides that were extracted from the hemolymph also exhibit antiviral activity (23). Hemocyanin is a large copper-containing glycoprotein (8,000 kDa), and its main function is to circulate, bind, and release oxygen in the animal's tissues (24). The native hemocyanin protein has two isoforms, contains high negative-charge density on its surface, and exists as didecamers that are comprised of 10 dimer subunits (with molecular mass of ∼800 kDa) (16, 25, 26). Although the antiviral activity of abalone hemocyanin on HSV has been demonstrated, its mechanism of action has yet to be elucidated (12).
The aim of this study was to provide a mechanistic understanding of the antiviral activity of hemocyanin from abalone Haliotis rubra against HSV-1 infections. Hemocyanin was shown to be the active compound after its isolation and concentration from the hemolymph by the use of ultrafiltration. More importantly, it was shown that hemocyanin inhibits viral attachment and entry, and the highest inhibition was obtained when it was preincubated with the virus rather than the cells. We demonstrated that this effect is due to hemocyanin binding selectively to gB, gC, and gD. In addition, we used mutant viral glycoproteins and demonstrated that the lysine-rich region in gB is the binding site for hemocyanin. Furthermore, hemocyanin appears to mimic nectin-1 in binding to gD. Our findings show that hemocyanin has a potential as a new therapeutic approach against HSV-1 by acting as a mimetic of cellular receptors for viral binding and attachment.
MATERIALS AND METHODS
Materials.
Hemolymph from Tasmanian abalone H. rubra was supplied by the Marine Biotechnology Australia Pty Ltd. African green monkey kidney (Vero) cells were propagated in Dulbecco modified Eagle medium (DMEM) (Lonza) supplemented with 5% fetal bovine serum (FBS) (Sigma) and 1% penicillin-streptomycin (Life Technologies). Viruses were HSV-1 F strain (27), vUL37-GFP (green fluorescent protein-labeled) HSV-1 (strain 17) (kindly provided by Frazer Rixon, MRC Virology Unit, Institute of Virology, United Kingdom) (28), and HSV F-GS2822 (kindly provided by Greg Smith, Northwestern University, USA) (29). The virus titer was calculated using a fluorescent focus assay on Vero cells (30). Polyclonal rabbit serum against gD (R7) (31), gB (R68) (32), and gC (R47), along with recombinant glycoproteins gD-1 (306t), gD-1 (rid), gC-1 (214t) (33–35), gB-1 (730t), and gB-1 (PK−) (36–38) were a kind gift of Gary H. Cohen and Roselyn J. Eisenberg (University of Pennsylvania, USA). Anti-hemocyanin antibody was raised against whole abalone hemocyanin (didecamer) in rabbits at Flinders University. Other antibodies included anti-rabbit IgG (whole molecule)–peroxidase antibody (Sigma), herpes simplex virus 1 antibody–horseradish peroxidase (HRP) conjugate (Thermo Scientific), Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen), rabbit polyclonal antibody against HSV-1 VP16 tegument (Abcam), rabbit anti-CMV-pp65 antibody (Abbiotec), and normal rabbit IgG (Invitrogen).
Virus purification.
Vero cells at 90% confluence were infected with HSV-1 at a multiplicity of infection (MOI) of 0.01 PFU/ml and were incubated for 2 to 3 days or until full cytopathic effect (CPE). For vUL37-GFP HSV-1 and HSV F-GS2822, cells were lysed by three cycles of freezing and thawing and two cycles of sonication (Branson Digital 450 cup sonifier) at 70% amplitude for 30 s in ice-cold water with a 20-s pause between each pulse. The cells were then pelleted at 1,000 × g (Heraeus Multifuge X3R centrifuge; Thermo Scientific) for 15 min at 4°C, and supernatant was aliquoted and stored at −80°C. For F strain, the infected cells were harvested and pelleted at 210 × g for 5 min and then lysed to release virus by three cycles of sonication under the same conditions as described above. Subsequently, the cells were centrifuged at 1,000 × g for 30 min to remove cell debris. The resulting supernatant was centrifuged for 2 h at 4°C using a type 70 Ti rotor at 23,000 × g (Optima XPN-100 ultracentrifuge; Beckman Coulter). The pellets were resuspended in phosphate-buffered saline (PBS) and layered onto a 20% sucrose cushion and centrifuged for 2 h at 4°C using an SW 32 Ti rotor at 26,000 × g with no brake. The pellet was resuspended in PBS and ultracentrifuged for 2 h at 4°C using a type 70 Ti rotor at 80,000 × g to remove sucrose. The pellet was resuspended in PBS and stored at −80°C prior to titering and subsequent use in an enzyme-linked immunosorbent assay (ELISA).
Purification of hemocyanin.
Hemolymph was collected and centrifuged at 4,000 × g at 4°C for 15 min to remove blood cells. Cell-free hemolymph is at neutral pH and is here called abalone serum. Purification of hemocyanin from abalone serum was performed by ultrafiltration as described elsewhere (39). The ultrafiltration was repeated for three cycles under the same conditions, and after each cycle, samples of retentate and permeate were pooled and the protein concentration and composition were determined using Qubit assay and blue native PAGE as described previously (39). Freshly prepared filtered protein samples, which consisted mainly of didecamer species but also lower-molecular-mass species, especially dimer subunits, were diluted in DMEM and used in in vitro tests.
Preparation of dimer subunits.
A region of the blue native PAGE gel containing hemocyanin dimer subunits (700- to 800-kDa region) was excised, cut into small pieces, and then placed in dialysis bags (molecular weight cutoff, 7,000 Da) with 1 to 1.5 ml native PAGE running buffer. The dialysis bags were submerged in the same buffer in a horizontal electrophoresis tank for elution at 200 V for 2 h. Subsequently, the native PAGE running buffer was pooled and filtered (Amicon ultracentrifugal filter units, 30 kDa; Millipore) to concentrate dimer subunits. The protein concentration in the resulting retentate was determined by reading the absorbance at 280 nm (Nano Drop 2000; Thermo Scientific). The molar extinction coefficient was calculated based on the number of tryptophan and tyrosine residues in the amino acid sequence of Haliotis. tuberculata available in the NCBI database. After that, 0.1% absorbance was calculated as the ratio of the molar extinction coefficient to the molecular mass of H. tuberculata subunit.
Gel filtration chromatography.
Gel filtration chromatography was applied to monitor the composition of hemocyanin before and after purification. The high-performance liquid chromatography (HPLC) system consisted of a Shimadzu CBM-20A module (Shimadzu, Kyoto, Japan) equipped with an LC-20AT delivery unit with a DGU-20As vacuum degasser, an SIL-20AC auto injector, and an SPD-M20A diode array detector. Chromatographic separation was conducted in size exclusion mode using a column packed with methacrylate hydrogel (PL aquagel-OH MIXED-H, 8 μm, 300 by 7.5 mm; Agilent Technologies) maintained at 40°C. The mobile phase consisted of 100 mM Tris-HCl buffer with a pH of 7.4 and was delivered isocratically at a flow rate of 0.8 ml/min. UV spectra were collected in the 200- to 400-nm range for peak detection and determination of peak purity. The injection volume was 30 μl, and samples were filtered through a 0.22-μm low protein binding nylon syringe filter prior to injection.
Cytotoxicity assay.
The effect of hemocyanin on cell viability was assessed using the WST-1 assay as described previously and according to the manufacturer's specifications (Roche) (39).
