Abstract
The CLSI epidemiological cutoff values (ECVs) of antifungal agents are available for various Candida spp., Aspergillus spp., and the Mucorales. However, those categorical endpoints have not been established for Fusarium spp., mostly due to the difficulties associated with collecting sufficient CLSI MICs for clinical isolates identified according to the currently recommended molecular DNA-PCR-based identification methodologies. CLSI MIC distributions were established for 53 Fusarium dimerum species complex (SC), 10 F. fujikuroi, 82 F. proliferatum, 20 F. incarnatum-F. equiseti SC, 226 F. oxysporum SC, 608 F. solani SC, and 151 F. verticillioides isolates originating in 17 laboratories (in Argentina, Australia, Brazil, Canada, Europe, Mexico, and the United States). According to the CLSI guidelines for ECV setting, ECVs encompassing ≥97.5% of pooled statistically modeled MIC distributions were as follows: for amphotericin B, 4 μg/ml (F. verticillioides) and 8 μg/ml (F. oxysporum SC and F. solani SC); for posaconazole, 2 μg/ml (F. verticillioides), 8 μg/ml (F. oxysporum SC), and 32 μg/ml (F. solani SC); for voriconazole, 4 μg/ml (F. verticillioides), 16 μg/ml (F. oxysporum SC), and 32 μg/ml (F. solani SC); and for itraconazole, 32 μg/ml (F. oxysporum SC and F. solani SC). Insufficient data precluded ECV definition for the other species. Although these ECVs could aid in detecting non-wild-type isolates with reduced susceptibility to the agents evaluated, the relationship between molecular mechanisms of resistance (gene mutations) and MICs still needs to be investigated for Fusarium spp.
INTRODUCTION
While the genus Fusarium and its teleomorphic (sexual) forms encompass a variety of species, only some have been associated with human disease. Identification of Fusarium isolates to the accepted phylogenetic species complex (SC) or species level is essential (1–4) but challenging, since important taxonomic changes have been made, and the taxonomy is still in a state of flux for some genera. Following the results of DNA-sequencing studies, well-known prevalent fungal genera were divided into several new genera. By 2013, the consensus was to continue using certain well-known generic names and to have a single name for each fungal species, including those in the genus Fusarium (5). In addition, the names of the well-known Fusarium anamorphs, as they have been used in the present paper, ought to be used instead of those of the known teleomorphs (e.g., Haemonectria and Gibberella) (1–3). However, the perception is that new generic changes may be suggested, such as the establishment of the genus Bisifusarium to include the more commonly known members of the Fusarium dimerum SC and the name Neocosmospora solani to replace Fusarium solani (6). The most frequent causes of fungal infections are members of three complexes, the F. solani species complex (SC), the F. oxysporum SC, and the Fusarium (Gibberella) fujikuroi SC (which includes, among others, F. verticillioides and F. proliferatum), and the next most frequent causes belong to the F. dimerum SC and F. incarnatum-F. equiseti SC; their distribution could be region dependent (4, 7–10). Common clinical presentations are onychomycosis, keratitis, allergic disease (sinusitis and bronchopulmonary disease) for nonimmunocompromised patients and disseminated disease, as well as other severe invasive infections, in immunocompromised hosts (e.g., patients with prolonged neutropenia and T-cell immunodeficiency) (1, 4, 7–13). Amphotericin B lipid formulations, voriconazole, posaconazole, and, to a lesser extent, itraconazole have been recommended or used for the treatment and prophylaxis of Fusarium infections, in addition to surgical debridement and reversal of immunosuppression (14–19). The survival rate is low, with some reports suggesting 30% or less, for fusariosis, especially among patients with persistent neutropenia (16–22). Successful therapeutic treatment of invasive disease is usually associated with neutrophil recovery, a major factor in making the setting of clinical breakpoints so challenging. The new formulations of itraconazole and posaconazole have improved bioavailability and reduced variability in exposure among subjects (23, 24). However, the efficacy of these formulations in the treatment of fusariosis has not been established.
