Abstract
Polyphosphate (polyP) has gained a wide interest in the food industry due to its potential as a decontaminating agent. In this study, we examined the effect of sodium tripolyphosphate (polyP3; Na5P3O10) against planktonic and biofilm cells of Prevotella intermedia, a major oral pathogen. The MIC of polyP3 against P. intermedia ATCC 49046 determined by agar dilution method was 0.075%, while 0.05% polyP3 was bactericidal against P. intermedia in time-kill analysis performed using liquid medium. A crystal violet binding assay for the assessment of biofilm formation by P. intermedia showed that sub-MICs of polyP3 significantly decreased biofilm formation. Under the scanning electron microscope, decreased numbers of P. intermedia cells forming the biofilms were observed when the bacterial cells were incubated with 0.025% or higher concentrations of polyP3. Assessment of biofilm viability with LIVE/DEAD staining and viable cell count methods showed that 0.05% or higher concentrations of polyP3 significantly decreased the viability of the preformed biofilms in a concentration-dependent manner. The zone sizes of alpha-hemolysis formed on horse blood agar produced by P. intermedia were decreased in the presence of polyP3. The expression of the genes encoding hemolysins and the genes of the hemin uptake (hmu) locus was downregulated by polyP3. Collectively, our results show that polyP is an effective antimicrobial agent against P. intermedia in biofilms as well as planktonic phase, interfering with the process of hemin acquisition by the bacterium.
INTRODUCTION
Prevotella intermedia is a black-pigmented anaerobic Gram-negative bacterium which has long been known to be associated with oral diseases, such as chronic periodontitis (1–3), aggressive periodontitis (4–6), puberty-associated gingivitis, acute necrotizing ulcerative gingivitis (7, 8), periapical periodontitis (9, 10), and noma (an acute gangrenous disease) (11, 12). Besides being involved in oral diseases, P. intermedia has also been reported to be associated with various systemic diseases, such as cystic fibrosis, chronic bronchitis (13–15), and atherosclerosis (16). Difficulty in controlling the bacterium has been attributed to resistance of P. intermedia to many antibiotics, including penicillins, cephalosporins, and tetracyclines (17, 18). Moreover, P. intermedia cells form a biofilm in which the bacterial cells become more resistant to antibiotics (19). Because biofilm can serve as a reservoir of antibiotic resistance (20), it is of clinical significance to develop alternative antimicrobial approaches for controlling antibiotic-resistant P. intermedia.
Inorganic polyphosphate (polyP) is a chain of few or many hundreds of phosphate (Pi) residues linked by high-energy phosphoanhydride (21). Intracellular polyP found in bacteria is considered a virulence factor since it performs various functions, such as serving as an ATP source and substitute, a regulator of the intracellular levels of metal ions, a channel for DNA entry, and a regulator that contributes to bacterial resistance and survival under stress conditions (21). Meanwhile, exogenous polyP has been known to possess antimicrobial effects against not only various Gram-positive bacteria, such as Sarcina lutea (22), Staphylococcus aureus (22–25), Listeria monocytogenes (25, 26), and Bacillus cereus (27), but also fungi, such as Aspergillus flavus (23). Due to its antimicrobial activity, polyP has gained a wide interest in the food industry as a decontaminating agent. In fact, several polyPs, such as sodium trimetaphosphate (STMP; Na3P3O9) and sodium tripolyphosphate (STPP, polyP3; Na5P3O10), are listed as generally recognized as safe (GRAS) food additives by the FDA. Its antimicrobial activity with GRAS-level safety has drawn our attention toward the clinical application of polyP in oral infectious diseases.
It has been reported that the antibacterial effect of polyP against Gram-positive bacteria, including mutans streptococci, is related to its ability to chelate divalent cations, resulting in cell division inhibition and loss of cell wall integrity (22, 23, 27–29). In contrast to the case with Gram-positive bacteria, large numbers of Gram-negative bacteria, including Escherichia coli and Salmonella enterica serovar Typhimurium, are able to grow in the presence of polyP at concentrations even up to 10% (22, 23, 30). These observations, together with less important role for the divalent cations in the membrane stability of Gram-negative bacteria, have led to the general assumption that polyP is ineffective against Gram-negative bacteria.
Recently, however, polyP was demonstrated to be bactericidal against Porphyromonas gingivalis, a black-pigmented anaerobic Gram-negative rod associated with periodontal disease (31). The MICs of polyP with different linear phosphorus chain lengths (3 to 75) for the bacterium were 0.06%, which is much lower than the MICs previously reported for Gram-positive bacteria (31). Notably, there is ample evidence to show that the mode of bactericidal action of polyP against Gram-negative bacteria is different from that against Gram-positive bacteria: antibacterial activity of polyP against P. gingivalis was independent of the bacterial growth phase, and no P. gingivalis cells with obviously aseptate and elongated morphology were observed when the bacterial cells were exposed to polyP (31).
Although polyP has fascinating properties required for ideal periodontal agents, such as a broad antimicrobial activity (22–25, 27, 29, 31), safety (32), and even bone forming activity (33), an antibacterial effect of polyP against periodontal pathogens except P. gingivalis has not been demonstrated. The aim of the present study was to observe the effects of polyP against periodontopathic P. intermedia in planktonic phase and biofilm.
MATERIALS AND METHODS
Bacterial strain and culture condition.
P. intermedia ATCC 49046 was obtained from the American Type Culture Collection (Manassas, VA). The bacterium was grown in brucella agar (Becton, Dickinson and Company, Sparks, MD) supplemented with 5% sheep blood or in brucella broth (Becton, Dickinson and Company). Both media were supplemented with 5 μg/ml of hemin (Sigma Chemical Co., St. Louis, MO) and 1 μg/ml of vitamin K1 (Sigma). The culture was incubated at 37°C in an anaerobic chamber (85% N2, 10% H2, and 5% CO2) (Forma Scientific Company, Marietta, OH).
Assessment of MICs.
polyP with chain length 3 (polyP3; Na5P3O10), which is listed as GRAS (polyP), was purchased from Sigma Chemical Co. polyP3 was dissolved in distilled water to 10% (wt/vol), sterilized using a 0.22-μm filter, and stored at −20°C until use. The MIC of polyP3 was determined by the agar dilution method as described previously (31). Briefly, various concentrations of polyP3 were prepared and added to brucella blood agar supplemented with 5 μg/ml of hemin and 1 μg/ml of vitamin K1. The final concentrations of polyP3 ranged from 0.025% to 0.4%. The agar plates were inoculated with approximately 105 to 106 cells/spot and incubated at 37°C for 3 days. The MIC was defined as the lowest concentration that inhibited the bacterial growth on brucella blood agar according to CLSI guidelines (34).
Time-kill analyses.
Time-kill experiments were performed in brucella broth supplemented with 5 μg/ml of hemin and 1 μg/ml of vitamin K1 (B-HK). Inocula of approximately 105 to 106 P. intermedia cells/ml grown to exponential phase were incubated with polyP3. Aliquots were removed from the cultures at 4-h intervals for 24 h, and viable cells were enumerated by plating them on brucella blood agar supplemented with 5 μg/ml of hemin and 1 μg/ml of vitamin K1.