Antiviral infectivity assay in Vero cells.
The antiviral activity of abalone serum and hemocyanin against vUL37-GFP HSV-1 was determined using a fluorescent focus assay as described before (39). Abalone serum/hemocyanin and HSV-1 were added to the cells together at 1:1 volume ratio, at final concentrations of 20, 37.5, 75, 156, and 312 nM and an MOI of 0.01 PFU/ml. An optimal virus concentration was determined to produce 100 to 150 fluorescent foci per well in the control (Vero cells infected with the virus but not treated with hemocyanin). The number of green fluorescent foci for each sample was counted manually with a Leica upright fluorescence microscope, and the percent reduction in the number of fluorescent foci was calculated relative to the control. This experiment was performed in triplicate.
Kinetics of antiviral activity.
For the experiments discussed below, hemocyanin was used at a concentration of 150 nM and vUL37-GFP HSV-1 was used at an MOI of 0.01 PFU/ml in 1% DMEM supplemented with 1% FBS (pH 7.5) unless otherwise stated. The hemocyanin concentration was 3-fold below the concentration of sample at which 50% of cells are viable (CC50) and 4-fold above the effective concentration of hemocyanin causing 50% reduction in the number of fluorescent foci (EC50) (Table 1). The MOI was adjusted to obtain 150 to 200 nonconfluent fluorescent foci per coverslip of the infected controls (cells infected but not treated with hemocyanin). These experiments were performed twice in triplicate.
TABLE 1.
Effect of repeated ultrafiltration on the protein concentration in the retentate and permeate
| Sample | Protein concn (mg/ml) after indicated no. of ultrafiltration cycles |
|||
|---|---|---|---|---|
| None | One | Two | Three | |
| Retentate | 8.81 | 11.96 | 12.91 | 11.79 |
| Permeate | 0.274 | 0.262 | 0.167 | |
Viral binding and entry versus postentry assay.
Vero cells were pretreated with hemocyanin for 2 h at 37°C, which was then replaced with virus and hemocyanin at a 1:1 volume ratio. The cultures were then incubated for 2 h at 37°C to study the effect of hemocyanin on viral attachment and entry. The cells were then washed with PBS three times to remove unbound virus and hemocyanin. To study the effect of hemocyanin on postentry events, virus was added to Vero cells at 37°C for 2 h. Unbound virus was removed by three washes in PBS, and then medium containing hemocyanin was added to the cells for 2 h at 37°C. Hemocyanin was then removed, and the cultures were washed with PBS three times. Fluorescent foci were counted manually as described above. The infected control consisted of cells infected but not treated with hemocyanin, and the mock control consisted of cells treated with hemocyanin but not infected with virus.
Effect of hemocyanin on cellular receptors.
Vero cells were treated with hemocyanin for 2 h at 37°C. Hemocyanin was removed, and the cells were washed before the addition of virus for 2 h at 37°C. Following the removal of unbound virus and incubation for a total of 48 h, the cells were fixed and fluorescent foci were counted as described above. The infected control consisted of cells that were not pretreated with hemocyanin, and the mock control consisted of cells that were treated with hemocyanin but not infected with virus.
Direct HSV-hemocyanin binding assay.
Virus was exposed to 300 nM hemocyanin or 10.5 μM heparin at a 1:1 volume ratio and incubated at 25°C or 4°C for 20 min. The mixtures were then added to Vero cells and incubated at 37°C for 2 h. The cells were washed three times to remove unbound virus and hemocyanin/heparin and incubated for 48 h at 37°C in carboxymethyl cellulose (CMC)-containing medium. Cells were then fixed, and fluorescent foci were counted. The infected control consisted of virus mixed with medium, and the mock control consisted of hemocyanin mixed with medium.
ELISA. (i) Optimization of surface coating with hemocyanin.
Serial dilutions of purified hemocyanin (from 0.002 to 3.14 nM) or dimer subunit (from 0.003 to 5.7 nM) in 0.1 M carbonate buffer (pH 9.4) were added to MaxiSorp 96-well plates (Nunc) in two replicates and incubated overnight at 4°C. The plate was then washed with washing solution POD (Enzygnost), blocked with StabilGuard immunoassay stabilizer, BSA free (SurModics), for 30 min at room temperature, and washed three times before the addition of anti-hemocyanin antibody (1:8,000) in PBS supplemented with 1% Tween 20 and 2% FCS (PBS-T-FCS). Following 2 h of incubation at room temperature, the plate was washed three times and anti-rabbit IgG (whole molecule)–peroxidase antibody (1:15,000) in PBS-T-FCS was added for 1 h at room temperature. The plate was then washed three times, followed by the addition of the substrate (tetramethylbenzidine [TMB]; Enzygnost) for 15 to 18 min at room temperature. The reaction was stopped with stopping solution POD (Enzygnost). The optical density (OD) was then read at 450 nm using a plate reader (Thermofisher Scientific). A background well contained hemocyanin/dimer subunit incubated with anti-rabbit IgG (whole molecule)–peroxidase antibody in the absence of anti-hemocyanin antibody.
(ii) Hemocyanin–HSV-1 binding assay.
Plates were coated with hemocyanin at 0.05 nM and the dimer subunit at 1.4 nM overnight at 4°C in two replicates. The plate was then washed three times, and 100 μl of two-times serially diluted purified HSV-1 (F strain, 3.3 × 108 PFU/ml) in 0.1 M carbonate buffer was added to the wells for 2 h at room temperature. The wells were blocked and washed as described above, followed by the addition of HSV-1 antibody–HRP conjugate (1:2,000) in PBS-T-FCS for 1 h at room temperature. The rest of the assay was performed as described above. Results are presented after subtracting background signal obtained from parallel mock assays in which no HSV-1 was added. Negative controls were wells that were coated with StabilGuard instead of hemocyanin.
(iii) Identification of hemocyanin-binding viral glycoproteins.
Purified HSV-1 (F strain) was mixed with different dilutions of polyclonal anti-gD (R7), -gB (R68), or -gC (R47) IgG in PBS-T-FCS for 1 h at room temperature. These mixtures were then added to immobilized hemocyanin at 0.1 nM for 2 h at room temperature. After washing three times, HSV-1 was detected with HSV-1 antibody–HRP conjugate raised against whole HSV-1, strain F. The percentage of inhibition of binding for each antibody was calculated, after adjusting background, based on the signal detected for the positive control. The positive control consisted of virus mixed with PBS-T-FCS. The negative control consisted of virus mixed with rabbit polyclonal antibody to HSV-1 tegument protein VP16 or normal rabbit IgG, and background was PBS-T-FCS mixed with polyclonal rabbit serum R7, R68, or R47 in the absence of virus.
(iv) Direct hemocyanin binding to recombinant viral glycoproteins.
