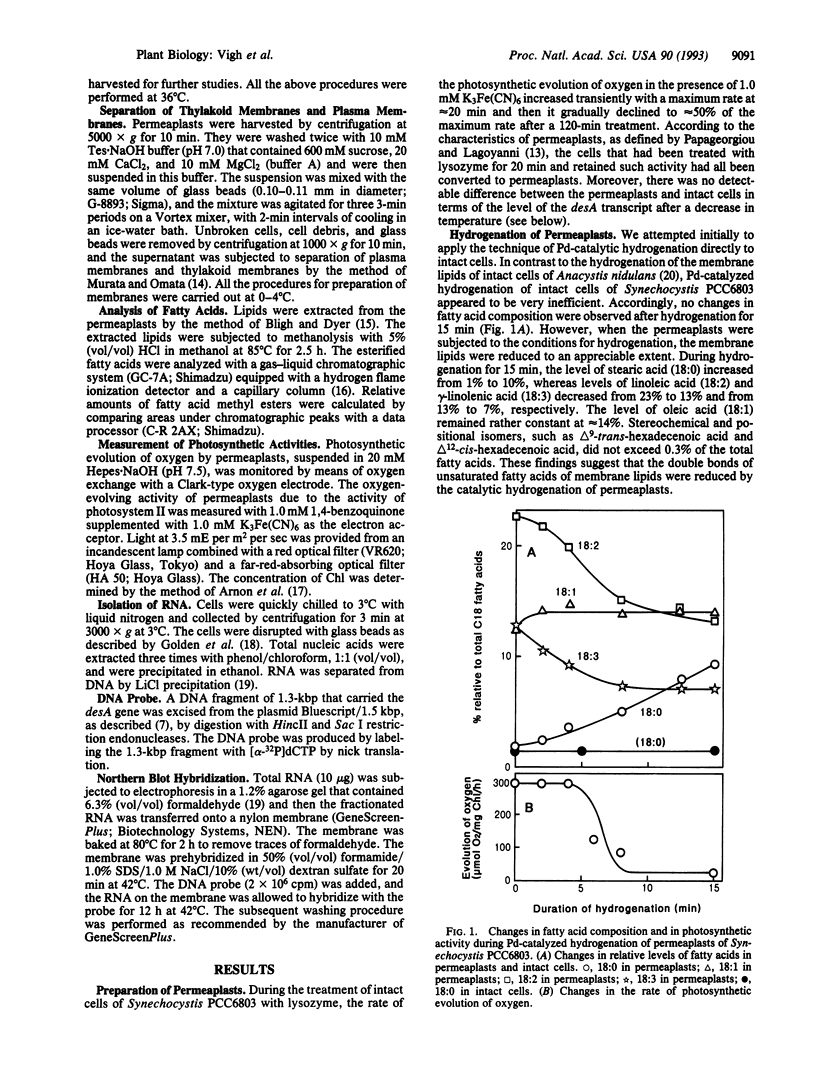
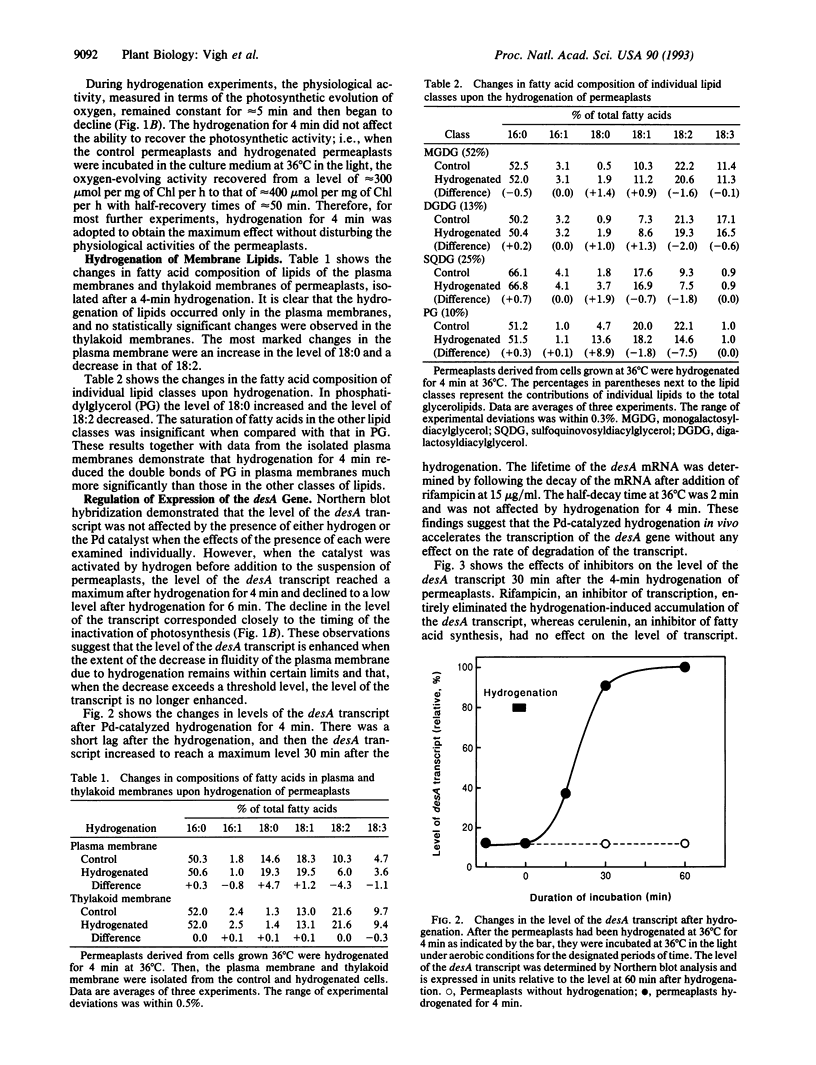
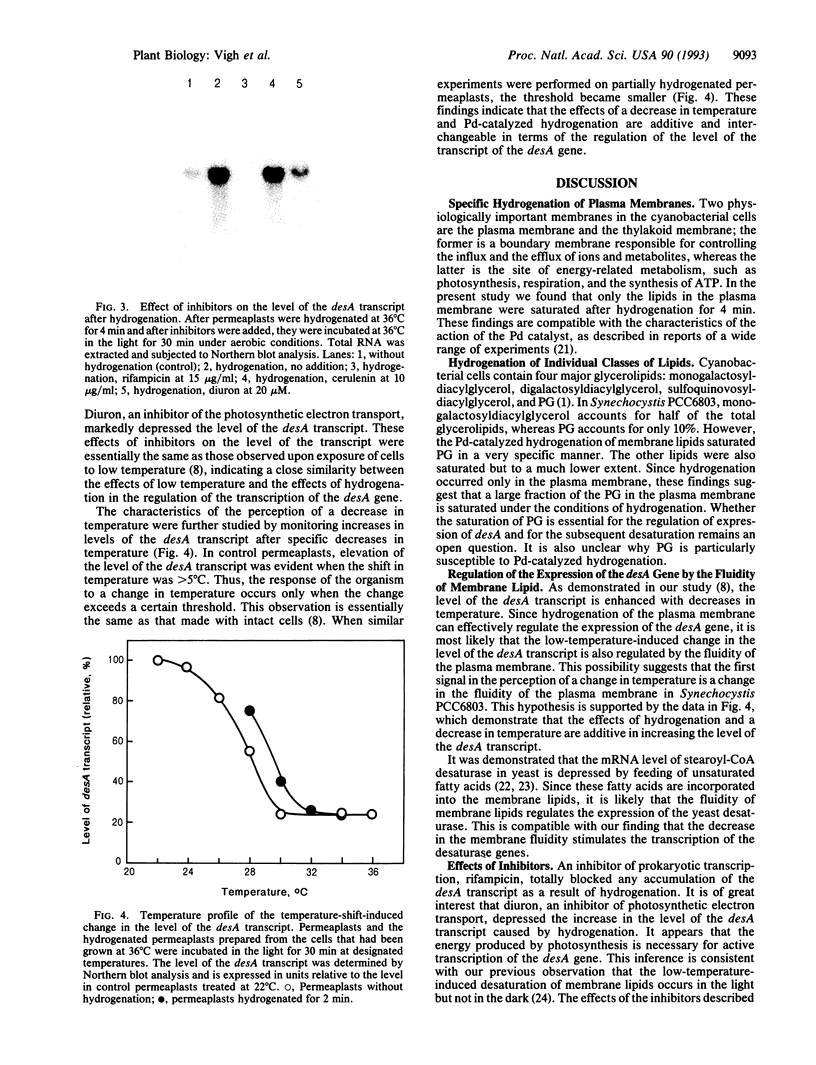
Abstract
One of the well-characterized phenomena associated with the acclimation of organisms to changes in ambient temperature is the regulation of the molecular motion or "fluidity" of membrane lipids via changes in the extent of unsaturation of the fatty acids of membrane lipids. The enzymes responsible for this process when the temperature is decreased are the desaturases, the activities of which are enhanced at low temperature. To examine whether the change in the fluidity of membrane lipids is the first event that signals a change in temperature, we studied the effect of the Pd-catalyzed hydrogenation of membrane lipids on the expression of the desA gene, which is responsible for the desaturation of fatty acids of membrane lipids in the cyanobacterium Synechocystis PCC6803. The Pd-catalyzed hydrogenation of plasma membrane lipids stimulated the expression of the desA gene. We also found that, for unexplained reasons, the hydrogenation was much more specific to a minor phospholipid, phosphatidylglycerol, than to members of other lipid classes. These results suggest that the organism perceives a decrease in the fluidity of plasma membrane lipids when it is exposed to a decrease in temperature.