Abstract
Oral candidiasis (OC), caused by the fungal pathogen Candida albicans, is the most common opportunistic infection in HIV+ individuals and other immunocompromised populations. The dramatic increase in resistance to common antifungals has emphasized the importance of identifying unconventional therapeutic options. Antimicrobial peptides have emerged as promising candidates for therapeutic intervention due to their broad antimicrobial properties and lack of toxicity. Histatin-5 (Hst-5) specifically has exhibited potent anticandidal activity indicating its potential as an antifungal agent. To that end, the goal of this study was to design a biocompatible hydrogel delivery system for Hst-5 application. The bioadhesive hydroxypropyl methylcellulose (HPMC) hydrogel formulation was developed for topical oral application against OC. The new formulation was evaluated in vitro for gel viscosity, Hst-5 release rate from the gel, and killing potency and, more importantly, was tested in vivo in our mouse model of OC. The findings demonstrated a controlled sustained release of Hst-5 from the polymer and rapid killing ability. Based on viable C. albicans counts recovered from tongues of treated and untreated mice, three daily applications of the formulation beginning 1 day postinfection with C. albicans were effective in protection against development of OC. Interestingly, in some cases, Hst-5 was able to clear existing lesions as well as associated tissue inflammation. These findings were confirmed by histopathology analysis of tongue tissue. Coupled with the lack of toxicity as well as anti-inflammatory and wound-healing properties of Hst-5, the findings from this study support the progression and commercial feasibility of using this compound as a novel therapeutic agent.
INTRODUCTION
The human fungal species Candida albicans is an opportunistic pathogen commonly colonizing human mucosal surfaces as a component of the normal microflora (1). However, when host defenses are weakened or when there is a disruption in the host environment, C. albicans can proliferate, causing an array of infections ranging from mucosal to systemic that are often life threatening (2–5). Oral candidiasis (OC), commonly known as thrush, is the most prevalent manifestation of candidiasis and is characterized by invasion of the mucosal tissue (6, 7). This condition is manifested as white lesions formed on the palate, buccal mucosa, and tongue or oropharynx causing oropharyngeal candidiasis. In HIV+ individuals, oropharyngeal candidiasis is considered an AIDS-defining illness, with 80 to 90% of these individuals suffering recurrent episodes during the course of their illness despite HIV therapy (2, 3, 8).
C. albicans is a highly adaptable fungal species, and its ability to transition from commensal to pathogen is primarily the result of its ability to morphologically switch between yeast and filamentous hyphal forms (1). In general, C. albicans infections are associated with its ability to form biofilms on host tissue and abiotic surfaces, where adhesion of yeast cells to the substrate is followed by proliferation and hyphal formation (9). Where the yeast form is associated with bloodstream and systemic disease, the hyphae are more adept at penetrating tissue and are therefore responsible for mucosal infections. Because of the high prevalence of candidiasis, there is intense interest in developing new approaches to prevent and treat this disease. Significantly, the increasing emergence of strains of C. albicans resistant to commonly used antifungal agents has made clinical management of candidiasis increasingly difficult (3, 10). Therefore, it has become crucial to identify alternative therapeutic agents.
Antimicrobial peptides (AMPs) are a group of natural compounds that have attracted considerable attention as potential therapeutic candidates for treatment of infections (11). An essential requirement for any antimicrobial agent is that it must have selective toxicity for the microbial target, which is an important feature of AMPs due to their preferential interaction with microbial cells, which makes them nontoxic to mammalian cells (12–15). Importantly, unlike conventional antimicrobials, AMPs do not induce resistance due to the profound changes in membrane structure warranted to confer the microbial cell resistance (12–14). These properties, combined with their broad range of activity and the short contact time required to induce killing, have led to their consideration as excellent candidates for development as novel pharmacologic agents (15).
Most notable among the antimicrobial peptides are the histatins, a family of histidine-rich, cationic proteins produced and secreted into saliva by human salivary glands considered to be an important part of host innate immunity (16, 17). In addition to defense of the oral cavity against microorganisms, several studies have also identified histatins as the prime agents that mediate the wound-healing activity of human saliva (18, 19). Among the histatins, histatin-5 (Hst-5) specifically, a 24-amino-acid peptide, has exhibited potent activity against C. albicans and other fungal species, including strains resistant to commonly used antifungal agents, indicating a different antifungal mode of action (20, 21). In addition, Hst-5 was also shown to exert a synergistic effect with azoles and amphotericin B antifungal drugs, indicating a potential use as a suitable candidate for combination therapy (22, 23). Histatin-5 is believed to exert its anticandidal effect through binding to receptor proteins (Ssa1 and Ssa2) on the fungal cell membrane (24). Once internalized, Hst-5 inhibits mitochondrial respiration, thus inducing the formation of reactive oxygen species leading to mitochondrial and cytoplasmic membrane damage, efflux of ATP, and cell death (25, 26).
Although Hst-5 has been shown to be efficacious on oral tissue by us and others using ex vivo and in vivo mouse infection models and its potential as an antifungal agent is appreciated, no studies have attempted to design a biocompatible delivery system for its application as a therapeutic agent (27, 28). For topical drug delivery, bioadhesive hydrogels are ideal as they can provide extended release of therapeutic agents such as antimicrobials to the oral mucosa. Hydrogels are three-dimensional polymer networks that have unique properties that can be modified to design a delivery system with the desired properties for treating infections (29–32). In fact, in a recent study by Håkansson et al. (33), a synthetic peptide, PXL150, with broad-spectrum antimicrobial activity, was incorporated into a hydroxypropyl cellulose gel and successfully used as topical treatment of infected wounds at surgical sites. Importantly, oral gels have been also used in studies involving drug administration via the buccal route (34, 35). To that end, in this study, we aimed to develop a novel bioadhesive hydrogel formulation of Hst-5 designed for topical oral application against OC. The new formulation was evaluated in vitro for Hst-5 release rate, stability, gel viscosity, and anticandidal activity; in addition, the new formulation was tested in vivo using our mouse model of OC (36).
