Abstract
Herpes simplex virus (HSV) and many other viruses, including HIV, initiate infection of host cells by binding to glycosaminoglycan (GAG) chains of cell surface proteoglycans. Although GAG mimetics, such as sulfated oligo- and polysaccharides, exhibit potent antiviral activities in cultured cells, the prophylactic application of these inhibitors as vaginal microbicides failed to protect women upon their exposure to HIV. A possible explanation for this failure is that sulfated oligo- and polysaccharides exhibit no typical virucidal activity, as their interaction with viral particles is largely electrostatic and reversible and thereby vulnerable to competition with GAG-binding proteins of the genital tract. Here we report that the cholestanol-conjugated sulfated oligosaccharide PG545, but not several other sulfated oligosaccharides lacking this modification, exhibited virucidal activity manifested as disruption of the lipid envelope of HSV-2 particles. The significance of the virus particle-disrupting activity of PG545 was also demonstrated in experimental animals, as this compound, in contrast to unmodified sulfated oligosaccharide, protected mice against genital infection with HSV-2. Thus, PG545 offers a novel prophylaxis option against infections caused by GAG-binding viruses.
INTRODUCTION
Since the original finding of WuDunn and Spear (1) that ubiquitous and negatively charged glycosaminoglycan (GAG) chains of cell surface proteoglycans provide the binding sites for attachment of herpes simplex virus (HSV) to cells, these oligosaccharides have been reported to assist infection of cells by a number of different viruses, including respiratory syncytial virus (RSV) (2, 3), HIV-1 (4), and Ebola virus (5). In HSV, glycoprotein C (gC) (6) and/or gB (7) mediates virus binding to cell surface GAGs. Sulfated polysaccharides and other polysulfonated compounds that mimic the structure of GAG chains are well-known inhibitors of the virus-GAG interaction in cultured cells (3, 8, 9). Due to extensive sulfonation, these compounds efficiently outcompete the binding of cell surface GAGs to the viral attachment proteins, thus preventing infection of cells. Notably, the same GAG mimetics can inhibit infectivity of different GAG-binding viruses (8, 9). In spite of potent antiviral activity in cultured cells, these inhibitors, i.e., cellulose sulfate, carrageenan, and PRO2000 (sulfonated poly-naphthalene), failed to protect women against contraction of HIV when tested as intravaginal virucides in several large clinical trials (10–12). Furthermore, there was an indication that one of these GAG mimetics, i.e., cellulose sulfate, increased the risk of contracting HIV (10). Although it is unclear why these compounds lack protective effects in humans (12, 13), some intrinsic features of the virus-GAG interaction, such as reversibility of the binding, may help to elucidate this issue. Virus binding to ubiquitous cell surface components, such as GAGs or sialic acid, has to be neatly balanced to avoid redundant dead-end interactions resulting in trapping of viral particles. Some sialic acid-binding viruses, such as influenza virus, express the receptor-destroying enzyme sialidase (14, 15), which cleaves sialic acid to ensure reversibility of redundant bindings during both virus attachment to and egress from cells. Pioneering studies using both neuraminidase inhibitors (16) and virus mutants deficient in expression of neuraminidase (17) showed the presence of large clumps of progeny virions trapped at the surface of infected cells. This vulnerable event in the viral life cycle was exploited for antiviral intervention, and the drugs currently approved for treatment of influenza virus infections are sialic acid mimetics that act as inhibitors of sialidase (18). In contrast, the GAG-binding viruses possess no GAG-destroying enzyme, since the reversibility of binding is a feature of the protein-GAG interaction that relies on weak but multiple electrostatic associations between clusters of positively charged amino acid residues of a viral attachment protein and the negatively charged sulfate/carboxylate groups of the GAG chains. Reversibility of this interaction enables the virus to surf along cellular protrusions in order to search for and bind to the more specific second receptor that is required for virus penetration into the cells (19). Although the GAG mimetics are more extensively sulfonated than the GAG chains, their inhibitory effects on the virus infection of cells are also reversible (8, 20). It has long been known that the efficient activity of GAG mimetics in cultured cells requires their permanent presence during virus attachment to cells and that a simple dilution of the virus-inhibitor complexes in culture medium can result in release of infectious virus (8). This indicates that interaction of GAG mimetics with viral particles is relatively weak and reversible and that in the genital tract, such electrostatic (ionic) associations are likely to be vulnerable to changes in the salt concentration and to competition with a number of other GAG-binding proteins (21, 22), such as growth factors, cytokines, and antimicrobial polypeptides that are present in genital mucosa and cervical secretions (23, 24).
We have previously found that muparfostat (formerly known as PI-88), a mixture of highly sulfated oligosaccharides, inhibited infectivity of HSV (25–27), RSV (28), and HIV-1 (29) in cultured cells without causing permanent inactivation of viral infectivity. However, the cholestanol-conjugated sulfated tetrasaccharide of muparfostat, also referred to as 14/P3 (27–29), and a related compound known as PG545 (30, 31) showed the capability to permanently inactivate the infectivity of HSV, RSV, and HIV-1 (27–29). In this study, we found that the permanent inactivation of viral infectivity by PG545 is due to “true” virucidal activity manifested as disruption of envelopes in HSV-2 particles and that PG545 but not muparfostat protects mice against genital HSV-2 infection.
MATERIALS AND METHODS
Compounds.
PG545, muparfostat, muparfostat tetrasaccharide fraction SM4, and sulfated alpha-1-azido-maltohexaose were provided by Progen Pharmaceuticals Ltd., Brisbane, Australia, and Medigen Biotechology Corporation, Taipei, Taiwan. The structure and antiviral properties of PG545, muparfostat, and SM4 were presented in earlier reports (25, 29), in which PG545 was referred to as compound P4 (29). PG545 was of ≥95% purity as determined by capillary electrophoresis, and full details of the synthesis, characterization, and biological activities of this compound were reported elsewhere (30, 31). All compounds were solubilized in deionized water, and stocks were stored at −20°C. Acyclovir (25 mg/ml) was purchased from Hospira Nordic AB and stored at 4°C.
Cells and viruses.
African green monkey kidney (GMK AH1) epithelial cells (32) were obtained from the Swedish Institute for Infectious Disease Control, Stockholm, Sweden. The cells were cultured in Eagle's minimum essential medium (EMEM) supplemented with 2% fetal calf serum (FCS), 0.05% Primaton RL substance (Kraft Inc., Norwich, CT), penicillin (60 μg/ml), and streptomycin (100 μg/ml). HSV-2 strain 333 (33) was used throughout the experiments. Clinical isolates VI03-1678, 90036, 90306, DE06-3942, and DE07-6820, derived from the collection at the Virus Laboratory (Sahlgrens University Hospital, Göteborg, Sweden), were used in some experiments. These viruses were isolated from specimens of genital secretions cultured in GMK AH1 cells and were identified as HSV-2 by using PCR (34). Regarding DE06-3942 and DE-07-6820, these two isolates were previously tested and found to be acyclovir resistant by an in-house cell culture assay and were included in this study for comparison. Viral stocks of the HSV-2 isolates were prepared from clinical isolates at low passage.
Antiviral assays.