Quantification of biofilm biomass.
The biofilm formation assay was performed as described previously (35, 36). Briefly, P. intermedia was grown to exponential phase and then adjusted to an optical density at 600 nm (OD600) of approximately 0.1. The bacterial suspension was dispensed (500 μl per well) into triplicate wells of 24-well polystyrene flat-bottom microtiter plates containing various concentrations of polyP3 in B-HK (500 μl) and incubated at 37°C anaerobically. Heat-killed bacterial cells that were initially killed by exposure to 100°C for 10 min were included as controls. Bacterial cell death was confirmed by culturing the heat-killed cells onto brucella blood agar. After 24 h, planktonic and loosely bound bacterial cells were removed by aspirating the spent media, followed by washing twice with physiological saline. Then, the remaining biofilm cells were stained with 0.1% crystal violet for 10 min. The plates were washed three times with distilled water and air dried. Then, 800 μl of 100% ethanol was added to release the crystal violet from the biofilm, and the absorbance of the released crystal violet was measured at a wavelength of 600 nm. The OD reading from sterile medium, dye, and ethanol was subtracted from all the test values.
Scanning electron microscopy (SEM).
Biofilms of P. intermedia were developed in the wells of 24-well polystyrene plates for 24 h in the presence of various concentrations of polyP3 as described above. After gentle washing with physiological saline three times, the biofilms were fixed by incubation for 1 h at room temperature with 2.5% (wt/vol) glutaraldehyde prepared in a filter-sterilized phosphate buffer (0.1 M; pH 7.4) and then rinsed three times for 10 min each in distilled water. The biofilms were postfixed with 1% (wt/vol) osmium tetroxide in 0.1 M phosphate buffer for 1 h, followed by a quick rinse in distilled water. The fixed biofilms were dehydrated in successive ethanol-water mixtures with increasing ethanol concentrations of 25%, 50%, and 75% by volume for 10 min each and then twice in pure ethanol for 10 min each. The biofilm samples were dried by critical point drying and then coated with gold using a sputter coater (IB-3; Eiko, Tokyo, Japan). Observations were performed at 15 kV with a scanning electron microscope (model S-4700; Hitachi High Technologies America, Inc., Pleasanton, CA).
Viscosity of spent culture media.
P. intermedia was grown to exponential phase and adjusted to an OD600 of approximately 0.3. The bacterial suspension was further incubated in the presence or absence of polyP3 at a concentration of 0.025% (1/3× MIC). After a 48-h incubation, the polyP3-treated culture medium was centrifuged to remove cells and sterilized using a 0.45-μm filter. The untreated culture medium was centrifuged, filter sterilized, and treated with polyP3 at various concentrations (0.05 to 1%) for 8 h. The viscosity of each culture medium was measured at 25°C using an SV-10 vibroviscometer (A&D Company Ltd., Tokyo, Japan) to determine the amount of extracellular polymeric substance (EPS) produced by P. intermedia.
Measurements of viability and biomass of preformed biofilms.
P. intermedia was grown to exponential phase and adjusted to an OD600 of approximately 0.3. The bacterial suspension was dispensed (200 μl per well) into a polystyrene 96-well plate. Several identical microtiter plates were prepared for measurements of viability and biomass of preformed biofilms. After 24 h of incubation at 37°C anaerobically, planktonic and loosely bound bacterial cells were removed by aspirating the spent media without disturbing the biofilms on the surface of the plates. Then, the preformed biofilms were further incubated in 200 μl of physiologic saline containing polyP3 at various concentrations. After 24 h, quantification of the biofilm biomass was performed as described above employing the crystal violet staining method. The enumeration of viable biofilm bacteria was also performed. Briefly, the plates were washed twice with physiological saline, and the biofilm cells in each well of the plates were detached by pipetting and scraping the surface of the well using a pipette tip. After 10-fold serial dilutions, the detached cells were subsequently enumerated by plating them on brucella blood agar supplemented with 5 μg/ml of hemin and 1 μg/ml of vitamin K1.
Confocal laser scanning microscopy (CLSM).
P. intermedia biofilms were established on glass-bottom confocal dishes (SPL Lifesciences, Republic of Korea) as described above. Following exposure to polyP3 at various concentrations for 24 h, the biofilms were stained with a LIVE/DEAD BacLight bacterial viability kit (Invitrogen, Eugene, OR) according to the manufacturer's instructions. Then the biofilms were examined under a confocal laser scanning microscope (Nikon D-Eclipse C1si; Nikon, Japan).
Hemolysis assay on solid medium.
Various concentrations of polyP3 were prepared and added to brain heart infusion agar (BHIA; Becton, Dickinson and Company) containing 5% horse blood but without hemin and vitamin K1. The horse blood BHIA plates were inoculated with approximately 108 P. intermedia cells/spot. The inoculated plates were then incubated at 37°C anaerobically. The area of the beta-hemolysis zone around the spot was observed at 24-h intervals for 5 days and then quantified using ImageJ version 1.49j (National Institutes of Health, Bethesda, MD).
qRT-PCR.
A P. intermedia culture grown to an OD600 of approximately 0.3 was divided into two aliquots. One was left unexposed, while the other was exposed to 0.05% polyP3. After anaerobic incubation for 2 h and 3 h, cells of each group were harvested. Total RNAs from P. intermedia cells were prepared using TRIzol reagent (Invitrogen). cDNA was synthesized from 0.5 μg of RNA using the RevertAid first-strand cDNA synthesis kit (Thermo Scientific, Wilmington, DE). To identify the expression value of genes related to hemin (oxidized form of heme) uptake and hemolysis, quantitative real-time PCR (qRT-PCR) was performed using specific primers for the selected genes (Table 1). The primers were designed based on the genome sequence of P. intermedia ATCC 49046 deposited in GenBank whole-genome shotgun sequence databases (BioProject no. PRJNA281562). qRT-PCR was carried out using 3 μl of diluted cDNA (∼40 ng/μl) and 250 nM primers for each 20-μl reaction mixture. Analysis was carried out on the MiniOpticon real-time PCR detection system (Bio-Rad Laboratories, CA) using SYBR Premix Ex Taq (TaKaRa, Kyoto, Japan) with the following conditions: 95°C for 3 min and then 40 cycles of 95°C for 25 s, 60°C for 25 s, and 72°C for 25 s. To confirm that a single PCR product was amplified, melting-curve analysis was performed under the following conditions: 65°C to 95°C, with a heating rate of 0.2°C per s. All PCRs and melting-curve experiments were always performed in triplicate, and each experiment included a negative control in which distilled water was added instead of template DNA. All quantifications were normalized to the P. intermedia 16S rRNA gene.
TABLE 1.