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bossie M. A., Martin C. E. Nutritional regulation of yeast delta-9 fatty acid desaturase activity. J Bacteriol. 1989 Dec;171(12):6409–6413. doi: 10.1128/jb.171.12.6409-6413.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Joó F., Balogh N., Horváth L. I., Filep G., Horváth I., Vígh L. Complex hydrogenation/oxidation reactions of the water-soluble hydrogenation catalyst palladium di (sodium alizarinmonosulfonate) and details of homogeneous hydrogenation of lipids in isolated biomembranes and living cells. Anal Biochem. 1991 Apr;194(1):34–40. doi: 10.1016/0003-2697(91)90147-l. [DOI] [PubMed] [Google Scholar]
- Los D., Horvath I., Vigh L., Murata N. The temperature-dependent expression of the desaturase gene desA in Synechocystis PCC6803. FEBS Lett. 1993 Feb 22;318(1):57–60. doi: 10.1016/0014-5793(93)81327-v. [DOI] [PubMed] [Google Scholar]
- McDonough V. M., Stukey J. E., Martin C. E. Specificity of unsaturated fatty acid-regulated expression of the Saccharomyces cerevisiae OLE1 gene. J Biol Chem. 1992 Mar 25;267(9):5931–5936. [PubMed] [Google Scholar]