A reproducible procedure for testing the antifungal susceptibilities of Fusarium spp. is described by the Clinical and Laboratory Standards Institute (CLSI) Subcommittee on Antifungal Susceptibility Tests in document M38-A2 (25). However, neither species-specific clinical breakpoints (BPs) nor epidemiological cutoff values (ECVs) have been established for this fungal group. The main reason, as for other, less-prevalent fungal species, is the lack of both clinical trials and knowledge regarding molecular resistance mechanisms for Fusarium spp. As a consequence, information on the relationships between resistance mechanisms, low and high MICs, and clinical response to therapy is not available. However, it is still possible to define ECVs. These are calculated on the basis of MIC distributions (≥100 MIC results per species and antifungal agent) from multiple (≥3) independent laboratories (26, 27; CLSI documents on ECVs under development). ECVs can identify non-wild-type (non-WT [often harboring molecular mechanisms of resistance]) isolates or isolates that are less susceptible to the antifungal agent being evaluated. Although amphotericin B and triazole MIC data have been reported for a variety of Fusarium spp., most available data were obtained for isolates identified only to the genus level or by nonmolecular methods, or the number of isolates evaluated was small (2, 4, 28–31). Therefore, there was a need to pool data from multiple laboratories in order to define ECVs for Fusarium spp.
The purposes of the present study were (i) to define the WT susceptibility endpoint MIC distributions of the three most prevalent species/species complexes (the F. oxysporum SC, the F. solani SC, and F. verticillioides) using aggregated CLSI M38-A2 broth microdilution MIC data originating from 16 of the 17 participating laboratories and (ii) to propose ECVs for amphotericin B, voriconazole, posaconazole, and itraconazole based on combinations of antifungal agents and species or complexes for which ≥113 isolates originating from ≥7 independent laboratories were used. Pooled distributions of MICs of amphotericin B, voriconazole, posaconazole, and itraconazole for 10 to 82 isolates belonging to less-prevalent species/complexes (e.g., F. dimerum SC, F. fujikuroi, F. incarnatum-F. equiseti SC, F. proliferatum) were also collated. We aggregated a total of 10 to 608 MICs (species and antifungal agent dependent) obtained in the 17 participating laboratories (in Argentina, Australia, Brazil, Canada, Europe, Mexico, and the United States).
MATERIALS AND METHODS
Isolates.
Each isolate was recovered from unique clinical specimens from patients most of whom had eye, skin (sometimes both a cutaneous infection and infection of a nail or other organ), sinus, or pulmonary infections or invasive disease (blood, lymph nodes). Antifungal susceptibility testing was performed according to the CLSI broth microdilution method (M38-A2) at the following medical centers: VCU Medical Center, Richmond, VA; Hospital São Paulo, Escola Paulista de Medicina—UNIFESP, São Paulo, Brazil; Instituto Nacional de Enfermedades Infecciosas “Dr. C. G. Malbrán,” Buenos Aires, Argentina; Institut National de Santé Publique du Québec, Laboratoire de Santé Publique du Québec, Sainte-Anne-de-Bellevue, Quebec, Canada; Provincial Laboratory, Alberta Health Services, Edmonton, Canada; University Hospitals Case Medical Center and Case Western Reserve University, Cleveland, OH; Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, Mexico; Facultat de Medicina, IISPV, URV, Reus, Spain; National Mycology Reference Centre, SA Pathology, Adelaide, Australia; Canisius Wilhelmina Hospital, Nijmegen, The Netherlands; The Instituto Adolfo Lutz Reference Center, São Paulo, Brazil; Hospital General Universitario Gregorio Marañón, Madrid, Spain; JMI Laboratories, North Liberty, IA; Mycology Department, The Instituto Adolfo Lutz Reference Center, São Paulo, Brazil; Grupo Fleury, São Paulo, Brazil; Department of Biomedical Sciences for Health, Università degli Studi di Milano, Milan, Italy; and the University of Texas Health Science Center, San Antonio, TX.