The effects of test compounds on HSV-2 infectivity in GMK AH1 cells were tested by the viral plaque reduction assay (27). Briefly, serial 5-fold dilutions of test compounds (0, 0.16, 0.8, 4, 20, and 100 μg/ml) in serum-free EMEM were mixed with ∼200 PFU of HSV-2 and incubated for 10 min at room temperature prior to the addition of the mixture to duplicate cultures of GMK AH1 cells seeded in 12-well cluster plates. Following incubation of the virus-compound mixture with cells for 90 min at 37°C in a humidified atmosphere comprising 5% CO2, the cells were rinsed twice with EMEM and overlaid with EMEM containing 1% methylcellulose solution and 2% FCS but no test compounds. After 3 days of incubation at 37°C, the cell cultures were stained with a 1% solution of crystal violet, and viral plaques were counted under a light microscope. The effect of acyclovir on HSV-2 infectivity was assayed in the same manner except that the drug was also present in the methylcellulose overlay. The virus inactivation assay (27) was performed by adding ∼105 PFU of HSV-2 to 5-fold serial dilutions of test compounds in 0.5 ml of serum-free EMEM. The virus-compound mixture was incubated for 15 min at 37°C in a water bath, and residual virus infectivity was titrated in the viral plaque assay in GMK AH1 cells. The cytotoxicity of test compounds was assessed by adding 100-μl volumes of 5-fold serial dilutions of test compounds in EMEM supplemented with 2% FCS to nearly confluent monolayer cultures of GMK AH1 cells growing in 96-well cluster plates. After incubation of cells with compounds for 72 h, the cell viability was determined by using the tetrazolium-based CellTiter96 AQueous One Solution cytotoxicity assay (Promega; G3580).
Virus purification.
Cultures of GMK AH1 cells in roller bottles were infected with HSV-2 333 at a multiplicity of infection (MOI) of 1. Following incubation of cells with the virus for 45 min at 37°C, the cells were washed with EMEM and incubated in EMEM supplemented with methyl-[3H]thymidine (10 μCi/ml; specific activity, 6.7 Ci per mmol; PerkinElmer) for 40 h at 37°C. The infectious culture medium was then harvested and clarified by low-speed centrifugation at 1,000 × g for 5 min and at 3,000 × g for 10 min. The supernatant fluid was collected and loaded onto the top of a three-step discontinuous sucrose gradient comprising 2 ml each of 50, 40, and 30% (wt/vol) sucrose in PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4). After centrifugation at 20,000 rpm (SW28.1 bucket rotor, Optima LE-80K ultracentrifuge; Beckman) for 2 h at 4°C, the virus band at the interphase of 40 and 50% sucrose was collected following side puncture of the tube.
Treatment of HSV-2 virions with PG545.
Purified HSV-2 virions were dialyzed against PBS to remove sucrose and then diluted with PBS to a final volume of 18 ml. Subsequently, PG545 in 100 μl of redistilled water was added to a 9-ml volume of the virus preparation to achieve a final drug concentration of 250 μg/ml. The virus-compound suspension was gently mixed by inverting the tube and incubated in a 37°C water bath for 30 min. The same volume of virus was also incubated with the PG545 solvent (redistilled water) to serve as a control. The mixtures were then centrifuged at 20,000 × g for 2 h (SW28.1 rotor) over the three-step discontinuous sucrose gradient consisting of 2 ml each of 50, 40, and 30% (wt/vol) sucrose in PBS, and 1-ml fractions were collected following side puncture of tube in the middle of the 50% sucrose layer. Each fraction was subjected to (i) titration of residual infectivity in the viral plaque assay, (ii) quantification of radioactivity in a scintillation counter, (iii) determination of the number of viral DNA copies based on quantitative PCR (qPCR) analysis (35), and (iv) determination of reactivity with mouse monoclonal antibodies against HSV glycoprotein B (gB) (clone B11D8; dilution, 1:100), gC (clone E5F7; dilution, 1:100), gE (clone B1E6; dilution, 1:100) and gG (clone O1C5; dilution, 1:100) and rabbit polyclonal antibody against the major capsid protein VP5 (NC1; dilution, 1:500) by an enzyme-linked immunosorbent assay (ELISA)-based method. The monoclonal antibodies were prepared and characterized in our laboratory (26, 36, 37), while polyclonal antibody NC1 was kindly provided by Gary Cohen and Roselyn Eisenberg (University of Pennsylvania).
Electron microscopy.
GMK AH1 cells were infected with HSV-2 333 at an MOI of 1, and infectious culture medium was harvested at 40 h after infection and clarified by using low-speed centrifugation, i.e., 200 × g, for 5 min, followed by centrifugation at 3,500 × g for 10 min. The clarified supernatant fluid was centrifuged at 20,000 × g for 2 h at 4°C, and the viral pellet was collected into 160 μl of PBS, incubated overnight at 4°C, and then gently resuspended by pipetting. Subsequently, 40-μl volumes of the virus preparation (∼3 × 107 PFU) were mixed with equal volumes of PG545 solutions or its solvent to achieve final drug concentrations of 500, 20, and 0 μg/ml. The mixtures were incubated for 30 min in a 37°C water bath or at room temperature and then supplemented with 8 μl of 2.5% solution of glutaraldehyde and left overnight at 4°C. Samples were then warmed briefly at 42°C, supplemented with 500 μl of melted (42°C) agarose, centrifuged at 1,000 × g for 5 min, and then processed for electron microscopy (38).
Intravaginal HSV-2 inoculation in mice.
Female, 6- to 8-week-old C57BL/6 mice were purchased from Harlan Laboratories (AN Venray, the Netherlands). Prior to the experiment, mice, in groups of 6 to 10, were rested for a week according to standard procedures at the animal facility of the microbiology laboratory at Sahlgrens University Hospital (Göteborg, Sweden). All animals were injected subcutaneously with 3 mg of Depo-Provera (Pfizer AB, Sollentuna, Sweden) (39) at 7 days prior to vaginal inoculation with HSV-2. In all experiments, mice, anesthetized with 3% isoflurane (Baxter Medical AB, Kista, Sweden), were infected intravaginally with 105 PFU of HSV-2 333 (∼24× the 50% lethal dose [LD50]) using a fine blunt plastic pipette tip. In the first series of experiments, the effect of prior treatment of a virus with PG545 on its capability to infect mice was investigated. The virus was incubated with a compound or its diluent (deionized water) for 15 min in room temperature at final drug concentrations of 0, 20, 100, and 500 μg/ml in a total volume of 20 μl of EMEM and then left on ice until instillation of the virus-compound mixture into mice in groups of 7. In the second series of experiments, the protective effects of PG545 were investigated as a function of the different times of addition of the compound relative to animal inoculation with HSV-2. PG545 or its solvent at concentrations of 5, 0.5, and 0 mg/ml in a volume of 10 μl of EMEM was instilled vaginally at 10 min prior to or 10 min after infection with HSV-2. At each time point, groups of 8 animals were used, and repetitions of the time point plus 10 min (0.5 mg/ml) and controls were performed and included in the results. Mice were monitored daily and scored for severity of vaginal inflammation and other disease symptoms as described earlier (40). Briefly, a lack of disease symptoms was graded as 0, genital erythema as 1, moderate genital inflammation with blisters as 2, severe and purulent genital lesions with loss of hair in the genital area as 3, and hind-limb paralysis and/or general bad condition as 4. All mice with a score of 4 were sacrificed. All experiments were assessed and approved by the regional ethics committee for animal experiments in Göteborg, Sweden (diary number 43-2012).