MATERIALS AND METHODS
Strains, growth conditions, and reagents.
The C. albicans standard strain SC5314 was used in all experiments (37). The strain was grown on yeast peptone dextrose agar and broth (YPD; Difco Laboratories) overnight at 30°C, and cells were equilibrated in fresh medium to an optical density of absorbance at 600 nm (A600) of 1.0. Cells were harvested, washed with 1 mM phosphate-buffered saline (PBS), and then suspended in PBS. For infection, C. albicans was used at a final cell density of 1 × 106 cells/ml. Hst-5 peptide was synthesized by GenScript (Piscataway, NJ) with >98% purity and reconstituted in 1 mM PBS. All experiments were performed on at least three separate occasions.
Development and optimization of the bioadhesive hydrogel formulation.
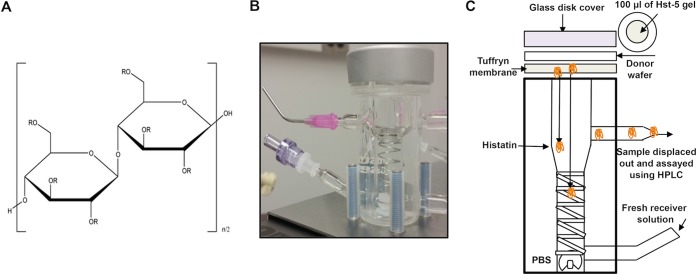
Hydroxypropyl methylcellulose (HPMC) K100 Premium LV (Dow Chemical, Midland, MI; donated by Colorcon Inc.) is a hydrophilic gelling polymer commonly used in FDA-approved formulations. Specifically, HPMC is widely used in the pharmaceutical industry for controlled release of drugs (Fig. 1A). HPMC grade K100 was selected due to its medium-length chain. An aqueous solution of Hst-5 was prepared by dissolving the peptide in 1 mM PBS, and HPMC was added to the solution at 4%, wt/wt, and was mixed until a clear gel solution formed. Hst-5 was incorporated at a final concentration of 2 mg/ml. Gels were stored at 4°C and tested regularly for stability and in vitro antimicrobial activity. Vehicle polymer with no Hst-5 incorporated was used as negative control in all experiments.
FIG 1.
(A) Hydroxypropyl methylcellulose (HPMC). R = H, OCH3, or OCH2CH(OH)CH3. K100 has an OCH3 substitution of 19.0 to 24.0% and an OCH2CH(OH)CH3 substitution of 4.0 to 12.0%. The chemical structure of HPMC has 6 R groups where substitutions could occur. The degree of substitution is in part responsible for the viscosity and release rate of Hst-5 from the gel. (B and C) The vertical diffusion cell (B) and a cartoon illustrating the diffusion process (C). A 100-μl aliquot of Hst-5 gel is placed on top of the Tuffryn membrane. A white wafer ring maintains a consistent surface area for diffusion. The glass disk cover is placed on top of the donor wafer to maintain temperature and humidity conditions. As illustrated, Hst-5 slowly diffuses out of the gel and through the cell and Tuffryn membrane and collects in the receiver compartment. Samples are collected from the receiver compartment for HPLC analysis at specified time intervals to maintain sink conditions. A helical stirring system is used to create constant stirring action throughout the solution to prevent stagnant layers of diffusant from occurring.
Rheological evaluation of the HPMC gels.
A cup and plate rheometer (Brookfield DV III; Middleboro, MA) was used to measure the viscosity of HPMC K100 LV Premium gels at 25°C, 37°C, and 40°C. A method was developed to measure the shear stress of the gel at different applied shear rates, which ranged from 7.5 to 67.5 cm−1 with a 7.5-cm−1 step size. The spindle was allowed to equilibrate at each shear rate for 1 min before the shear stress was measured, and this process was repeated from 67.5 cm−1 to 7.5 cm−1 to determine if hysteresis existed. Since no hysteresis was observed under these test conditions, we reported a single-point viscosity obtained at the shear rate of 37.5 cm−1.
Evaluation of Hst-5 diffusion from the gel.
A 7-ml vertical diffusion cell (Hanson Research Corp., Chatsworth, CA) was used to study the in vitro release of Hst-5 from the HPMC gel over time (Fig. 1B and C). The cell is equipped with a helix mixer for continuous mixing throughout the receiver solution. The arms of the glassware allow for sampling by displacement, and the glassware is jacketed for temperature control. An HT-450 Tuffryn membrane (P/N 66221; Pall Corporation, Timonium, MD) was used as the membrane barrier between receiver and donor solutions. This particular membrane is composed of hydrophilic polysulfone with a pore size of 0.45 μm and a 25-mm diameter and exhibits low binding affinity toward proteins. The membrane was washed in PBS buffer for several hours prior to use. The circulating water bath (Polyscience, Niles, IL) was set to 38°C to maintain the diffusion cells between 37 and 37.5°C, and 1 mM PBS buffer was used as receiver solution. A 100-μl aliquot of the Hst-5 gel was dispensed on the membrane with an automatic pipette, and samples were taken at 0, 0.25, 0.5, 0.75, 1, and 2 h. At each sampling interval, a 1-ml aliquot of fresh 1 mM PBS was added to the receiver solution. The first 0.5-ml of receiver solution to be displaced was discarded and the remainder 0.5 ml was collected for high-pressure liquid chromatography (HPLC) analysis.
Hst-5 assay.