- Murata N. Low-temperature effects on cyanobacterial membranes. J Bioenerg Biomembr. 1989 Feb;21(1):61–75. doi: 10.1007/BF00762212. [DOI] [PubMed] [Google Scholar]
- Ono T. A., Murata N. Chilling Susceptibility of the Blue-green Alga Anacystis nidulans: I. EFFECT OF GROWTH TEMPERATURE. Plant Physiol. 1981 Jan;67(1):176–181. doi: 10.1104/pp.67.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Joo F., Vigh L. The role of unsaturated lipids in membrane structure and stability. Prog Biophys Mol Biol. 1989;53(2):71–103. doi: 10.1016/0079-6107(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh L., Horváth I., Thompson G. A., Jr Recovery of Dunaliella salina cells following hydrogenation of lipids in specific membranes by a homogeneous palladium catalyst. Biochim Biophys Acta. 1988 Jan 13;937(1):42–50. doi: 10.1016/0005-2736(88)90225-8. [DOI] [PubMed] [Google Scholar]
- Wada H., Gombos Z., Murata N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature. 1990 Sep 13;347(6289):200–203. doi: 10.1038/347200a0. [DOI] [PubMed] [Google Scholar]
- Wada H., Murata N. Temperature-Induced Changes in the Fatty Acid Composition of the Cyanobacterium, Synechocystis PCC6803. Plant Physiol. 1990 Apr;92(4):1062–1069. doi: 10.1104/pp.92.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]