Recombinant gD-1 (306t), gB-1 (730t), gC-1 (214t), gD-1(rid), and gB-1 (PK−) were serially 2-fold diluted (0.03 to 16 μM) in PBS-T-FCS and added to immobilized hemocyanin or the dimer subunit on an ELISA plate at 0.05 nM or 1.4 nM, respectively, for 2 h at room temperature. The plates were then washed three times, and polyclonal anti-gD (R7), -gB (R68), or -gC (R47) IgG, which was diluted in PBS-T-FCS at 1:1,000, was added to their respective glycoproteins for 2 h at room temperature. The plate was then washed three times and incubated with anti-rabbit IgG (whole molecule)–peroxidase antibody at 1:15,000 in PBS-T-FCS for 1 h at room temperature. Detection was performed as described above. The positive control consisted of hemocyanin/dimer subunit only detected with anti-hemocyanin antibody and peroxidase-conjugated rabbit IgG. The negative control was prepared by the addition of a 2-fold serial dilution of cytomegalovirus (CMV) pp65 recombinant protein (Miltenyi Biotec) to hemocyanin/dimer subunit, which was detected with rabbit CMV-pp65 antibody at 1:1,000 and peroxidase-conjugated rabbit IgG. Background consisted of hemocyanin or the dimer subunit detected with anti-rabbit IgG (whole molecule)–peroxidase antibody.
(v) Competition ELISA with heparin.
Recombinant gD-1 (306t) at 8 μM and gB-1 (730t) at 2 μM were mixed with an increasing concentration of heparin in PBS-T-FCS for 1 h at room temperature. These mixtures were then added to immobilized hemocyanin at 0.1 nM for 2 h at room temperature. After washing three times, polyclonal anti-gD (R7) and -gB (R68) were added to their respective glycoproteins for 2 h at room temperature. The plate was then washed three times and incubated with anti-rabbit IgG for 1 h. Detection was performed as described above. The positive control consisted of gB/gD mixed with PBS-T-FCS. The negative control consisted of pp65 (8 μM) mixed with heparin, and background was PBS-T-FCS mixed with polyclonal rabbit serum R7 or R68 in the absence of gD/gB.
Immunofluorescence microscopy and image analysis: effect of virus exposure to hemocyanin on viral entry.
HSV F-GS2822 at an MOI of 20 was mixed with 300 nM hemocyanin for 20 min at 4°C. Hemocyanin-virus complex was then added to Vero cells grown on coverslips at 37°C for 1 h. Cultures on coverslips were then fixed and stained as described previously (40) with anti-hemocyanin antibody (1: 2,000) for detection of hemocyanin. The coverslips were then mounted on glass slides using Prolong Gold with 4′, 6′-diamidino-2-phenylindole (DAPI). The infection control consisted of virus mixed with medium, and the mock control consisted of hemocyanin mixed with medium. The slides were examined using a Leica SP5 confocal microscope.
Statistical analysis.
The results of the experiments were expressed as means ± standard deviations (SD). Comparisons between groups were made using one-way analysis of variance (ANOVA). Statistical significance was defined at a P value of <0.05.
RESULTS
Purification of hemocyanin.
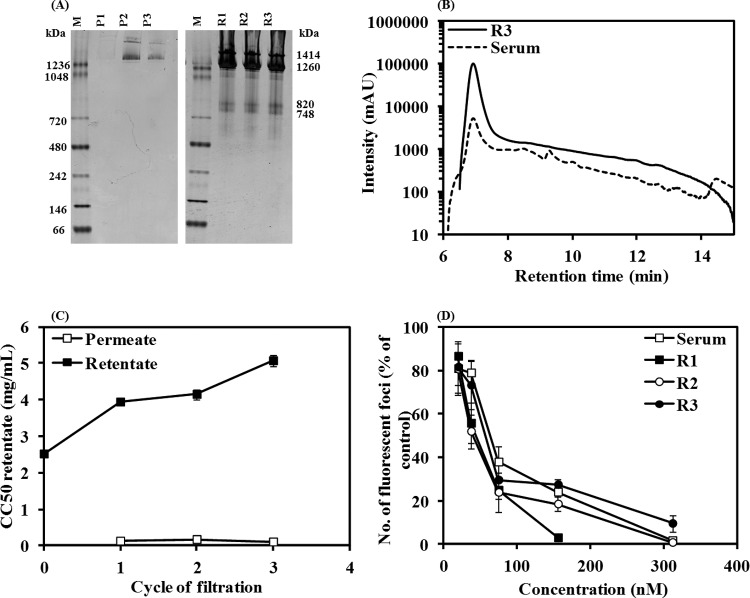
Purification of hemocyanin from abalone serum was conducted by ultrafiltration using 100-kDa-molecular-mass-cutoff filters to ensure that subsequent antiviral activity tests measured the efficacy of hemocyanin rather than the small peptides. Following each 20-min cycle of filtration, the protein compositions of retentate and permeate were monitored by blue native PAGE as shown in Fig. 1A. The electrophoretic profiles of retentate after one cycle (R1), two cycles (R2), and three cycles (R3) of ultrafiltration demonstrated the characteristic bands of the two isoforms of whole hemocyanin at 1,414 and 1,260 kDa and dimer subunits at 820 and 748 kDa. In contrast, the permeate contained a small amount of whole hemocyanin. In addition, the protein concentration in the permeate and retentate remained almost constant following each cycle of ultrafiltration, with retentate containing considerably more protein than permeate (Table 1). This suggests that only a negligible amount of hemocyanin protein was lost due to ultrafiltration. Filtered sample and abalone serum were then applied to gel filtration chromatography as shown in Fig. 1B. The single peak for filtered serum, as opposed to several peaks in abalone serum, confirmed the efficacy of ultrafiltration for purification of hemocyanin. The minor peaks that eluted after hemocyanin are most likely lower-molecular-weight forms of hemocyanin or possibly nonhemocyanin proteins.
FIG 1.
Anti-HSV-1 activity of purified hemocyanin from H. rubra. (A) Detection of characteristic bands of hemocyanin by blue native PAGE in the permeate after one (P1), two (P2), and three (P3) cycles of ultrafiltration and in the retentate after one (R1), two (R2), and three (R3) cycles of ultrafiltration using a filter with 100-kDa cutoff. Ultrapure water was used for elution in the ultrafiltration process. Lanes M, molecular mass marker. (B) Effect of purification on gel filtration chromatogram of abalone serum and R3. (C) Effect of purification on toxicity (CC50) of retentate and permeate as determined by a WST-1 assay on Vero cells. (D) Effect of addition of purified hemocyanin (R1, R2, and R3) and abalone serum with HSV-1 to cells on the infection as determined by a fluorescent focus assay on Vero cells. mAU, milli-absorbance units.
Testing of purified hemocyanin for cytotoxicity and antiviral activity.
First, we tested whether the purified hemocyanin retained the previously detected antiviral activity of hemocyanin as measured by the therapeutic index, equivalent to the EC50 divided by the CC50 (39). First, toxicity (CC50) of retentates and permeates was assessed using a WST-1 assay. As illustrated in Fig. 1C, the retentate was 25 to 47 times less toxic than its respective permeate when tested on Vero cells, indicating that toxic compounds had been removed with (repeated) ultrafiltrations. Furthermore, each 20-min cycle of ultrafiltration decreased the toxicity by 65, 12, and 16%, with the least toxic preparation obtained after three cycles of ultrafiltration (CC50 = 604 nM or 5.07 mg/ml).