Statistical analysis.
A sample size of 7 mice per group was calculated, assuming a power of 90% and a significance level of 5%, as sufficient to detect a 72% difference in survival rates between the control and the drug-treated animals. Fisher's exact test was used to analyze the mouse survival data. The Kruskal-Wallis, nonparametric, one-way analysis of variance (ANOVA) test was used to compare the amounts of viral DNA and infectious virus in vaginal lavage samples from mice. Student's t test was used for statistical analysis of data obtained in experiments with cultured cells. P values of ≤0.05 were considered statistically significant. Data were analyzed with the software GraphPad Prism version 5 for Mac OS X.
RESULTS
PG545 disrupts virus particles of HSV-2.
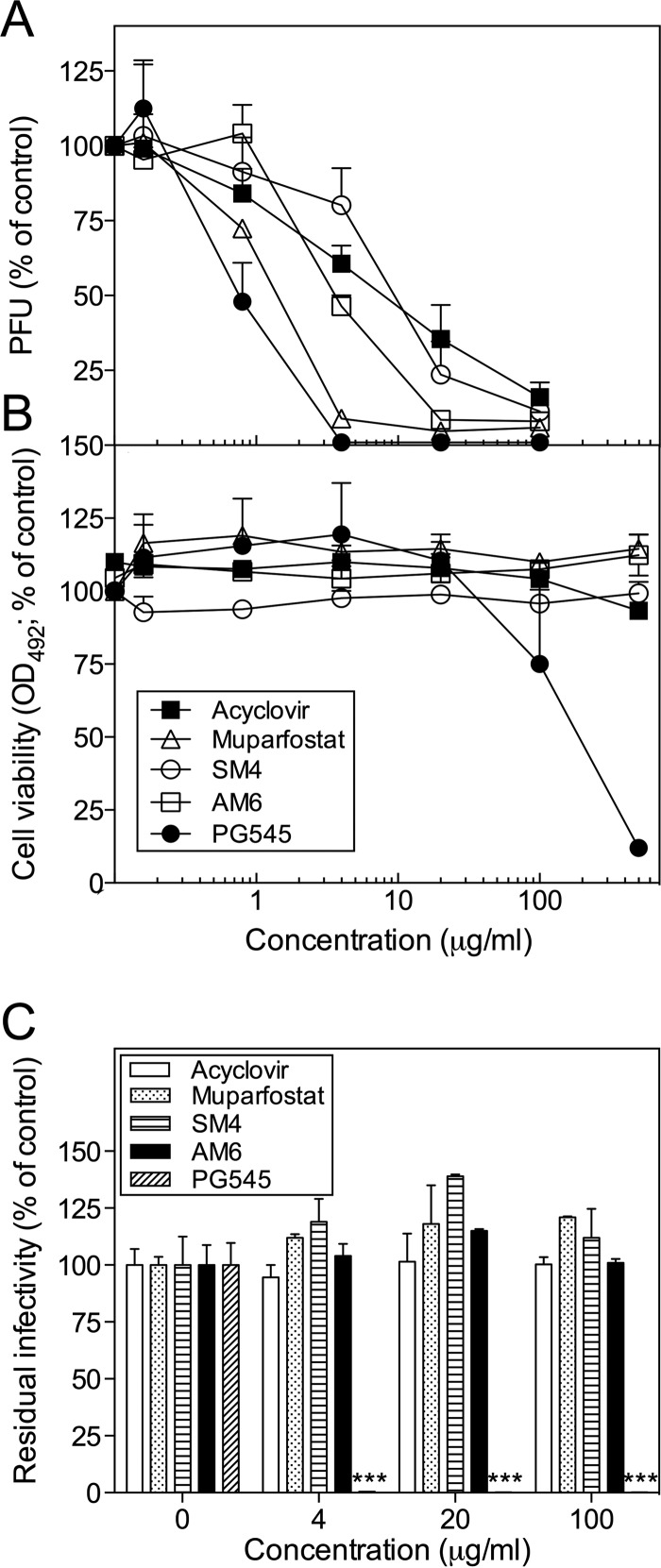
We compared the anti-HSV-2 activity of PG545, a cholestanol-conjugated sulfated tetrasaccharide (maltotetraose), with those of several other sulfated oligosaccharides lacking this modification, i.e., (i) muparfostat, a mixture of mannose-containing sulfated di- to hexasaccharides; (ii) SM4, a sulfated tetrasaccharide fraction of muparfostat; and (iii) AM6, an azide-conjugated sulfated maltohexaose (see Fig. S1 in the supplemental material). A known antiherpesvirus drug, acyclovir, was used as a control. The anti-HSV-2 (strain 333) activity of PG545, assessed by the viral plaque reduction assay in GMK AH1 cells (Fig. 1A) (50% inhibitory concentration [IC50], 0.8 μg/ml), was only slightly superior to that of muparfostat (IC50, 1.5 μg/ml) and ∼4- and ∼11-fold more pronounced than those of AM6 (IC50, 3.5 μg/ml) and SM4 (IC50, 9 μg/ml), respectively. Note that at concentrations of ≥4 μg/ml, PG545 inhibited HSV-2 completely, while ∼5% of the virus remained infectious in the presence of muparfostat even at the highest concentration tested. Similar to muparfostat, AM6 did not show a complete inhibitory effect at ≥20 μg/ml (Fig. 1A). PG545 was more toxic for GMK AH1 cells (50% cytotoxicity concentration [CC50], 200 μg/ml) than muparfostat, AM6, and SM4 (CC50, >500 μg/ml) (Fig. 1B). Hence, the selectivity of all these compounds for viral particles (CC50 per IC50) was high (∼250 for PG545 and >56 for remaining compounds). Furthermore, assessment of these compounds in the virus inactivation assay, based on incubation of the HSV-compound mixture prior to titration of residual virus infectivity, revealed that PG545 but not muparfostat, AM6, or SM4 exhibited virus-inactivating activity (Fig. 1C). These data suggest that the anti-HSV-2 activities of muparfostat, AM6, and SM4 in cultured cells (Fig. 1A) were due to reversible inhibition of viral particles, while that of PG545 was based on permanent inactivation of infectivity of HSV-2 particles. As expected, acyclovir did not inactivate the infectivity of free viral particles (Fig. 1C). The antiherpesviral activity of this drug (Fig. 1A) is known to be due to inhibition of viral DNA synthesis.
FIG 1.