Reverse-phase HPLC analysis was carried out using an Acquity UPLC H-Class system with fluorescence detection (Waters Corp., Milford, MA, USA) and an XBridge UPLC C18 column, 4.6 by 100 mm, 3.5-μm solid phase (Waters Corp., Milford, MA, USA). For this gradient method, the two mobile-phase compositions were 0.065% (wt/vol) trifluoroacetic acid (TFA) in water and 0.05% (wt/vol) TFA in acetonitrile. Histatin-5 was eluted using a linear gradient whereby the aqueous solution was varied from 95% to 35% over a 10-min interval and then from 35% to 5% over a 2.5-min interval. Finally, the mobile phase was brought back to its original composition by varying the aqueous solution from 5% to 95% over a 3.5-min interval followed by column equilibration for 5 min at a flow rate of 1 ml/min. For the fluorescence assay, excitation was at a wavelength (λex) of 230 nm, and detection was monitored at λem of 315 nm. The injection volume was 15.0 μl, and retention time for Hst-5 was 3.48 min. The method was linear in the range of 0.5 to 5 μg/ml.
In vitro evaluation of the formulation for anticandidal potency.
C. albicans cells at various cell densities were added to 50 μl of the Hst-5 hydrogel in microcentrifuge tubes, and the tubes were incubated at 37°C for 1 h. Following incubation, the reaction mixtures were emulsified in PBS and serially diluted, and aliquots from reaction mixtures were inoculated on YPD agar and incubated for 24 h at 37°C. The single colonies on each plate were counted, and killing was calculated based on CFU counts. Controls were included whereby cells were added to the vehicle gel with no Hst-5 incorporated. In order to demonstrate lack of toxicity of the polymer on C. albicans, experiments were also performed in PBS. No effect on C. albicans viability was noted for the polymer gel.
Animal studies.
All animal experiments were conducted at the AAALAAC accredited Animal Facility of the University of Maryland in accordance with the USA Animal Welfare Act as regulated by USDA. Animal studies were approved by the University of Maryland Animal Care and Use Committee (IACUC Protocol number 0814012). This institution has an Animal Welfare Assurance on file with the Office of Laboratory Animal Welfare, NIH. The Assurance Number is A-3199-01. Three-month-old pathogen-free female C57BL/6 mice (Charles River Laboratories, Shrewsbury, MA) were used in these studies. Mice were housed at a maximum of 5 per cage in the animal facility.
Infection model.
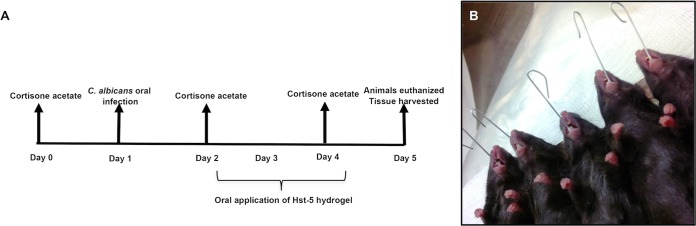
The infection mouse model was performed in accordance with an established model of OC with modification (38). The infection and treatment timeline can be seen in Fig. 2A. As immunocompetent mice are generally not colonized with C. albicans, mice were rendered susceptible to candidiasis by subcutaneous administration (0.2 ml) of cortisone acetate (200 mg/kg body weight) in the dorsum of the neck every other day starting 1 day before infection (total, 3 injections). Animals were divided into two groups with 5 mice in each group: one group was administered Hst-5 gel, and one group received a control gel. On the day of infection, mice were anesthetized by intraperitoneal injections (0.5 ml) of tribromoethanol (250 mg/kg body weight; Sigma-Aldrich). While under anesthesia, animals were placed under a heating lamp maintained at 37°C. Anesthetized animals were orally infected by placing calcium alginate swabs (Fisher Scientific) saturated for 5 min with C. albicans (1 × 106 cells/ml) yeast cell suspension sublingually for 50 min (Fig. 2B). Animals were placed in a supine position and monitored until they recovered from the anesthesia. One day postinfection with C. albicans, animals were anesthetized, and 100 μl of Hst-5 or control gel was applied to the oral cavity, covering the whole surface and sublingual areas. The same treatment was administered on the subsequent 2 days for a total of 3 applications. Animals were monitored daily for any developing clinical signs of distress and euthanized 4 days postinfection by CO2 inhalation followed by cervical dislocation. Tongues were harvested, weighed, homogenized, and cultured in triplicate on yeast chromogenic medium CHROMagar (DRG International, Inc.). Plates were incubated for 24 h at 37°C, and viable cells were enumerated and expressed as log CFU/gram tissue.
FIG 2.
Timeline for infection and Hst-5 treatment. (A) Animals were orally infected with a C. albicans cell suspension using calcium alginate swabs placed sublingually for 50 to 60 min. (B) One day prior to and following infection, the animals were immunosuppressed with cortisone acetate injections. One day postinfection, 100 μl of gel (control or Hst-5) was topically applied to the oral cavities, covering the surface and sublingual area of the tongue for 50 to 60 min. Animals similarly received daily applications for a total of 3 treatments and were euthanized 4 days postinfection, and tongues were harvested and processed.
Tissue histopathology analyses.
In order to visually assess fungal presence and tissue invasion, some tongues were cut in half longitudinally. Tongue tissue was fixed in paraformaldehyde, embedded in paraffin, and sectioned, and sections were deparaffinized with xylene and stained with periodic acid-Schiff (PAS). The whole periphery of each infected tongue section was examined by light microscopy and evaluated based on the presence and extent of adhering yeast cells and penetration of the epithelium by invasive hyphae.
SEM of infected tongue tissue.
Tongues from mice with OC were also subjected to scanning electron microscopy (SEM) analysis for better visualization of hyphal tissue invasion. Tongues were fixed in 2% paraformaldehyde–2.5% glutaraldehyde and, following washing steps with PBS, postfixed with 1% osmium tetroxide and then rinsed with PBS and dehydrated using a series of washes with ethyl alcohol (30 to 100%). Samples were dried by critical point drying using an Autosamdri-810 (Tousimius), mounted on aluminum stubs, and sputter coated with 10 to 20 nm of platinum-palladium and imaged with a Quanta 200 scanning electron microscope (FEI Co., Hillsboro, OR).
Data analysis.