Next, the antiviral activities (EC50) of the nontoxic concentrations of abalone serum, R1, R2, and R3 were assessed by a fluorescent focus assay on Vero cells. The GFP-tagged HSV-1 (vUL37-GFP HSV-1) and various concentrations of purified hemocyanin (R1 to R3) were added to Vero cells simultaneously for 2 h at 37°C, and the antiviral effect was compared to that of the control (cells infected but not treated with hemocyanin) after 48 h. Serum and R1 to R3 samples demonstrated a dose-dependent antiviral activity, as shown in Fig. 1D. The EC50 (effective concentration of hemocyanin causing 50% reduction in the number of fluorescent foci) was then calculated by regression analysis of these curves and is summarized in Table 2. The therapeutic index (Table 2) was then calculated for comparison of the antiviral efficacies of abalone serum, R1, R2, and R3. Abalone hemocyanin purified by one, two, or three cycles of ultrafiltration had a significantly higher therapeutic index than abalone serum, irrespective of the number of cycles. Therefore, one or two cycles of ultrafiltration was sufficient for obtaining a purified hemocyanin sample, which was 3-fold less toxic and more potent than abalone serum.
TABLE 2.
Effect of purification on the antiviral efficacy of hemocyanin
| Parameter | Resultd |
|||
|---|---|---|---|---|
| Serum | R1 | R2 | R3 | |
| CC50a | 157.4 ± 2.8 | 446.9 ± 10.3 | 507 ± 36.7 | 604 ± 44.3 |
| EC50b | 61.2 ± 4.1 | 45.1 ± 7.7 | 42.4 ± 8.6 | 62.2 ± 3.6 |
| TIc | 2.6 | 9.9 | 12 | 9.7 |
Concentration (nM) of sample at which 50% of cells are viable.
Concentration (nM) of sample causing 50% reduction in the number of fluorescent foci.
Therapeutic index (TI = CC50/EC50).
Results are for serum and retentate after one cycle (R1), two cycles (R2), or three cycles (R3) of ultrafiltration using an Amicon filter with a molecular mass cutoff of 100 kDa.
Hemocyanin inhibits HSV binding or entry but not postentry events.
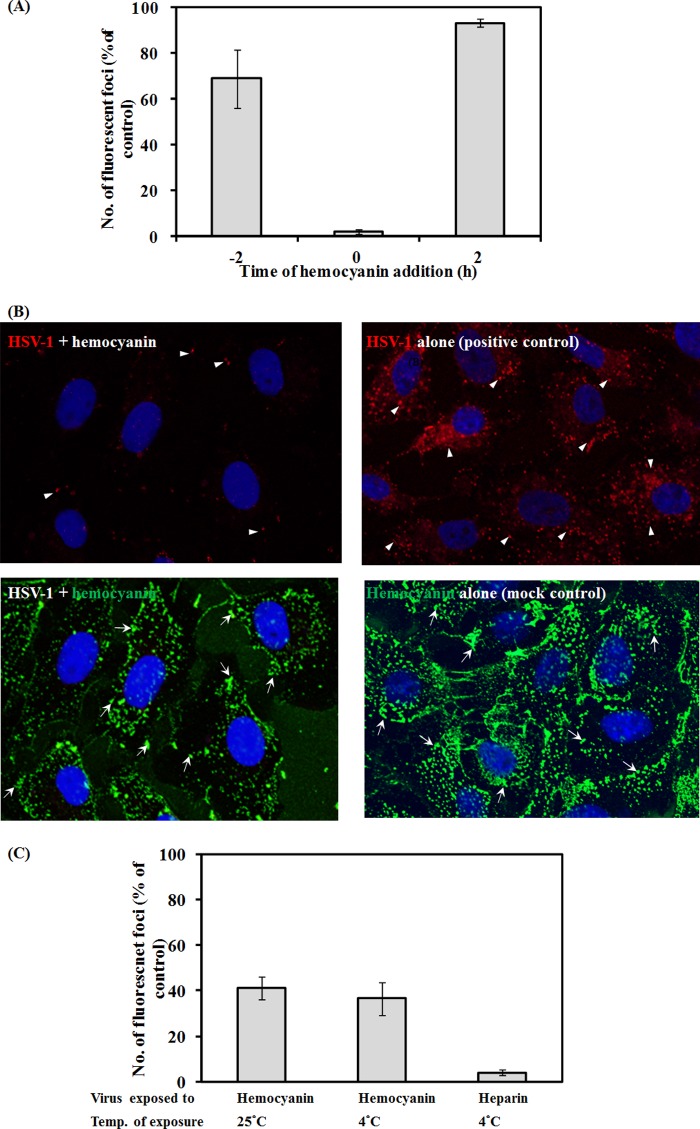
Figure 1D demonstrates that the anti-HSV-1 activity of hemocyanin occurs in a dose-dependent fashion when added to the cells simultaneously with the virus. This observation suggests that hemocyanin might inhibit early events of the viral life cycle, i.e., binding and entry or perhaps post-viral entry events. To test this hypothesis, hemocyanin was added to the cells before, during, or after virus addition. To test whether the observed inhibitory effect was due to hemocyanin blocking cellular receptors that are involved in viral binding and entry, cells were pretreated with hemocyanin for 2 h and washed off prior to infection. Alternatively, hemocyanin and virus were added to cells simultaneously for 2 h at 37°C and then removed, and finally HSV-1 was added to the cells for 2 h before the addition of hemocyanin for 2 h at 37°C. The effect of the addition of hemocyanin on viral infection under all the above-described test conditions was compared to the positive control (parallel experiment in the absence of hemocyanin) in Fig. 2A. As expected, the highest inhibition of HSV infection was observed when hemocyanin and HSV were added to the cells at the same time, but no effect was observed when hemocyanin was added before or after viral addition. This suggested that hemocyanin interacted with either HSV or perhaps its cellular receptors. However, the lack of effect of adding hemocyanin to cells immediately prior to the addition of HSV is not consistent with binding to cellular receptors. Thus, hemocyanin inhibits a step during viral attachment to and/or fusion with the cell membrane probably by interacting with the virus.
FIG 2.
Kinetics of antiviral activity of hemocyanin. (A) Effect of pretreating Vero cells with hemocyanin for 2 h before infection (time, −2 h) and addition of hemocyanin together with vUL37-GFP HSV-1 (time zero) or after removal of virus (time, +2 h) from Vero cells. Cells were incubated with virus (with or without hemocyanin) for 2 h at 37°C. (B) Confocal fluorescent micrographs showing Vero cell uptake of hemocyanin (green, arrows) and HSV F-GS2822 (red, arrowheads) that were preincubated for 20 min before incubation with cells for 1 h (left panel) or of HSV F-GS2822 alone (positive control, right panel) or hemocyanin alone (mock control, right panel). Hemocyanin was detected with anti-hemocyanin antibody and Alexa Fluor 488 anti-rabbit IgG. (C) Effect of 20 min of preincubation of vUL37-GFP HSV-1 with hemocyanin at either 4°C or 25°C on the number of fluorescent foci at 48 h postinfection compared to HSV-infected cells in the absence of hemocyanin as a positive control. Results represent two independent experiments in triplicate.
Hemocyanin interacts with infectious virus.