Primers used in this study
| Gene | Primer directiona | Primer sequence | Putative identification |
|---|---|---|---|
| 16S rRNA | F | TGTTACAATGGGAGGGACAAAGGG | |
| R | TTACTAGCGAATCCAGCTTCACGG | ||
| hmuY | F | CAACAACGACGACCCAAACC | Lipoprotein HmuY |
| R | TTCACCGTTGTTGATGCGTG | ||
| hmuR | F | CCTTGTTGTGGGCGGAAAAG | TonB-dependent receptor HmuR |
| R | CCGTATAGTAGAGGCGTGCG | ||
| hmuS | F | CAACAGGTGGCTGACATTGC | Hemin transport protein HmuS |
| R | TATCCTCGCGCTTCATCACC | ||
| hmuU | F | CTGCCTGCATTGCGAAGATG | Permease HmuU |
| R | GCCGAACCGACTTTACAACG | ||
| hmuV | F | TGATGGTGGCACTCGTAACC | Hypothetical protein HmuV |
| R | TCTGCTTGCCCTTCTTCGAG | ||
| hlyC | F | TCTTCCTGTCCTTTTCGCCC | Hemolysin C |
| R | AGTGGGGCAAAGAACGACAG | ||
| inpA | F | AGAGCTAACCACCACCAACG | Cysteine protease InterpainA |
| R | AGTTGCGGAGGGTGGTTATG |
F, forward; R, reverse.
Statistical analyses.
Statistical analyses were performed in accordance with the results of Shapiro-Wilk test of normal distribution. The data were further analyzed by use of 1-way analysis of variance (ANOVA), followed by the Tukey's honestly significant difference (HSD) multiple-comparison post hoc test. All values were expressed as means ± standard deviations (SDs). All statistical analyses were performed using IBM SPSS version 22 statistical software (IBM SPSS, Chicago, IL).
RESULTS
Effect of polyP3 against planktonic P. intermedia ATCC 4906.
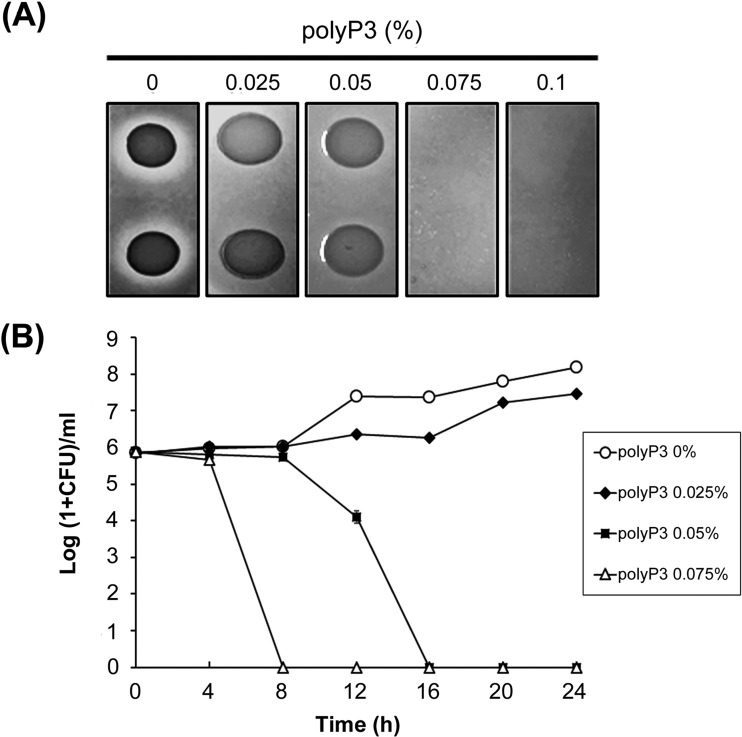
By the agar dilution method, the MIC of polyP3 against P. intermedia ATCC 49046 was determined to be 0.075% (Fig. 1A). The antibacterial effect of polyP3 on planktonic P. intermedia cells was further monitored in B-HK by counting viable cells at 4-h intervals for 24 h. As shown in Fig. 1B, 0.025% polyP3 had no apparent killing effect on planktonic P. intermedia cells. The bactericidal effect of polyP3 was observed at a concentration of 0.05%, which is lower than the MIC assessed on the brucella agar plate, and complete killing was observed at 16 h. In the presence of 0.075% polyP3, P. intermedia cells were completely killed after 8 h.
FIG 1.
Antibacterial effect of polyP3 against P. intermedia ATCC 4906. (A) MIC determination of polyP3 by agar dilution method. The bacterial cells were spot inoculated (approximately 105 to 106 cells/spot) onto brucella blood agar plates containing polyP3 at various concentrations and incubated at 37°C for 3 days anaerobically. The MIC was defined as the lowest concentration that inhibited the bacterial growth on the plate. (B) Time-kill curve of polyP3 against P. intermedia ATCC 49046 in liquid medium. Results are presented as the means ± SDs from three independent experiments. In the presence of 0.05 and 0.075% polyP3, complete killing of P. intermedia cells was observed at 16 h and 8 h, respectively.
Effect of polyP3 on the development and ultrastructure of biofilms.
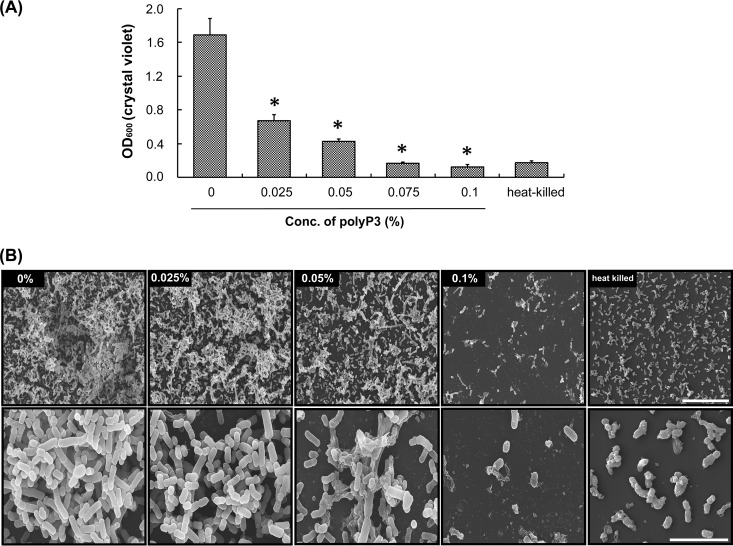
We investigated the effect of polyP3 on biofilm formation of P. intermedia by the crystal violet staining method using microtiter plates. As shown in Fig. 2A, polyP3 at concentrations of 0.025 to 0.1% decreased the amount of P. intermedia biofilm to 39.7 to 7.5% of the control. It was found that a small amount of the heat-killed P. intermedia cells adhered to the surface of the polystyrene culture plate. The amount of the surface-attached population formed by viable cells of P. intermedia that were incubated with 0.075 to 0.1% polyP3 was the same as or even lower than the amount of the heat-killed cells attached to the surface. SEM observation revealed that P. intermedia biofilm grew prolifically on the polystyrene surface of the culture plate, showing a multilayered structure in the absence of polyP3 (Fig. 2B). Incubation of P. intermedia with 0.025% polyP3 for 24 h did not cause any detectable change in bacterial cell shape but substantially decreased the density of the biofilm cells. Many fewer P. intermedia biofilm cells were observed on the plate when the bacterium was incubated with 0.05 and 0.1% polyP3 for 24 h, and some small pieces of cell debris were also found. Even the biofilm cells, especially when incubated with 0.1% polyP3, appeared to be irregular in shape and shorter than biofilm cells in the presence of 0 to 0.05% polyP3.