Anti-HSV-2 activity of PG545. (A) PG545, muparfostat, muparfostat tetrasaccharide (SM4), sulfated azido-maltohexaose (AM6), and acyclovir (1 μg/ml; ∼4 μM) were incubated with ∼200 viral PFU prior to infection of GMK AH1 cells. The results are expressed as percentages of the number of PFU found in drug-treated virus relative to that in mock-treated virus controls. Data are means ± SDs from two independent experiments carried out in duplicate wells. (B) Cytotoxicity of the test compounds for GMK AH1 cells was assessed by a tetrazolium-based cell viability assay (Promega). The results are expressed as percentages of absorbance values found in drug-treated cells relative to that developed in cells incubated with the drug solvent. Values are means ± SDs from two separate experiments carried out in duplicate or triplicate wells. (C) PG545 but not muparfostat, SM4, AM6, or acyclovir inactivates HSV-2 infectivity permanently. Approximately 105 PFU of HSV-2 was mixed with test compounds at specific concentrations and incubated for 15 min at 37°C. The results are expressed as percentages of residual infectivity of the compound-treated virus as related to that of the mock-treated virus control. Data are means ± SDs from two independent experiments carried out in duplicates. Triple asterisks indicate significant difference (P < 0.001) as calculated by Student's t test.
We also investigated whether the anti-HSV-2 activity of PG545, found with laboratory strain 333 (Fig. 1), could be reproduced with clinical isolates of HSV-2. PG545 potently inhibited infectivity of five clinical isolates of HSV-2, including two acyclovir-resistant strains (Table 1). Importantly, PG545 but not acyclovir exhibited virus particle-inactivating activity against all five clinical isolates tested (Table 1).
TABLE 1.
PG545 inhibits HSV-2 plaque-forming activity and inactivates infectivity of free virus particles
| HSV-2 strain or clinical isolate | IC50 (μg/ml) as determined by plaque assay |
Virus-inactivation activitya at indicated concn (μg/ml) of agent |
||||||
|---|---|---|---|---|---|---|---|---|
| PG545 | Acyclovir | PG545 |
Acyclovir |
|||||
| 100 | 20 | 4 | 100 | 20 | 4 | |||
| 333 | 0.8 | 7.3 | 0.0 | 0.0 | 0.0 | 103.5 | 113.8 | 100.0 |
| DE06-3942b | 0.4 | >100 | 0.0 | 0.0 | 0.0 | 85.3 | 100 | 88 |
| DE07-6820b | 0.5 | >100 | 0.0 | 0.0 | 2.8 | 108.7 | 104.9 | 102.9 |
| VI03-1678c | 0.6 | 3.6 | 0.0 | 0.0 | 0.0 | 113.4 | 104.9 | 108.5 |
| 90-036 | 0.4 | 3.0 | 0.0 | 0.0 | 52.2 | 99.4 | 108.5 | 100 |
| 90-306 | 0.4 | 2.3 | 0.0 | 0.0 | 73.6 | 99.4 | 103.2 | 93.6 |
Virus-inactivating activity is expressed as the percentage of residual infectivity found with PG545- or acyclovir-treated virus relative to that of the mock-treated virus control.
Clinical isolate of HSV-2 resistant to acyclovir.
Clinical isolate of HSV-2 from routine diagnostics.
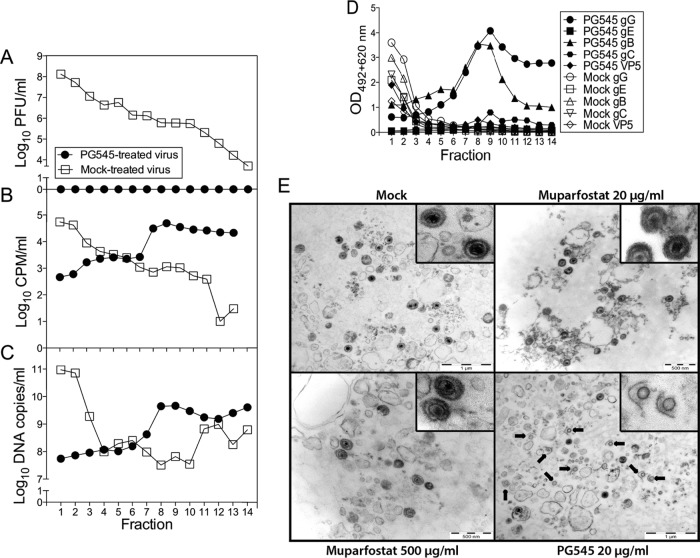
Because the virus-inactivating activity of PG545 increases the likelihood of its successful application as a topical virucide, we sought to elucidate the mode of the virus-inactivating activity of PG545. To this end, we studied the structural integrity of purified HSV-2 virions following their treatment with PG545. The [3H]thymidine-labeled HSV-2 particles, which during ultracentrifugation through the discontinuous sucrose gradient form a band at the interphase of 40 and 50% sucrose, were collected and incubated with PG545 or its solvent (redistilled water) for 30 min at 37°C. Subsequent recentrifugation of these preparations on identical sucrose gradients revealed that in mock-treated samples, most of the viral infectivity (Fig. 2A), [3H]thymidine label (Fig. 2B), viral DNA (Fig. 2C), and viral envelope glycoproteins B, C, G, and E and the viral capsid protein VP5 (Fig. 2D) were detected in fractions 1 and 2, which overlap the virus band at the 40 and 50% sucrose interphase. In contrast, in the PG545-treated sample, the virus infectivity was completely destroyed (Fig. 2A), and most of the [3H]thymidine label (Fig. 2B), viral DNA (Fig. 2C), and viral proteins (Fig. 2D) shifted to fractions 7 to 14. This suggested a substantial fragmentation of virions. The presence of a small peak of the viral capsid protein VP5 in fraction 8 (Fig. 2D) suggests that besides causing a disturbance to the lipid envelope, PG545 may affect the integrity of the viral capsid. To verify this interpretation, the extracellular HSV-2 virions were concentrated by pelleting and then treated with PG545 and assessed by electron microscopy. Treatment of these semipurified virions with a relatively low concentration of PG545 (20 μg/ml) resulted in disruption of the viral envelope in many virions (Fig. 2E; see also Fig. S2A in the supplemental material) and in release of viral DNA from capsids as observed by the absence of dark electron dense material in the disrupted virus particles (Fig. 2E). Treatment of virions with a relatively high concentration of PG545 (500 μg/ml) resulted in their complete lysis, with no discernible HSV-2 particles or their components (see Fig. S2A). In contrast, the number of damaged virions in samples treated with 20 or 500 μg/ml of muparfostat was low and similar to that of spontaneously disrupted viral particles in mock-treated HSV-2 sample (see Fig. S2B). Note that in a sample treated with 20 μg/ml of PG545, ∼60% of viral particles were disrupted (see Fig. S2B), while in the virus inactivation assay, all virus infectivity was lost after treatment with the same concentration of PG545 (Fig. 2A). This discrepancy could be explained by, in addition to the use of two different assays, the much larger amount of virus used in the electron microscopy study (∼3 × 107 PFU) than in the virus inactivation assay (∼105 PFU). However, we cannot exclude that at a relatively low PG545/virus particle ratio, this compound may inhibit virus infectivity without detectable alterations of the virion structure, e.g., by irreversible blockade or selective removal of the virus attachment proteins from the lipid envelope.
FIG 2.