The isolates were identified in each laboratory using conventional methods (both macroscopic and microscopic characteristics on potato dextrose agar) (1, 32) and were confirmed by DNA-PCR-based molecular assays (e.g., sequencing and amplification of β-tubulin [BenA], translation elongation factor 1α [TEF], or the largest and/or second largest subunit of RNA polymerase [RPB1 and/or RPB2, respectively], as well as analysis of the internal transcribed spacer 1 [ITS1] and ITS2 regions) (1, 4, 10, 33, 34). The CLSI MICs of each of the four antifungal agents were aggregated for 53 F. dimerum SC (including 1 F. delphinoides isolate), 10 F. fujikuroi, 82 F. proliferatum, 20 F. incarnatum-F. equiseti SC, 226 F. oxysporum SC, 608 F. solani SC (including 11 F. falciforme isolates), and 151 F. verticillioides isolates originating from 3 to 16 of the 17 independent laboratories (see Table 1). Additionally, insufficient MIC data (<10 isolates from 2 to 3 laboratories) were provided for other members of the F. fujikuroi SC, identified as F. sacchari, F. subglutinans, and F. thapsinum (data not shown). Since molecular resistance mechanisms have not been elucidated for Fusarium spp. and any antifungal agent, none of the isolates were evaluated for gene mutations.
TABLE 1.
Pooled MIC distributions of amphotericin B and three triazoles for species of Fusarium from 3 to 16 laboratories as determined by the CLSI broth microdilution methoda
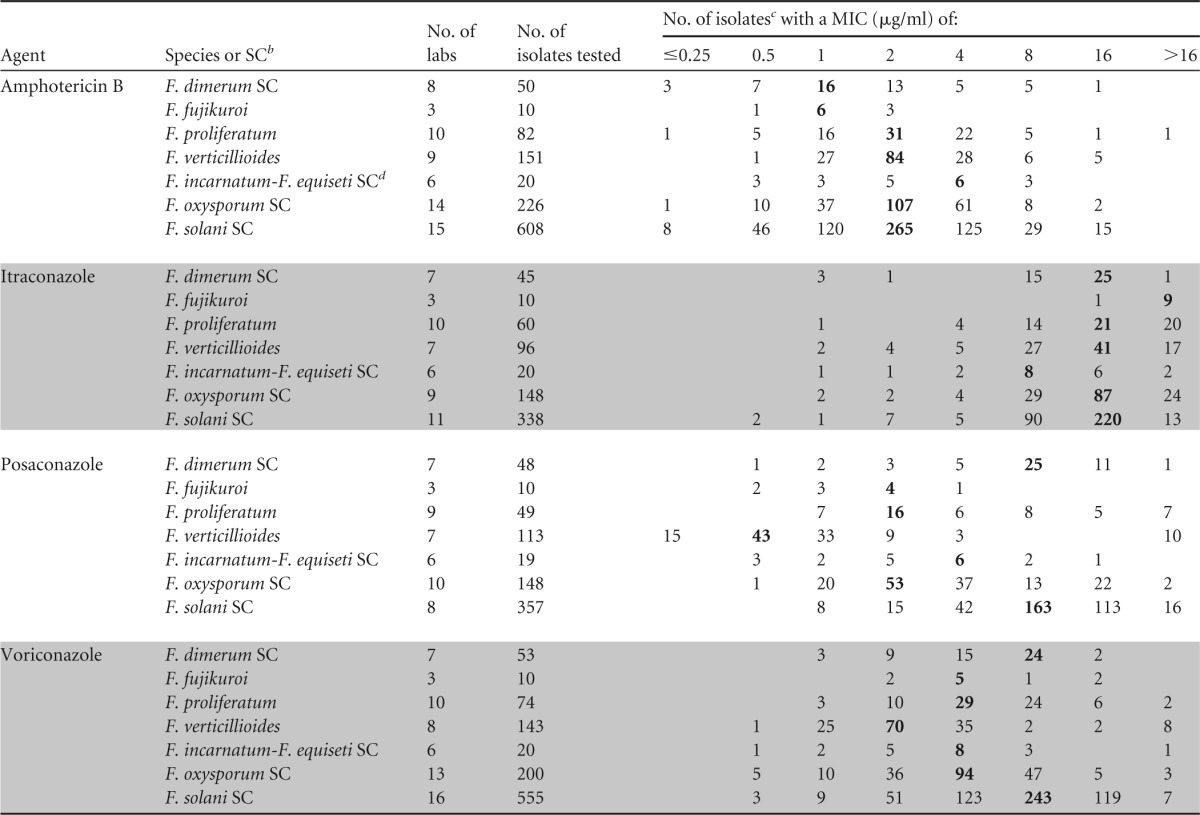
MICs were determined by CLSI method M38-A2 (25).