FIG 2.
Effect of polyP3 on biofilm formation and ultrastructure of P. intermedia ATCC 4906. A P. intermedia culture was diluted to an OD600 of approximately 0.1 in B-HK broth and then further incubated in 24-well plates with polyP3 at the indicated concentrations for 24 h. (A) Analyses of the biofilm biomass of P. intermedia ATCC 49046. The biofilm biomass was quantitated by crystal violet staining. Heat-killed bacterial cells that were initially killed by exposure to 100°C for 10 min were included as controls. Data are means ± SDs from two independent experiments performed in triplicate. *, P < 0.05, versus value for control. (B) SEM images of P. intermedia biofilm cells. Scale bars, 20 μm (upper) and 5 μm (lower).
Effects of polyP3 on preformed biofilms.
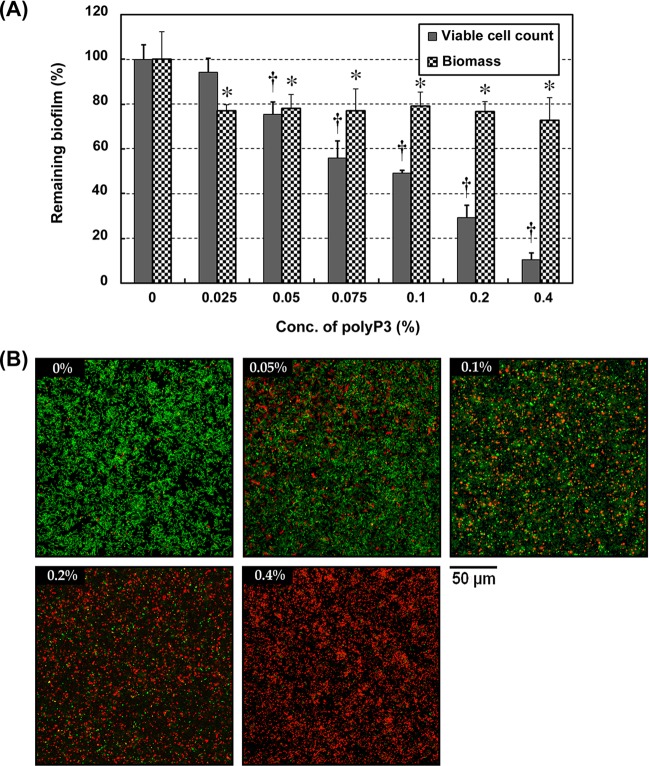
Bacteria that live inside the biofilm are strongly resistant to antimicrobials. High levels of antibiotic resistance were found in P. intermedia ATCC 49046 biofilms (19). To identify whether polyP3 also has inhibitory activity against already established P. intermedia ATCC 49046 biofilms in vitro, we measured the biomass and the viability of the biofilms by crystal violet staining and viable cell count, respectively. As shown in Fig. 3A, the biofilm biomass of the preformed biofilm exposed to polyP3 (0.025 to 0.4%) was decreased to 78.2 to 72.8% of the control. On the other hand, polyP3 (0.05 to 0.4%) significantly decreased the biofilm viability, to 75.4 to 10.6%, in a concentration-dependent manner. The viability of the preestablished P. intermedia biofilms exposed to polyP3 (0.05 to 0.4%) was further examined by CLSM. The biofilms were stained with a LIVE/DEAD BacLight bacterial viability kit consisting of green fluorescent SYTO 9, which stains live bacteria, and red fluorescent propidium iodide, which only penetrates nonviable bacterial cells with damaged or perturbed cell membranes (35). While unexposed control biofilm was shown to be predominantly green (viable), the fraction of red color (nonviable) increased in the polyP3-exposed biofilm (Fig. 3B).
FIG 3.
Effect of polyP3 on viability of preformed P. intermedia biofilm. Preestablished biofilms of P. intermedia ATCC 49046 were treated with polyP3 at the indicated concentrations for 24 h. (A) Biofilm biomass was quantitated by crystal violet staining. The viable biofilm bacteria were enumerated by detachment of cells, followed by agar plating count. The results are expressed as the means ± SDs from two independent experiments performed in triplicate. *, P < 0.05, versus value for control. (B) CLSM images of the P. intermedia biofilms. Magnification is ×600.
Effect of polyP3 on EPS.
EPS is the primary matrix material of biofilm, and viscosity of culture supernatant is an indication of the relative amount of EPS production in the culture (35, 37, 38). Yamanaka et al. (39, 40) reported that clinical isolates of P. intermedia designated strain 17 and strain OD1-16 produced mannose-rich EPSs, as revealed by the increased viscosity of the spent culture media. In contrast, the viscosity of the spent culture media of non-biofilm-forming bacteria, including P. intermedia ATCC 25611 and several P. gingivalis strains, was similar to that of the control medium without bacterial inoculation (40). As observed in our previous study (36), the viscosity of the spent culture medium of P. intermedia ATCC 49046 was significantly increased, at 1.31 ± 0.012 mPa · s, compared to that of the control B-HK medium without bacterial inoculation after 48 h of incubation. To identify whether polyP3 affects the amount of EPS produced by P. intermedia, we measured the viscosity of the spent culture media of the bacterium grown with and without 0.025% (1/3× MIC) polyP3. No significant difference was seen between the viscosities of the two spent culture media. The present study also measured the viscosity of the cell-free culture supernatants following treatment with polyP3 (0.05 to 1%) for 8 h. In the concentration range used, polyP3 did not significantly affect the viscosity of the bacterial spent culture medium. The degree of viscosity is strongly affected by the extent of polymer cross-linking (41, 42). It has been suggested that under appropriate conditions phosphates act as cation chelators, in which case they may reduce the viscosity of polysaccharide solutions as a result of decreased cation-induced molecular cross-linking (43). Therefore, it appears that polyP3 may not act as a cation chelator that can directly affect the cross-linking of polysaccharide in the EPS produced by P. intermedia.
Effects of polyP3 on hemolytic activity.
During the determination of MIC performed using brucella agar supplemented with sheep blood, hemin, and vitamin K1, differences in the zone sizes of alpha-hemolysis formed on brucella blood agar plates supplemented or not with polyP3 were often observed (Fig. 1A).