The database for statistical analysis was built up by incremental addition of experimental data. In order to determine the optimal variables and conditions (infectious doses, treatment protocol, endpoint of infection, etc.) and standardize the model, initial experiments were performed using small groups of animals. All in vitro analyses were performed on at least 3 separate occasions, whereas animal experiments were performed on 8 separate occasions in order to confidently establish the therapeutic efficacy of Hst-5, and averages from all experiments were used to present the data. All statistical analysis was performed using GraphPad Prism 5.0 software. A Student unpaired t test was used to compare differences between two samples. P values of <0.05 were considered to be significant and are marked with an asterisk in the figures.
RESULTS
Evaluation of Hst-5 release and formulation viscosity.
In vitro Hst-5 release testing from HPMC gel was performed using the vertical diffusion cell and HPLC analysis as described above. The percent release of Hst-5 from the gel was assessed at 37°C over a 2-h time period, which covers the duration of the animal treatment protocol. Based on the cumulative percent release (Fig. 3A) of Hst-5 from the HPMC gel, the profile indicated that Hst-5 was released rapidly, with 19.10% ± 3.99% of the initial amount released within 15 min and 87.21% ± 20.38% released by 1 h with a corresponding maximum concentration of drug in serum (Cmax) of 8.27 ± 2.74 μg/ml, showing a maximum concentration of 8 μg/ml to occur at 0.5 h (Fig. 3B). The fact that the majority of the Hst-5 in the polymer was released within the first 1 h is crucial, as this is the period of time that the mice were exposed to the gel while under anesthesia. Further, the viscosity of the gel was evaluated at different physiological temperatures; results demonstrated that the viscosity decreased with increasing temperature, whereby at room temperature the gel exhibited a viscosity of 317.84 ± 6.92 cP and at 37°C the viscosity decreased to 197.73 ± 0.76 cP (Fig. 3C).
FIG 3.
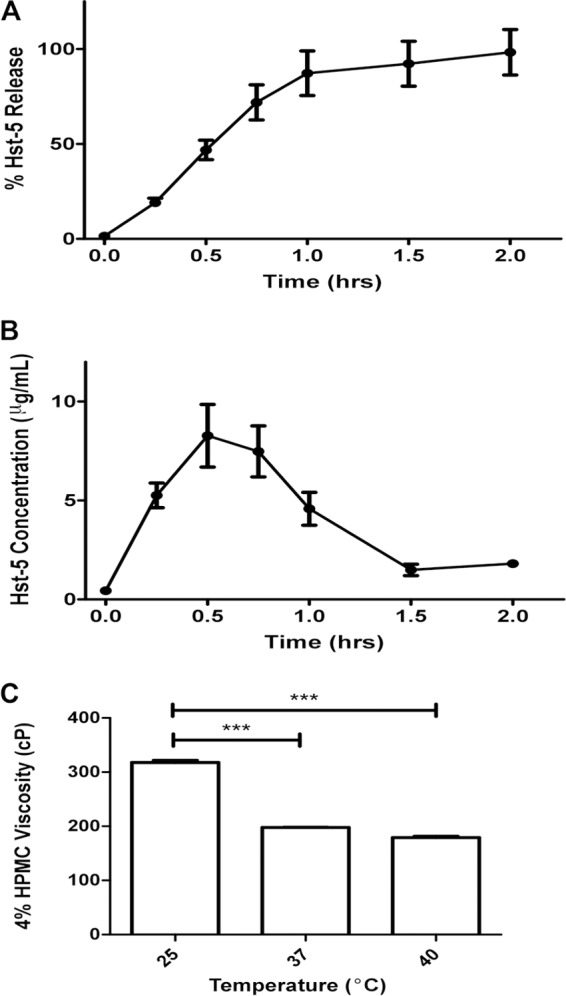
In vitro Hst-5 release testing from HPMC gel. Percent release of Hst-5 from the gel was assessed at 37°C using a vertical diffusion cell over a 2-h time period, and evaluation of cumulative release of Hst-5 indicated that at 1 h, 85% of Hst-5 is released from the gel (A) with a maximum concentration of 8 μg/ml occurring at 0.5 h (B). (C) Assessment of viscosity of the Hst-5 gel at different temperatures indicated that viscosity decreases with increasing temperature.
In vitro evaluation of the formulation for anticandidal potency.
Based on CFU counts from killing assays following 1 h of exposure of C. albicans to Hst-5 gel, a cell density-dependent killing effect was seen at all of the concentrations below 1 × 106 cells/ml compared to control polymer, indicating that the microbicidal effect of Hst-5 is preserved in the polymer (Fig. 4). The vehicle used in this study (HPMC gel) showed no effect by itself on C. albicans compared to PBS. It is important to note that the formulation was stored at 4°C and tested regularly for in vitro killing potency to evaluate antimicrobial stability. Thus far, there has been no indication of any deterioration of activity upon storage for over at least a 9-month period.
FIG 4.
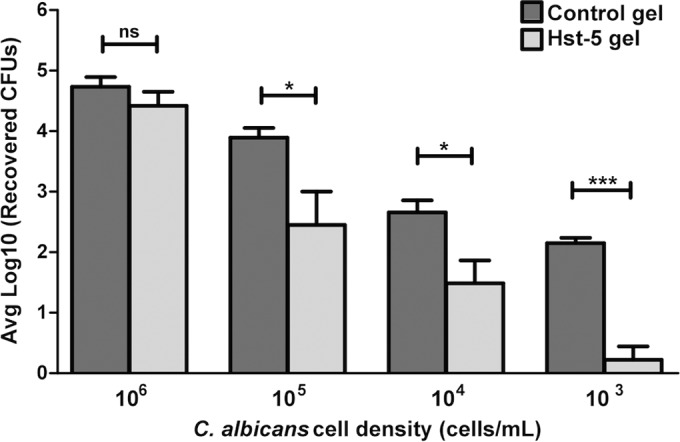
In vitro killing assay. Based on CFU counts from killing assays following 1 h of exposure of C. albicans to Hst-5 gel, a cell density-dependent killing effect was seen, with statistically significant killing activity observed at cell densities below 1 × 106 cells/ml, indicating that the microbicidal effect of Hst-5 is preserved in the polymer. The vehicle (HPMC alone) showed no killing effect by itself on C. albicans compared to PBS. ns, not significant.