PG545 disrupts HSV-2 virions. PG545- or mock-treated purified [3H]thymidine-labeled HSV-2 virions were centrifuged through the sucrose gradient, and fractions, collected from the bottom of the tube (fraction 1), were examined for viral infectivity by plaque titration assay (A), for radioactivity in a scintillation counter (B), for viral DNA by quantitative PCR (C), and for viral glycoproteins B, C, E, and G and the major capsid protein VP5 by ELISA (D). Fractions 1 and 2 correspond to the gradient position where most of the intact infectious virions concentrated. (E) Disruption of HSV-2 virions by PG545. Shown are electron microscopy images of semipurified preparations of HSV-2 virions subjected to mock, muparfostat, or PG545 treatment. Disrupted viral particles are indicated with arrows. Note the absence of dark, electron-dense material in capsids of disrupted virions. The images were selected from 10 images captured for each preparation. Insets show enlarged images of viral particles. More images are shown in Fig. S2A in the supplemental material. CPM, counts per minute.
PG545 but not muparfostat protects mice against HSV-2.
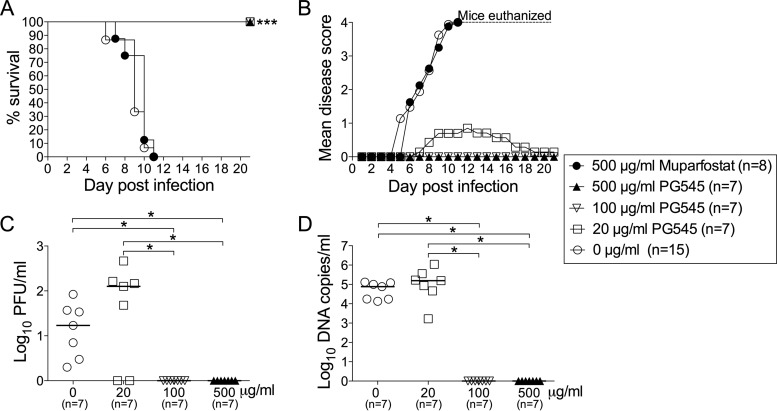
The finding that PG545 but not muparfostat exhibited HSV particle-destroying activity raised the question of whether this novel activity of PG545 could contribute to protection of mice against genital infection with HSV-2. In the first series of experiments, we investigated whether the treatment of the viral inoculum (105 PFU) with PG545 or muparfostat for 15 min at room temperature prior to administration of the mixture to mice would inactivate the virus, thus protecting animals. All mice in the control group that received the mock-treated virus developed severe disease and were sacrificed at day 6 to 11 postinfection (p.i.) (Fig. 3A and B). Pretreatment of virus with the high dose of muparfostat (500 μg/ml) offered no protection to mice, as the development of disease symptoms and the animal survival rate paralleled those in control animals (Fig. 3A and B). Note that the treatment of HSV-2 particles with the same concentration of muparfostat did not adversely affect the morphology of virions (Fig. 2E). In contrast, all mice inoculated with the virus treated with PG545 at 20 (PG545-20), 100 (PG545-100), and 500 (PG545-500) μg/ml survived the experiment (Fig. 3A), showing either mild (PG545-20) or no disease symptoms (Fig. 3B). This was accompanied by the absence of (PG545-500) or sporadic (PG545-100) virus replication in the genital tract, as the presence of viral DNA (Fig. 3D) and infectious virus (Fig. 3C) in the vaginal lavage fluids was limited to 1 or 2 animals only. These data are in line with our electron microscopy assessment which demonstrated that treatment of HSV-2 virions with PG545-500 caused complete lysis of virus particles (see Fig. S2A).
FIG 3.
Short virus treatment with PG545 protects mice against HSV-2. HSV-2 (105 PFU) was treated with PG545 or muparfostat for 15 min prior to intravaginal administration to mice. Animals were monitored for survival rates (A), disease scores (B), viral DNA (C), and infectious virus (D) in vaginal lavage fluids sampled at day 3 postinfection. The number of animals (n) in each group in panels A and B is indicated in the legend box. Fisher's exact test was used to analyze survival data in panel A. ANOVA was used to analyze the data in panels C and D, and the horizontal bars indicate median values of each group. Asterisks indicate significant difference as follows: *, P < 0.05, and ***, P < 0.001.
Interestingly, most of the mice that received virus treated with the lowest dose of compound, i.e., PG545-20, showed mild symptoms of local genital inflammation (scored as 1) at day 12, followed by nearly complete recovery at day 19 postinfection (Fig. 3B). This mild disease was accompanied by the presence in vaginal lavage fluids of viral DNA and infectious virus at quantities comparable to those of control mice (Fig. 3C and D). Although the amount of virus and the temperature of the PG545-virus preincubation were different in animal and electron microscopy studies, these data are supported by electron microscopy assessment demonstrating that on treatment of viral particles with PG545-20, some of the virions remained intact (Fig. 2E) and might therefore infect mice as shown in Fig. 3C and D.
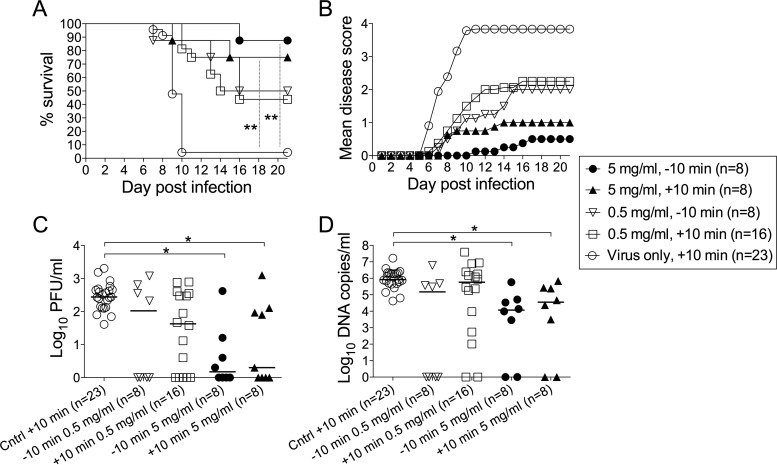
We also investigated the prophylactic potential of PG545 in a murine model of genital HSV-2 infection. To this end, PG545 was administered intravaginally to mice ∼10 min prior to inoculation with the virus, a time point that could resemble the vaginal use of microbicides in humans. Vaginal application of GAG mimetics prior to the virus inoculation requires much higher concentrations of the mimetics than those effective in cultured cells (41, 42), a finding most likely due to their neutralization by mucus. Out of eight mice that received PG545 at 5 mg/ml, only one developed genital disease (Fig. 4B), dying at day 16 p.i. (Fig. 4A), i.e., markedly later than most animals in the control group. The vaginal lavage fluid of this mouse contained infectious HSV-2 and viral DNA at quantities comparable to these of control animals (Fig. 4C and D). The rest of the animals that survived the experiments developed no genital disease and showed reduced amounts or no infectious virus or viral DNA in vaginal lavage fluid (Fig. 4). Instillation of PG545 at a 10-fold-lower concentration (0.5 mg/ml) conferred complete protection from HSV-induced disease in four out of eight mice (Fig. 4B), death (Fig. 4A), and virus replication in the vagina (Fig. 4C and D). Application of PG545 at ∼10 min p.i. also conferred protection in some animals against the disease symptoms, death, and virus replication in the genital tract (Fig. 4), although the extent of protection was less than in prophylactic use of this compound.