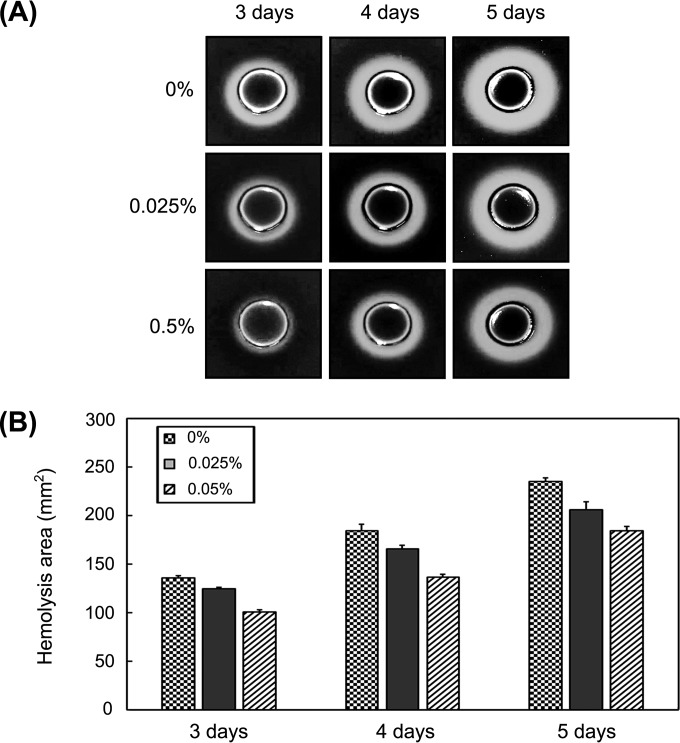
It has been reported that P. intermedia had stronger hemagglutinating and hemolytic activities with rabbit, horse, and human erythrocytes than sheep erythrocytes (44, 45). It has been also reported that the best hemolytic activity was observed for P. intermedia growing in BHIA in the absence of hemin and vitamin K1 (44). Hence, we further examined the effect of polyP3 on hemolytic activity of P. intermedia using horse blood BHIA without supplementation of hemin and vitamin K1, which is capable of inducing strong hemolytic activity of the bacterium. The minimum time of incubation for the detection of hemolysis on the horse blood BHIA was 3 days regardless of polyP3 addition. When zone sizes of beta-hemolysis on horse blood BHIA plates by P. intermedia were evaluated, they were found to be smaller on the blood agar plates containing polyP3 than those on the plates without polyP3 (Fig. 4).
FIG 4.
Effect of polyP3 on beta-hemolysis produced by P. intermedia ATCC 49046. Various concentrations of polyP3 were prepared and added to 5% horse blood BHIA in the absence of hemin and vitamin K1. P. intermedia was incubated on the agar plates for 5 days at 37°C. (A) Images of beta-hemolysis zone produced after 3, 4, and 5 days. (B) Area of the beta-hemolysis zone quantified using ImageJ version 1.49j (National Institutes of Health, Bethesda, MD). Results are expressed as means ± SDs from two independent experiments.
Differential expression of the genes related to hemolysis and hemin uptake.
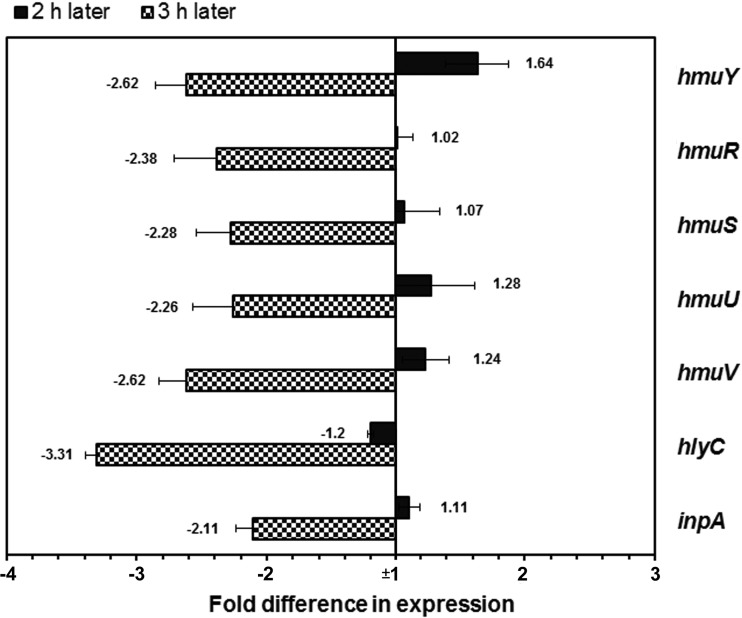
qRT-PCR was used to examine the expression of the genes encoding hemolysins (HlyC and InpA) and the genes of the hemin uptake hmu locus encoding HmuY, HmuR, HmuS, HmuU, and HmuV. When P. intermedia cells were exposed to 0.05% polyP3 for 2 h, hmuY was upregulated over 1.5-fold, but the other hmu genes were not changed; 3 h later, all of the genes tested were downregulated over 2-fold (Fig. 5).
FIG 5.
Expression levels of the genes related to hemin uptake and hemolysis in the presence of 0.05% polyP3. The expression of the genes was measured by qRT-PCR and normalized to that of the 16S rRNA gene. The expression level of each gene in the absence of polyP3 was set as 1-fold. The results are expressed as the means ± SDs from three independent experiments.
DISCUSSION
Black-pigmented anaerobes such as P. gingivalis, P. intermedia, and Prevotella nigrescens depend largely on external hemin as an iron source for their growth (45). Black pigment on the cell surface of the species is believed to result from accumulation of hemin (46). The redox potential of hemin, required as a prosthetic group of cytochrome b, allows it to mediate electron transfer with generation of cellular energy (31, 46). These bacteria, by virtue of their hemolytic activity, are capable of liberating the hemoglobin or hemin from erythrocytes, thereby acquiring the essential nutrient for their metabolism (44, 47, 48). It has been assumed that the high hemolytic and hemagglutinating activities of P. intermedia not only play a significant role in the growth of P. intermedia itself but also support the growth of other black-pigmented anaerobes, such as P. gingivalis, in subgingival sites (45).
In the present study, polyP3 was demonstrated to be an effective antibacterial agent against P. intermedia. The MIC of polyP3 determined by the CLSI agar dilution method was 0.075%. However, in time-kill analysis performed using liquid medium, complete killing of the bacterial cells was observed with polyP3 at the concentration of 0.05%, which is lower than the MIC assessed on the brucella agar plate (Fig. 1). Similarly, in a previous study using P. gingivalis W83, it was observed that the MIC of polyP with a chain length of 75 (polyP75) determined by the CLSI agar dilution method was 0.06%, while 0.03% polyP75 caused complete killing of P. gingivalis in broth (31). These findings are also in agreement with another study (49), in which agar dilution MICs tended to be slightly higher than the MICs determined by broth dilution methods.