Animal studies.
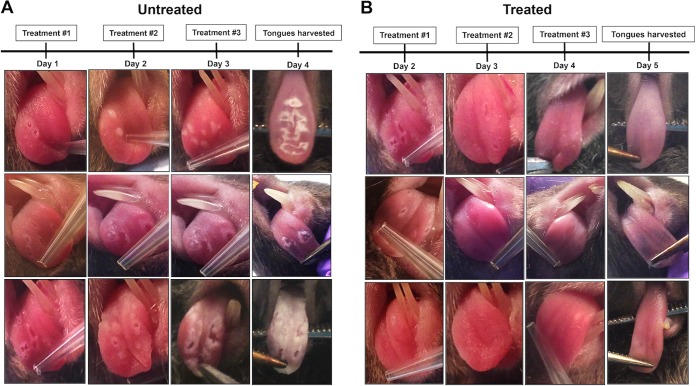
To determine whether the Hst-5 hydrogel protects the oral tissue from OC development, 4 days following infection with C. albicans, mice were euthanized and tongues were harvested for assessment of oral candidal burden. Findings demonstrated that all infected control mice developed clinical OC, whereby 1 day postinfection white lesions could be seen, which rapidly progressed in time into overt candidiasis (white plaques) on the tongues and other mucosal surfaces, including the pharynx (Fig. 5A). In contrast, the majority of the treated mice did not develop any clinical signs of infection, with no or minimal C. albicans recovered from the tongues. Interestingly, however, in several of the treated mice, although some white lesions could be seen on the day treatment was initiated, upon subsequent treatments the lesions disappeared, with low levels of C. albicans recovered from these mice (Fig. 5B). Based on CFU counts, a high level of C. albicans was recovered from all the untreated mice whereas in the treated groups, no C. albicans was recovered from 19 of the 41 treated mice, with low-level recovery from 11 mice of the 41 (Fig. 6). In the remaining 11 mice, levels comparable to those from untreated mice were recovered. Overall, the clinical pictures in both groups of animals were consistent with the tissue culture results.
FIG 5.
Effectiveness of the Hst-5 formulation against OC progression. Infected animals were administered topical applications of Hst-5 or control gels daily beginning 1 day postinfection for a total of 3 applications. (A) All untreated mice developed white lesions, which rapidly progressed into overt candidiasis (white plaques) covering the tongue and other mucosal surfaces. In contrast, the majority of the treated mice did not develop any clinical signs of infection. (B) However, in some of the treated mice, although some white lesions developed, upon subsequent treatments the lesions seemed to disappear and underlying tissue appeared normal.
FIG 6.
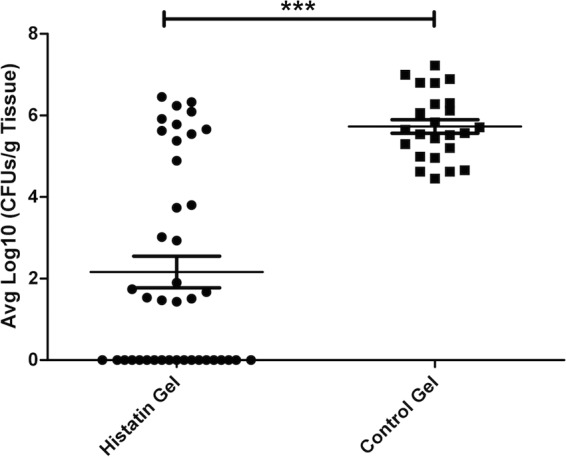
Recovery of C. albicans from homogenized tongue tissue from treated and control mice adjusted per gram tissue. In order to assess the fungal burden on infected tissue from treated and untreated animals, tongues were homogenized and cultured. Based on CFU counts, a high level of C. albicans was recovered from all the untreated mice whereas in the treated group, no C. albicans was recovered from 19 of the 41 treated mice, with low-level recovery of C. albicans from 11 of the 41 treated mice. In the remaining treated 11 mice, levels comparable to those from untreated mice were recovered. Data shown are the averages of data sets from experiments performed on 8 separate occasions.
Tissue histopathology analysis of tongue tissue.
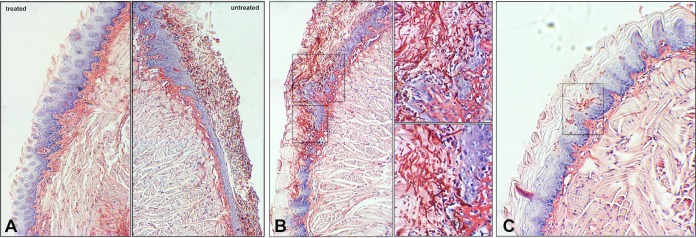
In addition to assessing microbial burden, tissue harvested from animals was also processed for histopathology. Microscopic analysis of PAS-stained tissue sections revealed extensive fungal adherence and hyphal penetration of the epithelial tissue in all the untreated mice, covering the whole periphery of the tongue with hyphae seen penetrating into the subepithelial tissue (Fig. 7A, right, and B). On the other hand, no evidence of C. albicans presence or sign of tissue damage was seen in the majority of the treated mice (Fig. 7A, left), although in a few of the treated mice, some C. albicans cells were seen sporadically adhering to limited areas of the tongue (Fig. 7C).
FIG 7.
Representative histopathology images of tongue tissue sections from infected treated and untreated mice. Half of the harvested tongues were fixed, embedded in paraffin, and stained with PAS, and sections were examined by light microscopy. (A) Representative images of sections of tongues from control and treated mice demonstrating the extensive presence of C. albicans around the periphery of the tongue along with hyphal invasion into the subepithelial tissue. (B) Magnified images of tongue tissue of a control mouse with OC revealing the massive amounts of invading hyphae (arrows) with the marked presence of host inflammatory cells. (C) Although no C. albicans was seen on the majority of the Hst-5-treated mice, some localized sporadic presence of C. albicans was seen in few of the treated animals. Bar, 20 μm.