FIG 4.
Prophylactic PG545 application protects mice against genital infection. PG545 was administered intravaginally to mice ∼10 min prior to or after infection with HSV-2, and animals were monitored for survival rates (A), disease scores (B), viral DNA (C), and infectious virus (D) in vaginal lavage fluids sampled at day 3 postinfection. Fisher's exact test was used to analyze survival data in panel A. ANOVA was used to analyze the data in panels C and D, and the horizontal bars indicate median values of each group. Asterisks indicate significant difference as follows: *, P < 0.05, and **, P < 0.01.
DISCUSSION
The GAG-mimicking inhibitors bind to the virus attachment components, thus disabling viral particles before infection of susceptible cells, a feature that advocates for their use as topical microbicides in the prevention of infections caused by many different GAG-binding viruses. However, two other functional features of these inhibitors, i.e., a nonvirucidal mode of activity (8, 20) and capability to interact with a plethora of heparin/GAG-binding proteins (22) that maintain the structural integrity and defense of the genital tract, have been largely neglected. In this study, we found that the cholestanol-conjugated sulfated oligosaccharide PG545, a clinical candidate under investigation in a phase I oncology trial (43), but not several other sulfated oligosaccharides lacking this modification, exhibited a distinctly altered mode of antiviral activity, i.e., from a simple, reversible inhibition to a virus particle-destroying activity. In addition to HSV, we have recently observed that PG545, unlike muparfostat, permanently inactivated the infectivity of HIV-1 and RSV virions (28, 29). However, it is not known whether this activity was due to disruption of viral particles. Other sulfated oligo-and polysaccharides (8), including those used in microbicidal trials, i.e., cellulose sulfate (42), and some carrageenans (44) did not show strong virus-inactivating activity, implying, similar to the case with muparfostat, a reversible mode of antiviral activity. Such nonvirucidal binding of GAG mimetics may result in coating and protection of infectious virions against proteolytic degradation in the mucosal milieu (45), a known feature of the protein-GAG interaction (22).
The virus particle-disrupting activity of PG545 is an essential feature of candidate topical microbicides that recalls the effect of surfactants on viral and cellular lipid membranes. The anionic surfactants docusate and sodium lauryl sulfate (46, 47) and the nonionic surfactant nonoxynol-9 (48) are known to exhibit virucidal activity against HSV and HIV in vitro. However, these compounds show poor selectivity toward viral particles. Therefore, the in vivo application of these compounds is frequently accompanied by perturbations of integrity of the genital epithelium, leading to increased susceptibility to microbial pathogens (49). We found that although PG545 exhibited greater cytotoxicity than muparfostat, its selectivity index was still larger than 100, implying a high preference for viral rather than cellular targets. Thus, the PG545—an amphiphilic molecule with polar and nonpolar groups—provides good selectivity toward viral particles, a feature of sulfated oligo- and polysaccharides, and surfactant-like virucidal effects. Although the exact sequence of events leading to PG545-mediated disruption of viral particles is not known, we previously found that muparfostat, PG545, and another cholestanol-conjugated oligosaccharide inhibited attachment to cells of HSV-2 (27), HIV-1 (29), and RSV (28), indicating that PG545 preserved this functional feature of sulfated oligo- and polysaccharides. In fact, the comparable anti-HSV-2 activities of PG545 and muparfostat found in the viral plaque assay (Fig. 1A) might be due to inhibition of the virus attachment to cells, since both of these compounds comprise structurally similar sulfated oligosaccharides (see Fig. S1 in the supplemental material). As discussed earlier, this inhibitory effect requires the continuous presence of muparfostat and is reversible, while in the case of PG545, binding to and blocking of the virus attachment proteins seem to be followed by disruption of the viral lipid envelope. Interestingly, while the muparfostat-resistant variants of HSV-1 (50), HSV-2 (26), and HIV-1 (51) featured, as expected, mutations in the viral attachment components or in modulators of this activity, we failed to select for PG545-resistant variants of HSV-2 (27) and HIV-1 (51). The inability of PG545 to generate drug-resistant viral variants, a marked feature of antivirals and selective microbicides, strongly suggests that PG545 targets several components of the viral particle, including envelope lipids. Besides disruption of the viral envelope, we frequently observed a lack of DNA inside the capsids of PG545-treated virions. Ejection of the HSV DNA from the capsid occurs through a single capsid site, the portal vertex, and requires virion uncoating, including removal of most of the tegument proteins (52). Therefore, it is likely that the virucidal activity of PG545 is not limited to the lipid envelope and that the outer tegument coat of proteins is disintegrated. It is noteworthy that the antiviral activities of the peptide-based inhibitors of HIV-1 entry into cells (53) and the acyclic nucleotide cidofovir (54) have been greatly potentiated following their conjugation to cholesterol or lipid.
Although PG545 and muparfostat exhibited comparable anti-HSV-2 activities in cultured cells, short treatment of HSV-2 virions with PG545 but not with muparfostat prior to the administration of the virus-compound mixture into mice conferred complete protection against HSV-2 disease, with no detectable virus or viral DNA in the genital tract. Likewise, prophylactic application of PG545 in mice at 10 min prior to HSV-2 challenge resulted in nearly complete protection against HSV-2-induced disease. The extent of anti-HSV-2 protection of mice by PG545 was comparable to that achieved by the GAG mimetics carrageenans (41, 55), cellulose sulfate (49), and PRO2000 (56) when used at concentrations ∼2 to 10 times higher than PG545 and as vaginal gel formulations rather than water solutions of test compounds. It should be noted that prophylactic application of PG545 and sulfated oligosaccharides as vaginal microbicides requires relatively high concentrations of these compounds. This requirement is most likely due to the neutralization of the compounds by different GAG-binding proteins (22) of the genital mucosa, such as epithelium- and neutrophil-derived antimicrobial polypeptides and components of seminal plasma (49) and cervical secretions (27), as well as some extracellular matrix and cell adhesion proteins (57). This implies that besides neutralization of an inhibitor, these interactions may impair local innate and adaptive immunity and perturb the structural integrity of the epithelium (57), i.e., alterations that most likely contributed to the failure of GAG mimetics in HIV clinical trials. Based on the premises that the GAG-binding proteins differ in their interactions with GAG chains (22) and that the lipid composition of the viral envelope is quantitatively different from that of the cell plasma membrane (58, 59), further studies to evaluate the suitability of targeting GAG-binding attachment proteins and lipids to develop therapeutics against HSV, RSV, and HIV-1 are warranted. It is noteworthy that PG545 is a known anticancer candidate agent that is currently under evaluation in a phase I clinical trial (Clinical Trials.gov identifier: NCT02042781) with patients with solid tumors, based on its inhibitory effects on heparanase and GAG-binding angiogenic growth factors (60).