The possibility that the interaction between HSV-1 and hemocyanin had an effect on the infectivity of virus was tested directly by immunofluorescence microscopy and fluorescent focus assay. The confocal images in Fig. 2B revealed that exposure of HSV F-GS2822 to hemocyanin for 20 min prior to incubation with Vero cells for 1 h at 37°C inhibited viral binding and entry into these cells. This was shown by the absence of red fluorescent protein (RFP)-labeled virus in the cytosol, as opposed to the infected control without hemocyanin, which showed abundant cytosolic labeled virus (Fig. 2B). This result was confirmed by quantifying the effect of preincubating vUL37-GFP HSV-1 with hemocyanin for 20 min at 4°C and 25°C on infection in Vero cells. Treated and untreated viruses were added to cells at 37°C for 2 h, and after removal of the unbound virus, the cells were incubated at 37°C for 48 h. The results in Fig. 2C show a significant decrease in the infection of Vero cells when the virus was pretreated with hemocyanin. As HSV-1 binds to negatively charged HSPG via its positively charged domains in gB and gC (7), the effect of exposing HSV-1 to heparin was tested as a positive control. As anticipated, preexposure to 10.5 μM heparin significantly impaired HSV-1 infection (Fig. 2C).
Hemocyanin inhibits infection by binding directly to HSV-1.
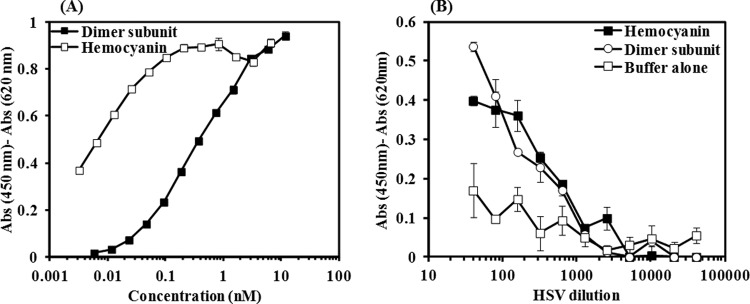
By varying the time of addition of hemocyanin with respect to HSV infection, we showed that hemocyanin blocks viral attachment and entry (Fig. 2A), possibly by interacting with the virus. The direct binding of hemocyanin to HSV-1 was confirmed by showing purified intact HSV-1 (F strain) bound to immobilized hemocyanin on ELISA plates. Dimer subunits (800 kDa, the structural component of hemocyanin didecamer) were included in these tests to verify that the binding is not just due to nonspecific trapping by the large size of the hemocyanin molecule (8 MDa). Gel electrophoresis showed that the isolated dimers were stable for 1 month and did not reassociate or degrade spontaneously. Various concentrations of hemocyanin/dimer subunit were used to optimize surface coating (Fig. 3A). Based on the results shown in Fig. 3A, coating with 0.02 to 0.4 nM hemocyanin and 1.4 to 5.7 nM dimer subunit yielded optimum coverage with minimum background signals.
FIG 3.
Hemocyanin binds to HSV-1. (A) ELISA to determine the lowest concentration of hemocyanin or its dimer subunit for sufficient surface coating of ELISA plates. The primary and secondary antibodies were anti-hemocyanin antibody and peroxidase-conjugated rabbit IgG antibody, respectively. Background consisted of a parallel mock control (no anti-hemocyanin antibody). (B) ELISA showing binding of purified HSV-1 to immobilized hemocyanin or its dimer subunit. HSV-1 was detected with HRP-conjugated anti-HSV-1 antibody raised against whole HSV-1, strain F. The negative control consisted of a plate coated with blocking buffer, and background consisted of a parallel mock control in which no HSV-1 was added. Experiments were performed in duplicate.
The binding of HSV-1 (F strain) was then tested by the addition of several dilutions of HSV-1 (Fig. 3B) to immobilized hemocyanin or the dimer subunit at 0.05 nM or 1.4 nM, respectively. Figure 3B shows that the dimer subunit bound to HSV-1 as efficiently as hemocyanin at most dilutions of HSV-1 and that the binding was significantly higher than that of the negative control (wells coated with blocking buffer). Therefore, we concluded that hemocyanin directly interacts with HSV-1 and that the binding characteristics of the whole molecule are preserved with the dimer subunit.
Hemocyanin binds to viral surface glycoproteins gB, gD, and gC.
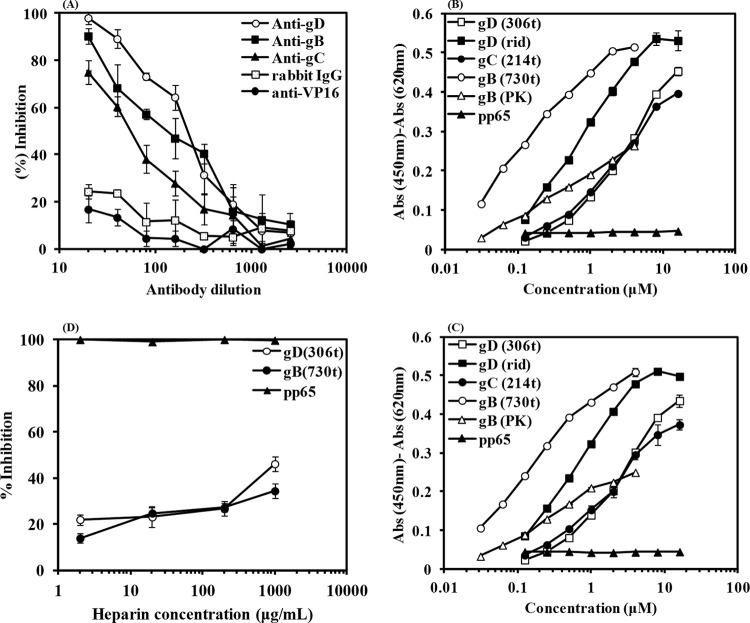
After hemocyanin was shown to bind to intact HSV-1, its capacity to bind to viral glycoproteins expressed on the surface of virus was also ascertained by ELISA. HSV-1 was incubated with decreasing concentrations of polyclonal anti-gD, -gB, or -gC IgG to inhibit HSV-1 binding to hemocyanin. As shown in Fig. 4A, incubation with these antibodies inhibited HSV-1 from binding to hemocyanin in a dose-dependent manner, indicating that hemocyanin interacted with gD, gB, and gC from HSV-1. In contrast, incubation of HSV-1 with an antibody directed toward the tegument protein VP16 or normal rabbit IgG failed to interfere with HSV-1 binding to immobilized hemocyanin.
FIG 4.
Hemocyanin binds to HSV-1 glycoproteins D, B, and C. (A) ELISA showing that preincubation of polyclonal anti-gD (R7), -gC (R47), and -gB (R68) IgG with purified HSV-1 inhibited binding to hemocyanin. Preincubated antibody and HSV-1 mixtures were added to immobilized hemocyanin. HSV-1 was then detected with HRP-conjugated anti-HSV-1 antibody. The percentage of inhibition was calculated based on the signal detected for the positive control in which HSV-1 was preincubated with buffer and then added to immobilized hemocyanin. The results are shown after adjusting for the background signal, which consisted of individual antibodies preincubated with buffer (PBS-T-FCS). Negative control consisted of HSV-1 preincubated with either antibodies to HSV-1 tegument VP16 protein or rabbit IgG. (B) Binding of recombinant surface glycoproteins B, C, and D and their gB-1 (PK−) and gD (rid1) mutants at various concentrations to immobilized hemocyanin or (C) the dimer subunit. Glycoproteins were detected with their respective antibodies as idescribed for panel A and peroxidase-conjugated rabbit IgG. The positive control consisted of hemocyanin detected with anti-hemocyanin antibody. The negative control consisted of hemocyanin/dimer subunit with the addition of pp65 and detection with anti-pp65 and peroxidase-conjugated rabbit IgG. (D) Competition ELISA with heparin. Preincubation of increasing concentrations of heparin with constant concentrations of gD (8 μM) or gB (2 μM) for 1 h at room temperature prior to addition to immobilized hemocyanin only partially inhibited the binding of gD/gB to hemocyanin. The percentage of inhibition was calculated based on the positive control, which was gD or gB preincubated with buffer. The negative control was pp65 (8 μM) preincubated with increasing concentrations of heparin.