Scanning electron microscopy of infected tongue tissue.
In addition, some of the infected tongues from untreated animals with OC were also subjected to SEM analysis. Consistent with histopathology, SEM analysis revealed a thick matrix covering the whole surface of the tongue of untreated mice, with C. albicans hyphae seen penetrating the outer epithelial layer of the tongue from the sublingual area where it was inoculated, leaving gaps in the tissue at sites of emergence (Fig. 8).
FIG 8.

Representative scanning electron microscopy image of a tongue from a mouse with oral candidiasis. Some sections of tongues from untreated mice with oral candidiasis were fixed and processed for scanning electron microscopy analysis. An overview image of the surface of the tongue showing the spiny layer with hyphae penetrating the tissue from the sublingual area where C. albicans was inoculated through the tongue surface.
DISCUSSION
The oral cavity is a primary target for opportunistic infections, particularly candidiasis. Despite traditional antifungal therapy, candidal infections are often reestablished soon after treatment ceases. Therefore, with the increasing number of predisposed populations coupled with the emergence of resistance to antifungal agents, it has become crucial to identify alternative antifungal agents. Specifically, the prospect of preventing C. albicans colonization and thus precluding candidiasis is becoming increasingly attractive.
Antimicrobial peptides are being widely used as blueprints for the development of innovative therapeutic agents that may be used as antimicrobials or modifiers of inflammation or in cancer therapy (11). Histatin-5 specifically is unique, as it is potent in killing C. albicans, including strains resistant to common antifungals, and importantly, does not induce resistance (14). Coupled with its lack of toxicity, these properties make Hst-5 an ideal candidate as a therapeutic agent (16, 23, 27, 28).
With any antimicrobial, an MIC is needed at the site of infection for a sufficient period of time to have a therapeutic effect. Therefore, to prevent OC development with Hst-5, a delivery system that allows distribution of Hst-5 to all affected tissues for a sufficient period of time to prevent infection is needed, and a bioadhesive delivery system is one of the best ways to accomplish this. For oral hydrogels, the principal performance criteria for optimal response are the resident time of the gel on the oral mucosa, the diffusion rate of Hst-5 out of the gel, and the stability of Hst-5 in the gel, properties that are controlled by the formulation composition (29). Hydrogel viscosity is a key factor in the rate of Hst-5 diffusion and retention of the gel on the oral mucosa, and therefore the gel composition needs to be optimized to meet all these performance criteria. Based on these criteria, HPMC grade K100 was selected due to its medium-length chain. This is important, as higher-molecular-weight polymers are thought to not bind effectively to the mucosa according to the interdiffusion theory of polymer attachment, and low-molecular-weight polymers would not be long enough for the polymer to entangle and control the release of Hst-5 (39, 40). In our study, HPMC was used at 4% concentration, as that level of viscosity was most suitable for application on oral tissue. Upon comprehensive in vitro evaluations, the Hst-5 formulation demonstrated potent killing activity against C. albicans and, importantly, stability and retention of antimicrobial activity in saliva and upon prolonged storage at 4°C.
Of more significance, we demonstrated the gel's efficacy in inhibiting the proliferation of colonizing C. albicans in vivo, thereby preventing the development of OC in mice receiving oral treatment. It is important to mention that release-testing assays monitoring the rate of Hst-5 diffusion from the polymer indicated that the highest percentage of release occurs within the first hour of testing. This was a crucial finding, as in our treatment protocol the gel is applied to the tissue for approximately 1 h, which is the period of time the mice are under anesthesia. Similarly, in the in vitro killing assays, C. albicans was also exposed to the gel for only 1 h. Therefore, the observed efficacy of the formulation against C. albicans within a short time frame supports the rapid and effective diffusion of Hst-5 onto the exposed tissue. Note that the gels tested in this study were optimized for our mouse model and the 1-h treatment duration, but if these gels were to be used in humans, the treatment duration would likely be longer and the gels would have to be optimized for this longer duration of treatment; however, with all the flexible formulation parameters described above, this can be readily done. In addition to efficacy, an important aspect of evaluating a novel drug is adverse effect to the host. Although Hst-5 is host produced and is known not to be toxic, in preliminary studies we confirmed its lack of toxicity on tissue by histopathological analyses for any signs of mucosal irritation prior to initiation of studies; similar testing was done using the HPMC polymer (data not shown).
Our previous studies have demonstrated that Hst-5 salivary levels are significantly reduced in HIV+ individuals concomitant with enhanced prevalence of OC in this patient population (41). Therefore, as a natural component of saliva, a therapeutic formulation of Hst-5 will ensure that a sufficiently protective level of Hst-5 is available in the oral cavity. Therefore, the availability of an Hst-5-based bioadhesive hydrogel formulation would also serve in prophylactically augmenting host natural mucosal defenses. This would be of particular importance for HIV+ individuals as well as patients with salivary dysfunction such as those with Sjögren's syndrome or head and neck cancer receiving radiotherapy, who are also predisposed to candidiasis. In addition, the elderly, infants, and patients who use inhaled corticosteroids for asthma are also at increased risk of developing OC, and therefore, these individuals would also benefit from the availability of the formulation.
It is noteworthy to mention that in addition to antimicrobial ability, Hst-5 was also shown to exhibit potent anti-inflammatory and wound-healing properties (19, 42). Therefore, it was very intriguing that in some of our treated mice, although some lesions were seen to develop early during infection, upon repeated applications of the Hst-5 gel, the lesions were resolved along with the associated inflammation to the affected tissue. Although further testing is warranted to explore the anti-inflammatory potential of Hst-5, these observations strongly support the reported anti-inflammatory and wound-healing activity of Hst-5. These findings are of significance, as in addition to antimicrobial effect, if an infection prevents tissue regeneration at the site of injury, the application of the hydrogel could accelerate the healing process by allowing tissue cell attachment and infiltration. This would be particularly useful in cases of Candida-associated denture stomatitis, a condition occurring in approximately 70% of denture wearers characterized by chronic inflammation induced by C. albicans infiltration of the denture-bearing mucosa (43, 44).