In contrast to HSV, in the case of which GAG chains promote virus attachment to cells, HIV-1 utilizes CD4 for attachment purposes (61), and GAG chains are believed to assist in or strengthen this interaction. However, in cells of the genital epithelium, where CD4 is not expressed, the GAG chains of proteoglycan syndecan were found to capture, protect, and translocate infectious HIV-1 to CD4-expressing T cells (45). PG545 may interfere with this process, since we have previously found that this compound permanently inactivated infectivity of HIV-1 strains that showed tropism for R5 as well as X4 coreceptors (29). As infection with HSV-2 is associated with an increased risk of transmission of HIV-1 (62–64), a dual PG545 activity against both viruses could be advantageous for prophylaxis of these sexually transmitted pathogens.
Supplementary Material
ACKNOWLEDGMENTS
We thank Keith Dredge (Progen Pharmaceuticals Ltd., Brisbane, Australia) for many helpful suggestions and comments on the manuscript.
Funding Statement
Financial support was provided by the Swedish Research Council, the LUA-ALF foundation of Sahlgren's University Hospital, the Swedish International Development Agency (SIDA), and the Torsten Söderberg Foundation. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We declare no competing financial interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02132-15.
REFERENCES
- 1.WuDunn D, Spear PG. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol 63:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krusat T, Streckert HJ. 1997. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol 142:1247–1254. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- 3.Hallak LK, Spillmann D, Collins PL, Peeples ME. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 74:10508–10513. doi: 10.1128/JVI.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol 75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Hearn A, Wang M, Cheng H, Lear-Rooney CM, Koning K, Rumschlag-Booms E, Varhegyi E, Olinger G, Rong L. 2015. Role of EXT1 and glycosaminoglycans in the early stage of filovirus entry. J Virol 89:5441–5449. doi: 10.1128/JVI.03689-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold BC, WuDunn D, Soltys N, Spear PG. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol 65:1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herold BC, Visalli RJ, Susmarski N, Brandt CR, Spear PG. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol 75(Part 6):1211–1222. [DOI] [PubMed] [Google Scholar]
- 8.Vaheri A. 1964. Heparin and related polyionic substances as virus inhibitors. Acta Pathol Microbiol Scand Suppl 171:171–198. [PubMed] [Google Scholar]
- 9.Witvrouw M, De Clercq E. 1997. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacol 29:497–511. doi: 10.1016/S0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- 10.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D, CS Study Group . 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med 359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. 2008. AIDS research. Microbicide fails to protect against HIV. Science 319:1026–1027. [DOI] [PubMed] [Google Scholar]
- 12.Pirrone V, Wigdahl B, Krebs FC. 2011. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res 90:168–182. doi: 10.1016/j.antiviral.2011.03.176. [DOI] [PubMed] [Google Scholar]
- 13.van de Wijgert JH, Shattock RJ. 2007. Vaginal microbicides: moving ahead after an unexpected setback. AIDS 21:2369–2376. doi: 10.1097/QAD.0b013e3282ef83fd. [DOI] [PubMed] [Google Scholar]
- 14.Hirst GK. 1942. Adsorption of influenza hemagglutinins and virus by red blood cells. J Exp Med 76:195–209. doi: 10.1084/jem.76.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottschalk A. 1957. Neuraminidase: the specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta 23:645–646. doi: 10.1016/0006-3002(57)90389-X. [DOI] [PubMed] [Google Scholar]
- 16.Palese P, Compans RW. 1976. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol 33:159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 17.Palese P, Tobita K, Ueda M, Compans RW. 1974. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology 61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 18.von Itzstein M, Wu W-Y, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron JM, Penn CR. 1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 19.Oh MJ, Akhtar J, Desai P, Shukla D. 2010. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun 391:176–181. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neyts J, De Clercq E. 1995. Effect of polyanionic compounds on intracutaneous and intravaginal herpesvirus infection in mice: impact on the search for vaginal microbicides with anti-HIV activity. J Acquir Immune Defic Syndr Hum Retrovirol 10:8–12. [PubMed] [Google Scholar]
- 21.Pomin VH. 2014. Biological findings from the recent NMR-based studies of glycosaminoglycan-protein interactions. Glycobiology 24:991–1003. doi: 10.1093/glycob/cwu065. [DOI] [PubMed] [Google Scholar]
- 22.Xu D, Esko JD. 2014. Demystifying heparan sulfate-protein interactions. Annu Rev Biochem 83:129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarbrough VL, Winkle S, Herbst-Kralovetz MM. 2015. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update 21:353–377. doi: 10.1093/humupd/dmu065. [DOI] [PubMed] [Google Scholar]
- 24.Kumamoto Y, Iwasaki A. 2012. Unique features of antiviral immune system of the vaginal mucosa. Curr Opin Immunol 24:411–416. doi: 10.1016/j.coi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyberg K, Ekblad M, Bergstrom T, Freeman C, Parish CR, Ferro V, Trybala E. 2004. The low molecular weight heparan sulfate-mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus. Antiviral Res 63:15–24. doi: 10.1016/j.antiviral.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Adamiak B, Ekblad M, Bergstrom T, Ferro V, Trybala E. 2007. Herpes simplex virus type 2 glycoprotein G is targeted by the sulfated oligo- and polysaccharide inhibitors of virus attachment to cells. J Virol 81:13424–13434. doi: 10.1128/JVI.01528-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekblad M, Adamiak B, Bergstrom T, Johnstone KD, Karoli T, Liu L, Ferro V, Trybala E. 2010. A highly lipophilic sulfated tetrasaccharide glycoside related to muparfostat (PI-88) exhibits virucidal activity against herpes simplex virus. Antiviral Res 86:196–203. doi: 10.1016/j.antiviral.2010.02.318. [DOI] [PubMed] [Google Scholar]
- 28.Lundin A, Bergstrom T, Andrighetti-Frohner CR, Bendrioua L, Ferro V, Trybala E. 2012. Potent anti-respiratory syncytial virus activity of a cholestanol-sulfated tetrasaccharide conjugate. Antiviral Res 93:101–109. doi: 10.1016/j.antiviral.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Said J, Trybala E, Andersson E, Johnstone K, Liu L, Wimmer N, Ferro V, Bergstrom T. 2010. Lipophile-conjugated sulfated oligosaccharides as novel microbicides against HIV-1. Antiviral Res 86:286–295. doi: 10.1016/j.antiviral.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Dredge K, Hammond E, Davis K, Li CP, Liu L, Johnstone K, Handley P, Wimmer N, Gonda TJ, Gautam A, Ferro V, Bytheway I. 2010. The PG500 series: novel heparan sulfate mimetics as potent angiogenesis and heparanase inhibitors for cancer therapy. Invest New Drugs 28:276–283. doi: 10.1007/s10637-009-9245-5. [DOI] [PubMed] [Google Scholar]