As identified by molecular methods (1, 4, 10, 33, 34). SC, species complex. F. fujikuroi, F. proliferatum, and F. verticillioides are members of the Fusarium (Gibberella) fujikuroi species complex.
The highest number in each row (showing the most frequently obtained MIC, or mode) is in boldface.
A synonym for the Fusarium incarnatum-F. equiseti species complex is Fusarium semitectum.
MIC data for at least one of the three quality control (QC) isolates Candida parapsilosis ATCC 22019, C. krusei ATCC 6258, and Paecilomyces variotii ATCC MYA-3630 and/or for the reference isolate Aspergillus flavus ATCC 204304 were reported by the participating laboratories (25).
Antifungal susceptibility testing.
The MICs of amphotericin B and the three triazoles (defined as the lowest drug concentrations that produced complete growth inhibition [100%] at 48 h [25]) for each available isolate in the total set (see Tables 1 and 2) were determined in each center by the CLSI broth microdilution method (with standard RPMI 1640 broth [0.2% dextrose] and final inoculum concentrations ranging from 0.4 × 104 to 5 × 104 CFU/ml). MICs for the Candida QC strains were determined after 48 h by using the 50% (triazoles) and 100% (amphotericin B) growth inhibition criteria (25). These MICs were within the recommended MIC limits with the following exceptions: discrepant MICs for both the C. krusei and C. parapsilosis QC isolates and the triazoles were observed, but the agreement (97.7 to 99.7%) was similar to or higher than those listed in the M38-A2 document (25); the modes were within 1 dilution.
TABLE 2.
Epidemiologic cutoff values of amphotericin B, itraconazole, posaconazole, and voriconazole for two clinically relevant Fusarium species complexes and F. verticillioides as determined by the CLSI broth microdilution methoda
| Species or species complex | Antifungal agentb | MIC (μg/ml) |
Calculated statistical ECV (μg/ml)c |
|||
|---|---|---|---|---|---|---|
| Range | Moded | ≥95% | ≥97.5% | ≥99% | ||
| F. verticillioides | AMB | 0.5–16 | 2 | 4 | 4 | 4 |
| ITR | 1–≥16 | 16 | ND | ND | ND | |
| POS | ≤0.25–≥16 | 0.5 | 2 | 2 | 2 | |
| VOR | 0.5–≥16 | 2 | 4 | 4 | 8 | |
| F. oxysporum SC | AMB | ≤0.25–16 | 2 | 4 | 8 | 8 |
| ITR | 1–≥16 | 16 | 32 | 32 | 32 | |
| POS | 0.5–16 | 2 | 8 | 8 | 8 | |
| VOR | 0.5–≥16 | 4 | 8 | 16 | 16 | |
| F. solani SC | AMB | ≤0.25–16 | 2 | 4 | 8 | 8 |
| ITR | 0.5–≥16 | 16 | 16 | 32 | 32 | |
| POS | 1–≥16 | 8 | 32 | 32 | 32 | |
| VOR | 0.5–≥16 | 8 | 16 | 32 | 32 | |
ECVs were defined for pooled distributions for ≥100 isolates from ≥3 laboratories using the methodology of CLSI document M38-A2 (25, 35).
AMB, amphotericin B; ITR, itraconazole; POS, posaconazole; VOR, voriconazole.
Calculated ECVs comprising ≥95%, ≥97.5%, and ≥99% of the statistically modeled population. ND, not determined (due to insufficient data).
MIC most frequently obtained for each distribution.
Definitions.
As defined in the introduction, the WT is the population of strains in a species-drug combination with no detectable acquired resistance mechanisms. The ECV (or WT cutoff value [COWT]) is the highest MIC that would categorize an isolate as WT (without known mechanisms of resistance) or, alternatively, the critical drug concentration value that may identify those strains that have decreased susceptibility to the agent being evaluated (non-WT isolates) or are potentially resistant (26, 27, 35).