Further, it is important to note that histatins in general have broad-spectrum antimicrobial properties and the formulation could therefore prove effective against oral bacterial pathogens such as those that cause periodontal and endodontic infections. In fact, histatins have been shown to inactivate proteases, neutralize toxins and lipopolysaccharides, and inhibit bacterial hemagglutination and coaggregation. Specifically, Porphyromonas gingivalis is the most important periodontal pathogen, and lipopolysaccharides from P. gingivalis have been shown to induce chemokines and interleukin 6 (IL-6) and IL-8 from the cells of the periodontium (45). However, recent studies demonstrated that synthetic Hst-5 suppressed IL-6 and IL-8 induction in human gingival fibroblast cells and significantly attenuated chemokines, thereby maintaining oral homeostasis and inflammation by downregulating host proinflammatory responses (18, 19, 42, 46).
In conclusion, although histatins have been acknowledged primarily as antimicrobial peptides in the oral defense against microorganisms, they are now also considered to be crucial for oral wound healing, tissue regeneration, and maintenance of the integrity of the oral soft tissues. Therefore, the combined findings from this study establish not only the experimental basis for the development of Hst-5 into a therapeutic agent to target oral Candida infections but also the associated inflammatory and tissue damage processes.
ACKNOWLEDGMENTS
We thank Ahmed Shawky for his contribution and Ru-Ching Hsia and the University of Maryland Core Imaging Facility for assistance with electron microscopy.
Funding Statement
This work was supported by National Institutes of Health grant DE14424 to M. A. Jabra-Rizk and a University of Maryland seed grant to M. A. Jabra-Rizk, Amy Karlsson, and Stephen Hoag. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Calderone RA. 2012. Candida and candidiasis. ASM Press, Washington, DC. [Google Scholar]
- 2.de Repentigny L, Lewandowski D, Jolicoeur P. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev 17:729–759. doi: 10.1128/CMR.17.4.729-759.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidel PL., Jr 2006. Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis. Adv Dent Res 19:80–84. doi: 10.1177/154407370601900116. [DOI] [PubMed] [Google Scholar]
- 4.Fidel PLJ. 2011. Candida-host interactions in HIV disease: implications for oropharyngeal candidiasis. Adv Dent Res 23:45–49. doi: 10.1177/0022034511399284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganguly S, Mitchell AP. 2011. Mucosal biofilms of Candida albicans. Curr Opin Microbiol 14:380–385. doi: 10.1016/j.mib.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DW, Jordan RPC, Wei X-Q, Alves CT, Wise MP, Wilson MJ, Lewis MAO. 2013. Interactions of Candida albicans with host epithelial surfaces. Oral Microbiol doi: 10.3402/jom.v5i0.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med 311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 9.Tournu H, Van Dijck P. 2012. Candida biofilms and the host: models and new concepts for eradication. Int J Microbiol 2012:845352. doi: 10.1155/2012/845352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlroth J, Choi B, Spellberg B. 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 11.Carmona-Ribeiro AM, de Melo Carrasco LD. 2014. Novel formulations for antimicrobial peptides. Int J Mol Sci 15:18040–18083. doi: 10.3390/ijms151018040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPhee JB, Hancock RE. 2005. Function and therapeutic potential of host defence peptides. J Peptide Sci 11:677–687. doi: 10.1002/psc.704. [DOI] [PubMed] [Google Scholar]
- 13.McPhee Scott JB MG, Hancock RE. 2005. Design of host defence peptides for antimicrobial and immunity enhancing activities. Combinational Chem High Throughput Screening 8:257–272. doi: 10.2174/1386207053764558. [DOI] [PubMed] [Google Scholar]
- 14.Kruse T, Kristensen H-H. 2008. Using antimicrobial host defense peptides as anti-infective and immunomodulatory agents. Expert Rev Anti Infect Ther 6:887–895. doi: 10.1586/14787210.6.6.887. [DOI] [PubMed] [Google Scholar]
- 15.Peters B, Shirtliff ME, Jabra-Rizk MA. 2010. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog 6:e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgerton M, Koshlukova SE, Lo TE, Chrzan BG, Straubinger RM, Raj PA. 1998. Candidacidal activity of salivary histatins. J Biol Chem 272:20438–20447. [DOI] [PubMed] [Google Scholar]
- 17.Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion. J Biol Chem 263:7472–7477. [PubMed] [Google Scholar]
- 18.Brand HS, Veerman EC. 2013. Saliva and wound healing. Chin J Dent Res 16:7–12. [PubMed] [Google Scholar]
- 19.Oudhoff MJ, Bolscher JGM, Nazmi K, Kalay H, van 't Hof W, Nieuw Amerongen AV, Veerman ECI. 2008. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J 22:3805–3812. doi: 10.1096/fj.08-112003. [DOI] [PubMed] [Google Scholar]
- 20.Nikawa H, Jin C, Fukushima H, Makihira S, Hamada T. 2001. Antifungal activity of histatin-5 against non-albicans Candida species. Oral Microbiol Immunol 16:250–252. doi: 10.1034/j.1399-302X.2001.160409.x. [DOI] [PubMed] [Google Scholar]
- 21.Helmerhorst EJ, Reijnders IM, van't Hof W, Simoons-Smit I, Veerman ECI, Nieuw Amerongen AV. 1999. Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob Agents Chemother 43:702–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koshlukova SE, Araujo MWB, Baev D, Edgerton M. 2000. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect Immun 68:6848–6856. doi: 10.1128/IAI.68.12.6848-6856.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van't Hof W, Reijnders IM, Helmerhorst EJ, Walgreen-Weterings E, Simoons-Smit IM, Veerman EC, Amerongen AV. 2000. Synergistic effects of low doses of histatin 5 and its analogues on amphotericin B anti-mycotic activity. Antonie Van Leeuwenhoek 78:163–169. doi: 10.1023/A:1026572128004. [DOI] [PubMed] [Google Scholar]