If hemocyanin is capable of binding to these glycoproteins expressed on the surface of intact virus, it should also be able to bind to the soluble ectodomain of gD, gB, and gC. To confirm that hemocyanin directly binds to viral glycoproteins, decreasing concentrations of recombinant gD-1 (306t), gB-1 (730t), and gC-1 (214t) were added to hemocyanin or the dimer subunit on ELISA plates. The results in Fig. 4B and C show that soluble gB bound to both hemocyanin and the dimer subunit more efficiently than gD and gC. In contrast, neither hemocyanin nor the dimer subunit bound to the tegument protein of human cytomegalovirus (pp65), which served as the negative control.
In order to identify the nature of interaction between gB/gD and hemocyanin/dimer subunit, we compared the binding of mutant gD, gD-1 (rid1), mutant gB, and gB-1 (PK−) molecules to hemocyanin and the dimer subunit (Fig. 4B and C). The rid1 mutation is a point mutation at position 27, which knocks out binding to HVEM but enhances binding to nectin-1 (5), and the gB PK- mutation comprises deletion of the HSPG binding site of gB, which is a 68-amino-acid, lysine-rich sequence (7). As illustrated in Fig. 4B and C, gD-1 (rid1) bound more efficiently to hemocyanin/dimer subunit than gD-1, suggesting that a motif in the hemocyanin or the dimer subunit mimics the gD binding domain in nectin-1. On the other hand, deletion of the HSPG binding domain from gB reduced binding to hemocyanin and the dimer subunit compared to that of gB without the deletion, suggesting that binding of gB to hemocyanin is mostly mediated by binding of the positively charged region in gB to negatively charged amino acids in hemocyanin. Furthermore, we performed a competition ELISA in which heparin competed with gB or gD in binding to hemocyanin, as shown in Fig. 4D. The result demonstrated that heparin only partially inhibited both interactions. This suggests that a conformational component in hemocyanin also mediates the binding and it is not just a charge effect. Thus, these findings strongly endorsed our hypothesis that hemocyanin impairs HSV-1 entry by interfering with gD and gB functions during viral entry. Hemocyanin and its dimer subunit consistently behaved similarly in binding to HSV-1, viral surface glycoproteins, and mutant viral glycoproteins, underlining that the interaction of hemocyanin with virus is not a nonspecific interaction resulting from the large size of the hemocyanin molecule and is not dependent on assembly of the dimers into the whole molecule.
DISCUSSION
The main objective of this study was to determine the mode of action of hemocyanin as an inhibitor of HSV-1 infection, using in vitro assays. The results of this study showed that hemocyanin has antiviral activity against HSV-1 in a dose-dependent manner. More importantly, our data show that hemocyanin binds specifically to viral surface glycoproteins, thereby inhibiting the attachment and entry of HSV-1 into host cells.
In this study, hemocyanin was separated from any compound smaller than 100 kDa by ultrafiltration to ensure that the measured antiviral activity was due to hemocyanin and not the peptides. The composition of serum from H. rubra has not yet been studied; however, we detected small proteins (<100 kDa) at very low concentrations in the serum (unpublished data). The exclusion of these peptides was necessary because they are considered effective components of the innate immune system of invertebrates in combating microbial attacks (41, 42). Therefore, they could exhibit antimicrobial and antiviral activity.
The cell viability assay before and after ultrafiltration demonstrated that purification of hemocyanin resulted in a significant decrease in the toxicity of retentate after each 20-min cycle of ultrafiltration, while the intensity of characteristic bands of hemocyanin remained stable in blue native PAGE gels. Measuring the toxicity of permeate also confirmed that the separated compounds were considerably more toxic than hemocyanin. The therapeutic index of purified samples was higher than that of innate abalone serum due to removal of the toxic residues. Therefore, we concluded that the observed antiviral activity was due to hemocyanin. This result is in agreement with previous studies, which demonstrate the activity of hemocyanin against HSV-1 (20–22).
The results of our in vitro tests (addition of hemocyanin at different time points with respect to infection) elucidated that hemocyanin blocks viral entry and attachment but not postentry events. Hemocyanin showed the greatest inhibitory effect when added to the cells simultaneously with the virus. Therefore, the interference with viral binding and fusion could be due to the interaction of hemocyanin with the virus and/or host cell surface proteins that are involved in viral entry. Interaction between hemocyanin and cell surface receptors was studied by treatment of cells with hemocyanin before infection. This treatment failed to inhibit the infection of Vero cells. This result strongly suggests that hemocyanin did not bind directly to the known cellular attachment and entry receptors for HSV-1, including HSPG, HVEM, nectin-1 and -2, and 3-O-sulfated heparan sulfate, to block infection.
Conversely, preincubation of HSV-1 with hemocyanin resulted in a significant decrease in the infection of Vero cells measured as fluorescent foci. Furthermore, our immunofluorescence studies showed that pretreating virus with hemocyanin blocked entry into Vero cells. These results strongly suggested that hemocyanin bound directly to HSV-1. Direct binding of hemocyanin to another virus was previously shown by Zhang et al. (43), who demonstrated that hemocyanin from the shrimp Penaeus monodon bound to white spot syndrome virus.
It is pivotal to discover the mechanism of interaction between hemocyanin and HSV-1 and to also determine whether this effect is due to nonspecific viral trapping by the very large hemocyanin molecule instead of a specific interaction with the virus. Hemocyanin specifically inhibited viral binding and attachment, and therefore, we hypothesize that it blocks viral surface glycoproteins that are involved in this process. In order to show that this effect is independent of the size of hemocyanin, we compared the binding capacity of the whole molecule to that of the dimer subunit, which is 10-times smaller than native didecamers of hemocyanin in ELISA.
In the present study, ELISA was used to confirm direct binding between hemocyanin/dimer subunit and HSV-1. It was shown that purified HSV-1 bound strongly to hemocyanin didecamers and dimer subunits. Two different experiments were then designed to assess whether hemocyanin bound to viral surface glycoproteins gB, gD, and gC. First, it was shown that incubation of HSV-1 with decreasing concentrations of antibodies against gD, gB, and gC interfered with HSV-1 binding to hemocyanin. More importantly, recombinant viral glycoprotein gD, gB, or gC expressed in baculovirus directly bound to hemocyanin as well as dimer subunits. Therefore, hemocyanin inhibits viral entry by interfering with the attachment of viral gD, gB, and gC to cellular receptors. These results are consistent with the in vitro antiviral tests, which showed that hemocyanin prevented viral attachment and entry.