Data analysis.
The data were analyzed as reported previously in various studies, by following the CLSI guidelines set forth for this purpose (26, 27, 35; CLSI documents on ECVs under development). Briefly, after the MIC distributions for each combination of an antifungal agent and a species or species complex from each laboratory were listed in an Excel spreadsheet, they were reviewed for skewed/abnormal distributions (e.g., the mode at the lowest concentration tested and/or bimodal [two modes in the same distribution]), which were not included in the statistical analysis. According to CLSI recommendations (CLSI documents on ECVs under development) and following the examination of global WT modal MIC variability, distributions for each antifungal agent and species or species complex were pooled with the qualifying data (abnormal distributions not included). ECVs were calculated for each pooled distribution by the previously reported iterative statistical technique that captured at least 95%, 97.5%, and 99% of the modeled WT population (not the observed MICs) (35). In addition, we evaluated the inherent variability (within approximately 1 doubling dilution) of susceptibility testing and the presence of outlier laboratories in each pooled distribution.
RESULTS AND DISCUSSION
Susceptibility testing should aid in predicting patient response to therapy, which is the specific role of the BP (36, 37). The CLSI has established species-specific BPs only for testing the susceptibilities of some Candida spp. to echinocandins, fluconazole, and voriconazole (38). The reason for this dearth of BPs is that their establishment requires particular steps: (i) WT MIC distributions and ECVs for each species and agent being evaluated, (ii) the pharmacokinetic and pharmacodynamic (PK/PD) parameters of the agent, (iii) knowledge of the relationship between mechanisms of resistance and MICs, and, most importantly, (iv) the correlation of MICs with clinical response to treatment with the specific agent in clinical trials (35–37). Data for these steps are not available for Fusarium spp. However, we have gathered MIC distributions for the F. oxysporum SC, the F. solani SC, and F. verticillioides (the three species or complexes most commonly associated with human disease) with three triazoles and amphotericin B. Although ECVs were not proposed for the other species evaluated due to insufficient data, their pooled MIC distributions are listed in Table 1; the CLSI criteria require a minimum of 100 MICs/species (MICs for 100 species-agent combinations) from at least three laboratories and ECVs calculated by the iterative statistical method (CLSI documents on ECVs under development). It is expected that the proposed ECVs would separate the two populations (WT and non-WT) that are present in the MIC distribution of a species-agent combination. Although they would not distinguish between susceptible (treatable) and resistant (nontreatable) isolates as BPs do, our proposed ECVs can help to identify those isolates that are more likely to harbor acquired molecular mutations conferring microbial resistance (non-WT isolates). This is important in the absence of BPs for Fusarium spp.
Table 1 depicts the pooled MIC distributions for the four agents and the Fusarium complexes/species evaluated. In general, the MIC distributions were typical for each antifungal agent and species, where 2 to 5 2-fold concentrations surround the modal MIC. The exceptions were itraconazole and some voriconazole distributions, which were skewed to the right. In addition, the distributions from the different laboratories were comparable, since their modal MICs for each combination of a species or species complex with an agent were within 1 2-fold dilution of one another, with three exceptions. The amphotericin B mode for F. oxysporum SC was 1 dilution higher in one of the contributing laboratories (4 μg/ml versus 1 to 2 μg/ml in the other laboratories), while the posaconazole and voriconazole modes were 1 dilution lower for F. oxysporum SC and F. verticillioides (1 μg/ml versus 2 to 4 μg/ml in the other laboratories) (data not shown in Tables 1 and 2). Most amphotericin B modes were 2 μg/ml; the exceptions were the lower modes for F. dimerum SC and F. fujikuroi and the higher mode for F. incarnatum-F. equiseti SC (Table 1). Among the triazoles, the highest values were observed when itraconazole was tested (modes, 8 to ≥16 μg/ml). Posaconazole and voriconazole modes ranged from 0.5 to 8 μg/ml and 2 to 8 μg/ml, respectively, with the lowest modes for F. verticillioides and the highest for both the F. solani SC and the F. dimerum SC. The MIC data (agent dependent) for 2 to 11 isolates of F. falciforme were similar to those of their F. solani SC with one exception: the eight posaconazole MICs for this species were >16 μg/ml. The same applied to the 4 to 8 isolates of the other three members of the F. fujikuroi SC (F. sacchari, F. subglutinans, and F. thapsinum), for which all itraconazole MICs were >16 μg/ml (data not shown in Table 1). Although some of the distributions for the less prevalent species are small, these results underline the need for identification to the species or complex level in addition to antifungal susceptibility testing.