- 24.Li XS, Reddy MS, Baev D, Edgerton M. 2003. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J Biol Chem 278:28553–28561. doi: 10.1074/jbc.M300680200. [DOI] [PubMed] [Google Scholar]
- 25.Helmerhorst EJ, Breeuwer P, van't Hof W, Walgreen-Weterings E, Oomen LCJM, Veerman ECI, Nieuw Amerongen AV, Abee T. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem 274:7286–7291. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 26.Baev D, Li XS, Dong J, Keng P, Edgerton M. 2002. Human salivary histatin 5 causes disordered volume regulation and cell cycle arrest in Candida albicans. Infect Immun 70:4777–4784. doi: 10.1128/IAI.70.9.4777-4784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters BM, Fidel PL Jr, Scheper MA, Hackett W, El Shaye S, Jabra-Rizk MA. 2010. Protection of the oral mucosa by salivary histatin-5 against Candida albicans in an ex vivo murine model of oral infection. FEMS Yeast Res 10:597–604. doi: 10.1111/j.1567-1364.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tati SLR, Puri S, Kumar R, Davidow P, Mira Edgerton M. 2014. Histatin 5-spermidine conjugates have enhanced fungicidal activity and efficacy as a topical therapeutic for oral candidiasis. Antimicrob Agents Chemother 58:756–766. doi: 10.1128/AAC.01851-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermonden TCR, Hennink WE. 2012. Hydrogels for protein delivery. Chem Rev 112:2853–2888. doi: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- 30.Mathiowitz E, Chickering DE, Lehr C-M. 1999. Bioadhesive drug delivery systems: fundamentals, novel approaches, and development. Marcel Dekker, New York, NY. [Google Scholar]
- 31.Tatavarti AS, Hoag SW. 2006. Microenvironmental pH modulation based release enhancement of a weakly basic drug from hydrophilic matrices. J Pharm Sci 95:1459–1468. doi: 10.1002/jps.20612. [DOI] [PubMed] [Google Scholar]
- 32.Tatavarti AS, Mehta KA, Augsburger LL, Hoag SW. 2004. Influence of methacrylic and acrylic acid polymers on the release performance of weakly basic drugs from sustained release hydrophilic matrices. J Pharm Sci 93:2319–2331. doi: 10.1002/jps.20129. [DOI] [PubMed] [Google Scholar]
- 33.Håkansson J, Bjorn C, Lindgren K, Sjöström E, Sjöstrand V, Mahlapuu M. 2014. Efficacy of the novel topical antimicrobial agent PXL150 in a mouse model of surgical site infections. Antimicrob Agents Chemother 58:2982–2984. doi: 10.1128/AAC.00143-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones DS WA, Brown AF, O'Neill M. 1997. Mucoadhesive, syringeable drug delivery systems for controlled application of metronidazole to the periodontal pocket: in vitro release kinetics, syringeability, mechanical and mucoadhesive properties. J Control Release 49:71–79. doi: 10.1016/S0168-3659(97)00060-6. [DOI] [Google Scholar]
- 35.Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin C. 2000. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release 67:357–368. doi: 10.1016/S0168-3659(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 36.Kong E KS, Van Dijck P, Peters BM, Shirtliff ME, Jabra-Rizk MA. 2015. Clinical implications of oral candidiasis: host tissue damage and disseminated bacterial disease. Infect Immun 83:604–613. doi: 10.1128/IAI.02843-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine'5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 38.Solis NV, Filler SG. 2012. Mouse model of oropharyngeal candidiasis. Nat Protoc 7:637–642. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YLW, Foss A, Peppas NA. 2000. Molecular aspects of muco- and bioadhesion: tethered structures and site-specific surfaces. J Control Release 65:63–71. doi: 10.1016/S0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 40.Andrews GP, Laverty TP, Jones DS. 2009. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm 71:505–518. doi: 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Khan SA, Fidel PL Jr, Al Thunayyan A, Meiller TF, Jabra-Rizk MA. 2013. Impaired histatin-5 level and salivary antimicrobial activity against C. albicans in HIV-infected individuals. J AIDS Clin Res 4(193):pii:1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imatani TKT, Minaguchi K, Okuda K. 2000. Histatin 5 inhibits inflammatory cytokine induction from human gingival fibroblasts by Porphyromonas gingivalis. Oral Microbiol Immunol 15:378–382. doi: 10.1034/j.1399-302x.2000.150607.x. [DOI] [PubMed] [Google Scholar]
- 43.Webb BC, Thomas CJ, Willcox MDP, Harty DWS, Knox KW. 1998. Candida-associated denture stomatitis. Aetiology and management: a review. Part 3. Treatment of oral candidosis. Australian Dent J 43:244–249. [DOI] [PubMed] [Google Scholar]
- 44.Nett JE, Marchillo K, Spiegel CA, Andes DR. 2010. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun 78:3650–3659. doi: 10.1128/IAI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan SA KE, Meiller TF, Jabra-Rizk MA. 2015. Periodontal diseases: bug induced, host promoted. PLoS Pathog 11:e1004952. doi: 10.1371/journal.ppat.1004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borgwardt DS MA, Van Hemert JR, Yang J, Fischer CL, Recker EN, Nair PR, Vidva R, Chandrashekaraiah S, Progulske-Fox A, Drake D, Cavanaugh JE, Vali S, Zhang Y, Brogden K. 2014. Histatin 5 binds to Porphyromonas gingivalis hemagglutinin B (HagB) and alters HagB-induced chemokine responses. Sci Rep 4:3904. doi: 10.1038/srep03904. [DOI] [PMC free article] [PubMed] [Google Scholar]