Several studies have demonstrated that the antibacterial mechanism of polyP against Gram-positive bacteria is associated with its metal ion-chelating nature. polyP induces direct lysis of S. aureus by chelation of structurally essential metal ions in their membranes (24, 28). Another mechanism for antimicrobial activity of polyP, suggested by Maier et al. (27), is that polyP may affect the ubiquitous bacterial cell division protein FtsZ, whose GTPase activity is strictly dependent on divalent metal ions. Indeed, polyP-induced cell lysis was not observed in stationary-phase B. cereus, which lacks active growth and cell division (27). Moreover, B. cereus cells exposed to sublethal concentration of polyP exhibited aseptate and elongated morphology (27). Recently, unlike most Gram-negative bacteria tested, P. gingivalis was demonstrated to be susceptible to polyP (31). In the study, polyP exerted an antibacterial effect against P. gingivalis cells in stationary as well as exponential phase. Furthermore, no P. gingivalis cells with obviously aseptate and elongated morphology were observed when the bacterial cells were exposed to polyP (31). Instead, it was observed in the study that utilization of hemin in P. gingivalis was disturbed by polyP.
In the present study, the zone sizes of beta-hemolysis produced by P. intermedia were decreased on horse blood agar containing polyP3 at sub-MICs (Fig. 4). These findings were confirmed by our qRT-PCR, in which hlyC and inpA encoding representative P. intermedia hemolysins (50) were downregulated by polyP3 (Fig. 5). These results clearly indicate that polyP3 inhibited hemolytic activity of P. intermedia. Once released from host proteins, hemin is transported into the cell, across two membranes of the Gram-negative bacterium, to be utilized. The selected genes involved in the hemin uptake system (20), i.e., hmuY, hmuR, hmuS, hmuU, and hmuV, were also downregulated in P. intermedia exposed to polyP3 for 3 h (Fig. 5). This result is similar to a previous observation with P. gingivalis W83, whose expression of the genes involved in hemolysis and hemin transportation was downregulated by polyP75 (31). All of these results suggest that polyP may affect the hemin acquisition process in the black-pigmented anaerobes, from hemolysis to transportation of hemin into cell.
Notably, the expression of hmuY was temporarily increased 1.64-fold at 2 h but was decreased 2.62-fold at 3 h (Fig. 5). It has been known that iron affects the transcription of as many as 10 to 20% of genes identified in P. gingivalis genomes, and the hmu locus is significantly upregulated under iron-limited conditions (51). HmuY and HmuR are considered outer membrane hemin receptors, while HmuSTUV proteins are involved in processing and transport of the hemin molecule across the inner membrane (51). Especially, HmuY lipoprotein is preferentially observed as the major outer membrane protein of P. gingivalis under iron-depleted conditions (52). Therefore, it seems that a temporal increase in expression of hmuY reflects the struggle for iron in P. intermedia whose hemin utilization is disturbed.
It should be noted that hemolysin production by P. gingivalis was significantly increased when the bacterial cells were grown in the presence of limiting hemin or in the absence of hemin (48) and that hemolytic activity of P. intermedia was decreased in solid media in the presence of hemin (44). In the present study, hemolytic activity of P. intermedia was significantly decreased in the presence of polyP3, as revealed by hemolysis assay on solid media and qRT-PCR (Fig. 4 and 5). Based on all these results, we speculate that antibacterial effect of polyP3 against black-pigmented Gram-negative bacteria is attributed to a disturbance of the heme or hemin acquisition process either in the immediate vicinity or on the surface of the bacterial cells, rather than induction of hemin or iron depletion conditions through chelating hemin or iron in the extracellular milieu of the bacterial cells.
Biofilm-grown cells express properties distinct from those of planktonic cells, one of which is an increased resistance to antimicrobial agents (53). As for some antibiotics, the concentration required to kill sessile bacteria in biofilm may be higher than a thousand times the concentration required to kill planktonic bacteria of the same strain (54). Biofilm-forming bacteria possess high resistance to antimicrobial agents by virtue of decreased metabolic activity, expression of resistant genes, or production of EPS through which penetration of antimicrobial agents is restricted (55–58).
In the present study, the biofilm formation by an inoculum of P. intermedia ATCC 49046 incubated in B-HK for 24 h was completely inhibited in the presence of polyP3 at concentrations of 0.075% (1× MIC) and 0.1% (Fig. 2). It was also observed that the amount of the biofilm formed by the bacterium was significantly decreased by polyP3 even at a concentration of 0.025% (1/3× MIC). On the other hand, EPS production by P. intermedia cells was not affected by 0.025% polyP3, and even higher concentrations of polyP3 (up to 1%) appeared not to affect the extent of cross-linking in the EPS, as reflected by the viscosity of the culture media. Therefore, it is quite conceivable that the effect of polyP3 to reduce biofilm formation of P. intermedia is mostly due to its inhibitory effect on bacterial cell growth, thereby decreasing the total number of bacterial cells. We also observed the effect of polyP3 on already-formed P. intermedia biofilm. Higher concentrations of polyP (0.05 to 0.4%) reduced the survival rates of the preformed biofilms by up to 89.4% in a concentration manner, while lower concentrations of polyP (0.025 to 0.4%) caused a relatively small but statistically significant decrease in the biofilm biomass, by as much as 27.2% (Fig. 3). It seems that polyP3 is able to easily diffuse through EPS and kill the bacteria in the biofilms.
Collectively, our results show that polyP is an effective antimicrobial agent against P. intermedia in biofilms as well as planktonic phase. Moreover, the bactericidal effect of polyP against P. gingivalis has previously been confirmed. These results indicate that polyP can be an alternative antibacterial agent for controlling black-pigmented Gram-negative oral pathogens. It is noteworthy that polyP not only has an excellent safety profile (32) but also can enhance bone formation (33) and promote the growth, differentiation, and angiogenic potential of human dental pulp cells (59). Therefore, use of polyP seems to be a fascinating and safe strategy in the prevention and treatment of oral diseases like chronic periodontitis and endodontic infection-periapical periodontitis, in which black-pigmented anaerobic bacteria are implicated, with an expectation of its additional beneficial effects of promoting bone formation and engineering dental pulp tissue. Applying such a strategy, however, requires further study to ensure the effectiveness of polyP against multispecies oral biofilms in vivo.