The mechanism of hemocyanin interaction with viral glycoproteins is not yet fully defined. It is known that hemocyanins are glycosylated (20, 44, 45), and some of the important features of hemocyanins in clinical applications such as immunogenicity and antigenicity are related to their high carbohydrate content and unusual glycans (46–48). Furthermore, Dolashka-Angelova et al. (20) demonstrated that one functional unit (50 kDa; the building blocks of hemocyanin) of Rapana thomasiana hemocyanin that was natively devoid of glycans lacked antiviral activity against poliovirus type 1. Therefore, the interaction of hemocyanin and viral surface glycoproteins might be related to glycosylation. However, our results do not support this. gB, gD, and gC do not bind to glycans on their cellular receptors but to regions of charged amino acids on sulfated proteoglycans (9). Thus, charge and conformation might play more important roles than glycosylation. The results of competition ELISA with heparin were in agreement with this hypothesis showing that the interaction between hemocyanin and gB or gD was partially inhibited in the presence of heparin. Furthermore, hemocyanin is a highly acidic protein (pKa 3.5) because of the predominance of Asp and Glu residues, often in stretches. As HSV-1 glycoproteins gB, gC, and gD contain highly basic complementary regions, which bind the cellular HSPG and HVEM receptors (49), hemocyanin may interfere with these interactions. The contribution of charge was shown by incorporating a gB mutant deleted for the positively charged region, which is the HSPG binding domain. This mutation decreased binding to gB, indicating that a negatively charged region in hemocyanin or the dimer subunit regulates binding to gB. On the other hand, the rid1 mutation in gD enhanced binding of hemocyanin and the dimer subunit to gD. The rid1 mutation prevents the usage of HVEM for entry by interfering with the formation of a hairpin loop at the N terminus of gD, which is necessary for formation of the gD-HVEM complex. However, gD (rid1) binds to nectin-1 with increased affinity, which is due to a weaker interaction between gD N and C termini than that in native gD, which promotes binding to nectin-1 (5). This observation suggested that the hemocyanin binding site in gD resembles the nectin-1 binding site. Nectin-1 interacts with a large surface on gD involving mostly residues from the C-terminal extension and a few amino acids from the N-terminal region (50). Thus, there may be two regions on abalone hemocyanin which bind to HSV-1 via gD and/or gB. However, as the change of conformation of the rid1 mutant is extensive, more experiments are required to confirm this conclusion. Future studies will focus on defining these glycoprotein binding regions on hemocyanin, using proteolytically cleaved functional units or recombinantly expressed proteins.
Therefore, the definition of binding of abalone hemocyanin and hemocyanin dimers to HSV-1 glycoproteins is encouraging for the development of one or more hemocyanin analogues as potential inhibitors of HSV-1 infections. In the future, we aim to develop small-molecule inhibitors of the hemocyanin-gD or -gB interactions as potential antiviral inhibitors of HSV binding and entry. They would have a distinct mode of action compared to that of current antiviral compounds and could be used in synergy with current drugs.
ACKNOWLEDGMENTS
We thank Marine Biotechnology Australia Pty Ltd. for the supply of abalone serum and Gary H. Cohen and Roselyn J. Eisenberg for their kind gift of recombinant viral glycoproteins, the mutant, and antibodies to these glycoproteins and also their advice and guidance in preparing the manuscript. We are thankful to Laurence Cantrill for technical assistance in confocal microscopy and Valerie Marsden for help with virus purification. We thank members of the Arbovirus Emerging Diseases Unit at Pathology West-ICPMR Westmead for their help with ELISA.
Funding Statement
This work was supported by the Australian Research Council and Marine Biotechnology Australia Pty Ltd. (grant number LP100100799). N.T.Z. acknowledges the USydIS scholarship award from the University of Sydney.
REFERENCES
- 1.De Clercq E. 2002. Strategies in the design of antiviral drugs. Nat Rev Drug Discov 1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 2.Biswas S, Sukla S, Field HJ. 2014. Helicase-primase inhibitors for herpes simplex virus: looking to the future of non-nucleoside inhibitors for treating herpes virus infections. Future Med Chem 6:45–55. doi: 10.4155/fmc.13.192. [DOI] [PubMed] [Google Scholar]
- 3.Spear PG. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol 6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazear E, Whitbeck JC, Zuo Y, Carfi A, Cohen GH, Eisenberg RJ, Krummenacher C. 2014. Induction of conformational changes at the N-terminus of herpes simplex virus glycoprotein D upon binding to HVEM and nectin-1. Virology 448:185–195. doi: 10.1016/j.virol.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatta V, Petrovic B, Campadelli-Fiume G. 2015. The engineering of a novel ligand in gH confers to HSV an expanded tropism independent of gD activation by its receptors. PLoS Pathog 11:e1004907. doi: 10.1371/journal.ppat.1004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laquerre S, Argnani R, Anderson DB, Zucchini S, Manservigi R, Glorioso JC. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol 72:6119–6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold BC, Gerber SI, Polonsky T, Belval BJ, Shaklee PN, Holme K. 1995. Identification of structural features of heparin required for inhibition of herpes simplex virus type 1 binding. Virology 206:1108–1116. doi: 10.1006/viro.1995.1034. [DOI] [PubMed] [Google Scholar]
- 9.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai XM, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Glycobiology 9:1142–1142. [DOI] [PubMed] [Google Scholar]
- 10.Zhong MG, Xiang YF, Qiu XX, Liu Z, Kitazato K, Wang YF. 2013. Natural products as a source of anti-herpes simplex virus agents. RSC Adv 3:313–328. doi: 10.1039/C2RA21464D. [DOI] [Google Scholar]
- 11.Dang VT, Benkendorff K, Green T, Speck P. 2015. Marine snails and slugs: a great place to look for antiviral drugs. J Virol 89:8114–8118. doi: 10.1128/JVI.00287-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang VT, Benkendorff K, Speck P. 2011. In vitro antiviral activity against herpes simplex virus in the abalone Haliotis laevigata. J Gen Virol 92:627–637. doi: 10.1099/vir.0.025247-0. [DOI] [PubMed] [Google Scholar]
- 13.Dang VT, Speck P, Doroudi M, Smith B, Benkendorff K. 2011. Variation in the antiviral and antibacterial activity of abalone Haliotis laevigata, H. rubra and their hybrid in South Australia. Aquaculture 315:242–249. doi: 10.1016/j.aquaculture.2011.03.005. [DOI] [Google Scholar]
- 14.Dang VT, Speck P, Benkendorff K. 2012. Influence of elevated temperatures on the immune response of abalone, Haliotis rubra. Fish Shellfish Immunol 32:732–740. doi: 10.1016/j.fsi.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Dolashka P, Voelter W. 2013. Antiviral activity of hemocyanins. Invertebrate Surviv J 10:120–127. [Google Scholar]