- 31.Ferro V, Liu L, Johnstone KD, Wimmer N, Karoli T, Handley P, Rowley J, Dredge K, Li CP, Hammond E, Davis K, Sarimaa L, Harenberg J, Bytheway I. 2012. Discovery of PG545: a highly potent and simultaneous inhibitor of angiogenesis, tumor growth, and metastasis. J Med Chem 55:3804–3813. doi: 10.1021/jm201708h. [DOI] [PubMed] [Google Scholar]
- 32.Guenalp A. 1965. Growth and cytopathic effect of rubella virus in a line of green monkey kidney cells. Proc Soc Exp Biol Med 118:85–90. doi: 10.3181/00379727-118-29763. [DOI] [PubMed] [Google Scholar]
- 33.Duff R, Rapp F. 1971. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol 233:48–50. [DOI] [PubMed] [Google Scholar]
- 34.Namvar L, Olofsson S, Bergstrom T, Lindh M. 2005. Detection and typing of herpes simplex virus (HSV) in mucocutaneous samples by TaqMan PCR targeting a gB segment homologous for HSV types 1 and 2. J Clin Microbiol 43:2058–2064. doi: 10.1128/JCM.43.5.2058-2064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Görander S, Harandi AM, Lindqvist M, Bergstrom T, Liljeqvist JA. 2012. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J Virol 86:7544–7553. doi: 10.1128/JVI.00186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergström T, Sjogren-Jansson E, Jeansson S, Lycke E. 1992. Mapping neuroinvasiveness of the herpes simplex virus type 1 encephalitis-inducing strain 2762 by the use of monoclonal antibodies. Mol Cell Probes 6:41–49. doi: 10.1016/0890-8508(92)90070-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liljeqvist JA, Trybala E, Svennerholm B, Jeansson S, Sjogren-Jansson E, Bergstrom T. 1998. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J Gen Virol 79(Part 5):1215–1224. [DOI] [PubMed] [Google Scholar]
- 38.Widehn S, Kindblom LG. 1990. Agarose embedding: a new method for the ultrastructural examination of the in-situ morphology of cell cultures. Ultrastruct Pathol 14:81–85. doi: 10.3109/01913129009050876. [DOI] [PubMed] [Google Scholar]
- 39.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest 70:369–380. [PubMed] [Google Scholar]
- 40.Morrison LA, Da Costa XJ, Knipe DM. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 41.Carlucci MJ, Scolaro LA, Noseda MD, Cerezo AS, Damonte EB. 2004. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antiviral Res 64:137–141. doi: 10.1016/j.antiviral.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Cheshenko N, Keller MJ, MasCasullo V, Jarvis GA, Cheng H, John M, Li JH, Hogarty K, Anderson RA, Waller DP, Zaneveld LJ, Profy AT, Klotman ME, Herold BC. 2004. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob Agents Chemother 48:2025–2036. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winterhoff B, Freyer L, Hammond E, Giri S, Mondal S, Roy D, Teoman A, Mullany SA, Hoffmann R, von Bismarck A, Chien J, Block MS, Millward M, Bampton D, Dredge K, Shridhar V. 2015. PG545 enhances anti-cancer activity of chemotherapy in ovarian models and increases surrogate biomarkers such as VEGF in preclinical and clinical plasma samples. Eur J Cancer 51:879–892. doi: 10.1016/j.ejca.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlucci MJ, Ciancia M, Matulewicz MC, Cerezo AS, Damonte EB. 1999. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antiviral Res 43:93–102. doi: 10.1016/S0166-3542(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 45.Bobardt MD, Saphire AC, Hung HC, Yu X, Van der Schueren B, Zhang Z, David G, Gallay PA. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27–39. doi: 10.1016/S1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 46.Gong Y, Wen A, Cheung D, Wong M, Sacks SL. 2001. Preclinical evaluation of docusate as protective agent from herpes simplex viruses. Antiviral Res 52:25–32. doi: 10.1016/S0166-3542(01)00156-5. [DOI] [PubMed] [Google Scholar]
- 47.Piret J, Roy S, Gagnon M, Landry S, Desormeaux A, Omar RF, Bergeron MG. 2002. Comparative study of mechanisms of herpes simplex virus inactivation by sodium lauryl sulfate and n-lauroylsarcosine. Antimicrob Agents Chemother 46:2933–2942. doi: 10.1128/AAC.46.9.2933-2942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malkovsky M, Newell A, Dalgleish AG. 1988. Inactivation of HIV by nonoxynol-9. Lancet i:645. [DOI] [PubMed] [Google Scholar]
- 49.Segarra TJ, Fakioglu E, Cheshenko N, Wilson SS, Mesquita PM, Doncel GF, Herold BC. 2011. Bridging the gap between preclinical and clinical microbicide trials: blind evaluation of candidate gels in murine models of efficacy and safety. PLoS One 6:e27675. doi: 10.1371/journal.pone.0027675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekblad M, Adamiak B, Bergefall K, Nenonen H, Roth A, Bergstrom T, Ferro V, Trybala E. 2007. Molecular basis for resistance of herpes simplex virus type 1 mutants to the sulfated oligosaccharide inhibitor PI-88. Virology 367:244–252. doi: 10.1016/j.virol.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 51.Said J, Andersson E, Trybala E, Bergstrom T. 2013. HIV-1 variants with reduced sensitivity to sulfated oligosaccharide muparfostat contain mutations in the envelope glycoproteins gp120 and gp41. J Antivir Antiretrovir 5:50–56. doi: 10.4172/jaa.1000063. [DOI] [Google Scholar]
- 52.Newcomb WW, Cockrell SK, Homa FL, Brown JC. 2009. Polarized DNA ejection from the herpesvirus capsid. J Mol Biol 392:885–894. doi: 10.1016/j.jmb.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ingallinella P, Bianchi E, Ladwa NA, Wang YJ, Hrin R, Veneziano M, Bonelli F, Ketas TJ, Moore JP, Miller MD, Pessi A. 2009. Addition of a cholesterol group to an HIV-1 peptide fusion inhibitor dramatically increases its antiviral potency. Proc Natl Acad Sci U S A 106:5801–5806. doi: 10.1073/pnas.0901007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson VA, Smith SK, Foster S, Li Y, Lanier ER, Gates I, Trost LC, Damon IK. 2014. In vitro efficacy of brincidofovir against variola virus. Antimicrob Agents Chemother 58:5570–5571. doi: 10.1128/AAC.02814-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zacharopoulos VR, Phillips DM. 1997. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diagn Lab Immunol 4:465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourne N, Bernstein DI, Ireland J, Sonderfan AJ, Profy AT, Stanberry LR. 1999. The topical microbicide PRO 2000 protects against genital herpes infection in a mouse model. J Infect Dis 180:203–205. doi: 10.1086/314853. [DOI] [PubMed] [Google Scholar]
- 57.Mesquita PM, Cheshenko N, Wilson SS, Mhatre M, Guzman E, Fakioglu E, Keller MJ, Herold BC. 2009. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J Infect Dis 200:599–608. doi: 10.1086/600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Genderen IL, Godeke GJ, Rottier PJ, van Meer G. 1995. The phospholipid composition of enveloped viruses depends on the intracellular membrane through which they bud. Biochem Soc Trans 23:523–526. doi: 10.1042/bst0230523. [DOI] [PubMed] [Google Scholar]
- 59.Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR. 2008. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J Virol 82:11228–11238. doi: 10.1128/JVI.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, Vincent C, Brandt R, Ferro V, Bytheway I. 2011. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br J Cancer 104:635–642. doi: 10.1038/bjc.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 62.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 63.Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 64.Holmberg SD, Stewart JA, Gerber AR, Byers RH, Lee FK, O'Malley PM, Nahmias AJ. 1988. Prior herpes simplex virus type 2 infection as a risk factor for HIV infection. JAMA 259:1048–1050. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.