ACKNOWLEDGMENTS
This work was supported by the Basic Science Research Program through the National Foundation of Korea funded by the Ministry of Education, Science and Technology (MRC, 2012R1A5A2051384).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Ashimoto A, Chen C, Bakker I, Slots J. 1996. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol 11:266–273. doi: 10.1111/j.1399-302X.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 2.Petit MD, Steenbergen TJ, Timmerman MF, de Graaff J, van der Velden U. 1994. Prevalence of periodontitis and suspected periodontal pathogens in families of adult periodontitis patients. J Clin Periodontol 21:76–85. doi: 10.1111/j.1600-051X.1994.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 3.Polson AM, Garrett S, Stoller NH, Bandt CL, Hanes PJ, Killoy WJ, Southard GL, Duke SP, Bogle GC, Drisko CH, Friesen LR. 1997. Multi-center comparative evaluation of subgingivally delivered sanguinarine and doxycycline in the treatment of periodontitis. II. Clinical results. J Periodontol 68:119–126. [DOI] [PubMed] [Google Scholar]
- 4.Albandar JM, Brown LJ, Löe H. 1997. Putative periodontal pathogens in subgingival plaque of young adults with and without early-onset periodontitis. J Periodontol 68:973–981. doi: 10.1902/jop.1997.68.10.973. [DOI] [PubMed] [Google Scholar]
- 5.Kuru B, Yilmaz S, Noyan U, Acar O, Kadir T. 1999. Microbiological features and crevicular fluid aspartate aminotransferase enzyme activity in early onset periodontitis patients. J Clin Periodontol 26:19–25. doi: 10.1034/j.1600-051X.1999.260104.x. [DOI] [PubMed] [Google Scholar]
- 6.Kamma JJ, Nakou M, Gmür R, Baehni PC. 2004. Microbiological profile of early onset/aggressive periodontitis patients. Oral Microbiol Immunol 19:314–321. doi: 10.1111/j.1399-302x.2004.00161.x. [DOI] [PubMed] [Google Scholar]
- 7.Rowland RW, Mestecky J, Gunsolley JC, Cogen RB. 1993. Serum IgG and IgM levels to bacterial antigens in necrotizing ulcerative gingivitis. J Periodontol 64:195–201. doi: 10.1902/jop.1993.64.3.195. [DOI] [PubMed] [Google Scholar]
- 8.Novak MJ. 1999. Necrotizing ulcerative periodontitis. Ann Periodontol 4:74–78. doi: 10.1902/annals.1999.4.1.74. [DOI] [PubMed] [Google Scholar]
- 9.Gomes BP, Drucker DB, Lilley JD. 1994. Associations of specific bacteria with some endodontic signs and symptoms. Int Endod J 27:291–298. doi: 10.1111/j.1365-2591.1994.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 10.Jacinto RC, Gomes BP, Ferraz CC, Zaia AA, Filho FJ. 2003. Microbiological analysis of infected root canals from symptomatic and asymptomatic teeth with periapical periodontitis and the antimicrobial susceptibility of some isolated anaerobic bacteria. Oral Microbiol Immunol 18:285–292. doi: 10.1034/j.1399-302X.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 11.Bolivar I, Whiteson K, Stadelmann B, Baratti-Mayer M, Gizard Y, Mombelli A, Pittet D, Schrenzel J. 2012. Bacterial diversity in oral samples of children in Niger with acute noma, acute necrotizing gingivitis, and healthy controls. PLoS Negl Trop Dis 6:e1556. doi: 10.1371/journal.pntd.0001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkler WA Jr, Enwonwu CO, Idigbe EO. 1999. Microbiological understandings and mysteries of noma (cancrum oris). Oral Dis 5:150–155. [DOI] [PubMed] [Google Scholar]
- 13.Shinzato T, Saito A. 1994. A mechanism of pathogenicity of “Streptococcus milleri group” in pulmonary infection: synergy with an anaerobe. J Med Microbiol 40:118–123. doi: 10.1099/00222615-40-2-118. [DOI] [PubMed] [Google Scholar]
- 14.Ulrich M, Beer I, Braitmaier P, Dierkes M, Kummer F, Krismer B, Schumacher U, Gräpler-Mainka U, Riethmüller J, Jensen PØ, Bjarnsholt T, Høiby N, Bellon G, Döring G. 2010. Relative contribution of Prevotella intermedia and Pseudomonas aeruginosa to lung pathology in airways of patients with cystic fibrosis. Thorax 65:978–984. doi: 10.1136/thx.2010.137745. [DOI] [PubMed] [Google Scholar]
- 15.Brook I, Frazier EH. 2003. Immune response to Fusobacterium nucleatum and Prevotella intermedia in the sputum of patients with acute exacerbation of chronic bronchitis. Chest 124:832–833. doi: 10.1378/chest.124.3.832. [DOI] [PubMed] [Google Scholar]
- 16.Fiehn NE, Larsen T, Christiansen N, Holmstrup P, Schroeder TV. 2005. Identification of periodontal pathogens in atherosclerotic vessels. J Periodontol 76:731–736. doi: 10.1902/jop.2005.76.5.731. [DOI] [PubMed] [Google Scholar]
- 17.Andrés MT, Chung WO, Roberts MC, Fierro JF. 1998. Antimicrobial susceptibilities of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens spp. isolated in Spain. Antimicrob Agents Chemother 42:3022–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fosse T, Madinier I, Hannoun L, Giraud-Morin C, Hitzig C, Charbit Y, Ourang S. 2002. High prevalence of cfxAβ-lactamase in aminopenicillin-resistant Prevotella strains isolated from periodontal pockets. Oral Microbiol Immunol 17:85–88. doi: 10.1046/j.0902-0055.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi N, Ishihara K, Kimizuka R, Okuda K, Kato T. 2006. The effects of tetracycline, minocycline, doxycycline and ofloxacin on Prevotella intermedia biofilm. Oral Microbiol Immunol 21:366–371. doi: 10.1111/j.1399-302X.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu F, Anaya C, Lewis JP. 2007. Outer membrane proteome of Prevotella intermedia 17: identification of thioredoxin and iron-repressible hemin uptake loci. Proteomics 7:403–412. doi: 10.1002/pmic.200600441. [DOI] [PubMed] [Google Scholar]
- 21.Kornberg A, Rao NN, Ault-Riché D. 1999. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem 68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 22.Post FJ, Krishnamurty GB, Flanagan MD. 1963. Influence of sodium hexametaphosphate on selected bacteria. Appl Microbiol 11:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knabel S, Walker H, Harman P. 1991. Inhibition of Aspergillus flavus and selected gram-positive bacteria by chelation of essential metal cations by polyphosphates. J Food Prot 54:360–365. [DOI] [PubMed] [Google Scholar]
- 24.Lee RM, Hartman PA, Olson DG, Williams FD. 1994. Bactericidal and bacteriolytic effects of selected food-grade phosphates, using Staphylococcus aureus as a model system. J Food Prot 57:276–283. [DOI] [PubMed] [Google Scholar]
- 25.Zaika L, Kim A. 1993. Effect of sodium polyphosphates on growth of Listeria monocytogenes. J Food Prot 56:577–580. [DOI] [PubMed] [Google Scholar]
- 26.Rajkowski KT, Calderone SM, Jones E. 1994. Effect of polyphosphate and sodium chloride on the growth of Listeria monocytogenes and Staphylococcus aureus in ultra-high temperature milk. J Dairy Sci 77:1503–1508. doi: 10.3168/jds.S0022-0302(94)77089-2. [DOI] [PubMed] [Google Scholar]
- 27.Maier SK, Scherer S, Loessner MJ. 1999. Long-chain polyphosphate causes cell lysis and inhibits Bacillus cereus septum formation, which is dependent on divalent cations. Appl Environ Microbiol 65:3942–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RM, Hartman PA, Stahr HM, Olson DG, Williams FD. 1994. Antibacterial mechanism of long-chain polyphosphates in Staphylococcus aureus. J Food Prot 57:289–294. [DOI] [PubMed] [Google Scholar]