- 16.Coates CJ, Nairn J. 2014. Diverse immune functions of hemocyanins. Dev Comp Immunol 45:43–55. doi: 10.1016/j.dci.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Charlet M, Chernysh S, Philippe H, Hetru C, Hoffmann JA, Bulet P. 1996. Innate immunity—isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J Biol Chem 271:21808–21813. doi: 10.1074/jbc.271.36.21808. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 19.Dolashka P, Moshtanska V, Borisova V, Dolashki A, Stevanovic S, Dimanov T, Voelter W. 2011. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 32:1477–1483. doi: 10.1016/j.peptides.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Dolashka-Angelova P, Lieb B, Velkova L, Heilen N, Sandra K, Nikolaeva-Glomb L, Dolashki A, Galabov AS, Van Beeumen J, Stevanovic S, Voelter W, Devreese B. 2009. Identification of glycosylated sites in Rapana hemocyanin by mass spectrometry and gene sequence, and their antiviral effect. Bioconjug Chem 20:1315–1322. doi: 10.1021/bc900034k. [DOI] [PubMed] [Google Scholar]
- 21.Dolashka P, Velkova L, Shishkov S, Kostova K, Dolashki A, Dimitrov I, Atanasov B, Devreese B, Voelter W, Van Beeumen J. 2010. Glycan structures and antiviral effect of the structural subunit RvH2 of Rapana hemocyanin. Carbohydr Res 345:2361–2367. doi: 10.1016/j.carres.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Genova-Kalou P, Dundarova D, Idakieva K, Mohmmed A, Dundarov S, Argirova R. 2008. Anti-herpes effect of hemocyanin derived from the mollusk Rapana thomasiana. Z Naturforsch C 63:429–434. [DOI] [PubMed] [Google Scholar]
- 23.Green TJ, Robinson N, Chataway T, Benkendorff K, O'Connor W, Speck P. 2014. Evidence that the major hemolymph protein of the Pacific oyster, Crassostrea gigas, has antiviral activity against herpesviruses. Antiviral Res 110:168–174. doi: 10.1016/j.antiviral.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Vanholde KE, Miller KI. 1982. Hemocyanins. Q Rev Biophys 15:1–129. doi: 10.1017/S0033583500002705. [DOI] [PubMed] [Google Scholar]
- 25.Cheng K, Koeck PJB, Elmlund H, Idakieva K, Parvanova K, Schwarz H, Ternstrom T, Hebert H. 2006. Rapana thomasiana hemocyanin (RtH): comparison of the two isoforms, RtH1 and RtH2, at 19 angstrom 16 angstrom resolution. Micron 37:566–576. doi: 10.1016/j.micron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Gatsogiannis C, Markl J. 2009. Keyhole limpet hemocyanin: 9-angstrom cryoEM structure and molecular model of the KLH1 didecamer reveal the interfaces and intricate topology of the 160 functional units. J Mol Biol 385:963–983. doi: 10.1016/j.jmb.2008.10.080. [DOI] [PubMed] [Google Scholar]
- 27.LaVail JH, Tauscher AN, Sucher A, Harrabi O, Brandimarti R. 2007. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience 146:974–985. doi: 10.1016/j.neuroscience.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saksena MM, Wakisaka H, Tijono B, Boadle RA, Rixon F, Takahashi H, Cunningham AL. 2006. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J Virol 80:3592–3606. doi: 10.1128/JVI.80.7.3592-3606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antinone SE, Smith GA. 2010. Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J Virol 84:1504–1512. doi: 10.1128/JVI.02029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weidmann M, Sall AA, Manuguerra JC, Koivogui L, Adjami A, Traore FF, Hedlund KO, Lindegren G, Mirazimi A. 2011. Quantitative analysis of particles, genomes and infectious particles in supernatants of haemorrhagic fever virus cell cultures. Virol J 8:81. doi: 10.1186/1743-422X-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isola VJ, Eisenberg RJ, Siebert GR, Heilman CJ, Wilcox WC, Cohen GH. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol 63:2325–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handler CG, Eisenberg RJ, Cohen GH. 1996. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol 70:6067–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicola AV, Willis SH, Naidoo NN, Eisenberg RJ, Cohen GH. 1996. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol 70:3815–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krummenacher C, Rux AH, Whitbeck JC, Ponce-De-Leon M, Lou H, Baribaud I, Hou WF, Zou CH, Geraghty RJ, Spear PG, Eisenberg RJ, Cohen GH. 1999. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J Virol 73:8127–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sisk WP, Bradley JD, Leipold RJ, Stoltzfus AM, Deleon MP, Hilf M, Peng C, Cohen GH, Eisenberg RJ. 1994. High-level expression and purification of secreted forms of herpes-simplex virus type-1 glycoprotein gD synthesized by baculovirus-infected insect cells. J Virol 68:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shelly SS, Cairns TM, Whitbeck JC, Lou H, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. The membrane-proximal region (MPR) of herpes simplex virus gB regulates association of the fusion loops with lipid membranes. mBio 3(6):e00429-12. doi: 10.1128/mBio.00429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bender FC, Whitbeck JC, Ponce de Leon M, Lou H, Eisenberg RJ, Cohen GH. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J Virol 77:9542–9552. doi: 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, Cohen GH. 2011. Capturing the herpes simplex virus core fusion complex (gB-gH/gL) in an acidic environment. J Virol 85:6175–6184. doi: 10.1128/JVI.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanjani NT, Sairi F, Marshall G, Saksena MM, Valtchev P, Gomes VG, Cunningham AL, Dehghani F. 2014. Formulation of abalone hemocyanin with high antiviral activity and stability. Eur J Pharm Sci 53:77–85. doi: 10.1016/j.ejps.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Miranda-Saksena M, Armati P, Boadle RA, Holland DJ, Cunningham AL. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J Virol 74:1827–1839. doi: 10.1128/JVI.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Destoumieux D, Munoz M, Bulet P, Bachere E. 2000. Penaeidins, a family of antimicrobial peptides from penaeid shrimp (Crustacea, Decapoda). Cell Mol Life Sci 57:1260–1271. doi: 10.1007/PL00000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tincu JA, Taylor SW. 2004. Antimicrobial peptides from marine invertebrates. Antimicrob Agents Chemother 48:3645–3654. doi: 10.1128/AAC.48.10.3645-3654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang XB, Huang CH, Qin QW. 2004. Antiviral properties of hemocyanin isolated from shrimp Penaeus monodon. Antiviral Res 61:93–99. doi: 10.1016/j.antiviral.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Velkova L, Dolashka P, Lieb B, Dolashki A, Voelter W, Van Beeumen J, Devreese B. 2011. Glycan structures of the structural subunit (HtH1) of Haliotis tuberculata hemocyanin. Glycoconj J 28:385–395. doi: 10.1007/s10719-011-9337-2. [DOI] [PubMed] [Google Scholar]
- 45.Kurokawa T, Wuhrer M, Lochnit G, Geyer H, Markl J, Geyer R. 2002. Hemocyanin from the keyhole limpet Megathura crenulata (KLH) carries a novel type of N-glycans with Gal(beta1-6)Man-motifs. Eur J Biochem 269:5459–5473. doi: 10.1046/j.1432-1033.2002.03244.x. [DOI] [PubMed] [Google Scholar]
- 46.Wirguin I, Suturkovamilosevic L, Briani C, Latov N. 1995. Keyhole limpet hemocyanin contains Gal(beta 1-3)-GalNac determinants that are cross-reactive with the T antigen. Cancer Immunol Immunother 40:307–310. doi: 10.1007/BF01519630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqui NI, Idakieva K, Demarsin B, Doumanova L, Compernolle F, Gielens C. 2007. Involvement of glycan chains in the antigenicity of Rapana thomasiana hemocyanin. Biochem Biophys Res Commun 361:705–711. doi: 10.1016/j.bbrc.2007.07.098. [DOI] [PubMed] [Google Scholar]
- 48.Siddiqui NI, Yigzaw Y, Preaux G, Gielens C. 2009. Involvement of glycans in the immunological cross-reaction between alpha-macroglobulin and hemocyanin of the gastropod Helix pomatia. Biochimie 91:508–516. doi: 10.1016/j.biochi.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Pertel PE, Fridberg A, Parish ML, Spear PG. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313–324. doi: 10.1006/viro.2000.0713. [DOI] [PubMed] [Google Scholar]
- 50.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7(9):e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]