- 29.Shibata H, Morioka T. 1982. Antibacterial action of condensed phosphates on the bacterium Streptococcus mutans and experimental caries in the hamster. Arch Oral Biol 27:809–816. doi: 10.1016/0003-9969(82)90034-6. [DOI] [PubMed] [Google Scholar]
- 30.Obritsch JA, Ryu D, Lampila LE, Bullerman LB. 2008. Antibacterial effects of long-chain polyphosphates on selected spoilage and pathogenic bacteria. J Food Prot 71:1401–1405. [DOI] [PubMed] [Google Scholar]
- 31.Moon JH, Park JH, Lee JY. 2011. Antibacterial action of polyphosphate on Porphyromonas gingivalis. Antimicrob Agents Chemother 55:806–812. doi: 10.1128/AAC.01014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanigan RS. 2001. Final report on the safety assessment of sodium metaphosphate, sodium trimetaphosphate, and sodium hexametaphosphate. Int J Toxicol 3:75–89. [DOI] [PubMed] [Google Scholar]
- 33.Hacchou Y, Uematsu T, Ueda O, Usui Y, Uematsu S, Takahashi M, Uchihashi T, Kawazoe Y, Shiba T, Kurihara S, Yamaoka M, Furusawa K. 2007. Inorganic polyphosphate: a possible stimulant of bone formation. J Dent Res 86:893–897. doi: 10.1177/154405910708600917. [DOI] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard, 7th ed Document M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 35.Moon JH, Jang EY, Shim KS, Lee JY. 2015. In vitro effects of N-acetyl cysteine alone and in combination with antibiotics on Prevotella intermedia. J Microbiol 53:321–329. doi: 10.1007/s12275-015-4500-2. [DOI] [PubMed] [Google Scholar]
- 36.Moon JH, Kim C, Lee HS, Kim SW, Lee JY. 2013. Antibacterial and antibiofilm effects of iron chelators against Prevotella intermedia. J Med Microbiol 62:1307–1316. doi: 10.1099/jmm.0.053553-0. [DOI] [PubMed] [Google Scholar]
- 37.Denny TP, Makini FW, Brumbley SM. 1988. Characterization of Pseudomonas solanacearum Tn5 mutants deficient in extracellular polysaccharide. Mol Plant Microbe Interact 1:215–223. doi: 10.1094/MPMI-1-215. [DOI] [Google Scholar]
- 38.Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamanaka T, Furukawa T, Matsumoto-Mashimo C, Yamane K, Sugimori C, Nambu T, Mori N, Nishikawa H, Walker CB, Leung KP, Fukushima H. 2009. Gene expression profile and pathogenicity of biofilm-forming Prevotella intermedia strain 17. BMC Microbiol 16:11. doi: 10.1186/1471-2180-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamanaka T, Yamane K, Furukawa T, Matsumoto-Mashimo C, Sugimori C, Nambu T, Obata N, Walker CB, Leung KP, Fukushima H. 2011. Comparison of the virulence of exopolysaccharide-producing Prevotella intermedia to exopolysaccharide non-producing periodontopathic organisms. BMC Infect Dis 11:228. doi: 10.1186/1471-2334-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopkins MJ, Englyst HN, Macfarlane S, Furrie E, Macfarlane GT, McBain AJ. 2003. Degradation of cross-linked and non-cross-linked arabinoxylans by the intestinal microbiota in children. Appl Environ Microbiol 69:6354–6360. doi: 10.1128/AEM.69.11.6354-6360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isobe Y, Endo K, Kawai H. 1992. Properties of a highly viscous polysaccharide produced by a Bacillus strain isolated from soil. Biosci Biotechnol Biochem 56:636–639. doi: 10.1271/bbb.56.636. [DOI] [PubMed] [Google Scholar]
- 43.Molins RA. 1990. Phosphates in food, p 66–70. CRC Press, Boca Raton, FL. [Google Scholar]
- 44.Silva TA, Rodrigues PH, Ribeiro RN, Noronha FS, Farias Lde M, Carvalho MA. 2003. Hemolytic activity of Prevotella intermedia and Prevotella nigrescens strains: influence of abiotic factors in solid and liquid assays. Res Microbiol 154:29–35. doi: 10.1016/S0923-2508(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto M, Maeda N, Kondo K, Leung KP. 1999. Hemolytic and hemagglutinating activities of Prevotella intermedia and Prevotella nigrescens. FEMS Microbiol Lett 178:299–304. doi: 10.1111/j.1574-6968.1999.tb08691.x. [DOI] [PubMed] [Google Scholar]
- 46.Lewis JP, Dawson JA, Hannis JC, Muddiman D, Macrina FL. 1999. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J Bacteriol 181:4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beem JE, Nesbitt WE, Leung KP. 1998. Identification of hemolytic activity in Prevotella intermedia. Oral Microbiol Immunol 13:97–105. doi: 10.1111/j.1399-302X.1998.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 48.Chu L, Bramanti TE, Ebersole JL, Holt SC. 1991. Hemolytic activity in the periodontopathogen Porphyromonas gingivalis: kinetics of enzyme release and localization. Infect Immun 59:1932–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenny MT, Dulworth JK, Brackman MA. 1989. Comparison of the agar dilution, tube dilution, and broth microdilution susceptibility tests for determination of teicoplanin MICs. J Clin Microbiol 27:1409–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruan Y, Shen L, Zou Y, Qi Z, Yin J, Jiang J, Guo L, He L, Chen Z, Tang Z, Qin S. 2015. Comparative genome analysis of Prevotella intermedia strain isolated from infected root canal reveals features related to pathogenicity and adaptation. BMC Genomics 16:122. doi: 10.1186/s12864-015-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis JP. 2010. Metal uptake in host-pathogen interactions: role of iron in Porphyromonas gingivalis interactions with host organisms. Periodontol 2000 52:94–116. doi: 10.1111/j.1600-0757.2009.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis JP, Plata K, Yu F, Rosato A, Anaya C. 2006. Transcriptional organization, regulation and role of the Porphyromonas gingivalis W83 hmu haemin-uptake locus. Microbiology 152:3367–3382. doi: 10.1099/mic.0.29011-0. [DOI] [PubMed] [Google Scholar]
- 53.Mah TF, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 54.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. 2002. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res 66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 55.Rice LB. 2006. Antimicrobial resistance in gram-positive bacteria. Am J Med 119:S11–S19; discussion, S64–S73. [DOI] [PubMed] [Google Scholar]
- 56.Tenover FC. 2006. Mechanisms of antimicrobial resistance in bacteria. Am J Med 119:S3–10; discussion S62–70. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Frank KL, Reichert EJ, Piper KE, Patel R. 2007. In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob Agents Chemother 51:888–895. doi: 10.1128/AAC.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M. 2011. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis 15:305–311. doi: 10.1590/S1413-86702011000400002. [DOI] [PubMed] [Google Scholar]
- 59.Bae WJ, Jue SS, Kim SY, Moon JH, Kim EC. 2015. Effects of sodium tri- and hexametaphosphate on proliferation, differentiation, and angiogenic potential of human dental pulp cells. J Endod 41:896–902. doi: 10.1016/j.joen.2015.01.038. [DOI] [PubMed] [Google Scholar]