While the in vitro activities of the four antifungal agents evaluated are similar to those reported previously for Fusarium isolates (both CLSI and EUCAST [European Committee on Antimicrobial Susceptibility Testing] MICs) (2, 4, 28–31), overall, our MIC ranges are wider (Tables 1 and 2). In addition to our aggregated itraconazole data, this was evident with amphotericin B MICs for both the F. proliferatum and F. oxysporum SCs and with voriconazole MICs for the F. solani SC. However, the number of isolates for each pooled distribution was higher than those tested in prior studies (2, 4, 28–31) (15 to 22 isolates for more-prevalent species) and perhaps better represented the range of susceptibilities to these agents. Nevertheless, the most frequent MICs (when provided) were similar to those in the present study. To our knowledge, pooled MIC data are not available for the less-prevalent species. Based on these data and the widespread geographical regions from which our pooled MIC data originated, we assume that our data are valid.
As mentioned above, the CLSI has set forth criteria for the calculation of species-specific ECVs based on unmodified CLSI methodologies for MIC determination (≥100 isolates originating in at least three independent laboratories per species-agent combination) and for the calculation of the ECV percentage (≥97.5% values) by the iterative statistical technique (CLSI documents on ECVs under development). Since ≥97.5% values risk classifying some isolates with acquired resistance mechanisms as WT, we have also provided the ≥95% and ≥99% ECVs. Either the values were the same or the ≥97.5 and ≥99% ECVs were separated by 1 dilution. Table 2 depicts the ECVs for the aggregated MIC distributions that met the CLSI criteria: amphotericin B, itraconazole, posaconazole, and voriconazole versus the F. oxysporum SC, the F. solani SC, and F. verticillioides. Insufficient data precluded the calculation of ECVs for the combination of itraconazole and F. verticillioides or any other species. The ECVs of amphotericin B were 4 μg/ml (F. verticillioides) and 8 μg/ml (F. oxysporum SC and F. solani SC); these values are actually above what is anecdotally considered the notional “breakpoint” for resistance among some Aspergillus spp. (2 μg/ml). Similarly high ECVs were observed among Aspergillus spp., Mucor circinelloides, and Rhizopus arrhizus (26, 39). As expected, the highest ECVs were those of the three triazoles for the F. solani SC (32 μg/ml). Lower posaconazole and voriconazole ECVs were calculated for F. verticillioides (2 and 4 μg/ml, respectively) and the F. oxysporum SC (8 and 16 μg/ml, respectively). These triazole ECVs are mostly higher than the expected maximal, variable, and dose-dependent trough levels of each of the agents (23, 24, 40) and highlight the intrinsically resistant nature of Fusarium spp. The same applies to amphotericin B values.
Although case series of Fusarium infections have been reported throughout the years (4, 7, 9, 16, 20–22), only in a recent report was an indication of a potential correlation between MICs for Fusarium spp. and response to treatment found (22), where CLSI MICs for seven Fusarium isolates identified by molecular methods, antifungal therapy (voriconazole or both voriconazole and amphotericin B), and clinical response were documented for patients with invasive fusariosis. Favorable clinical responses were reported for two of the seven patients infected with F. verticillioides (voriconazole MICs, 2 and 4 μg/ml, respectively); according to our voriconazole ECV for this species, both infecting strains would be considered WT isolates (Table 2). Of the four patients infected with F. solani, the correlation was evident for only one (a favorable clinical response and a voriconazole MIC of 4 μg/ml, or another WT strain). The remaining three patients failed therapy; two of them were treated with both voriconazole and amphotericin B (amphotericin B MICs, 4 μg/ml; voriconazole MICs, >8 μg/ml or >16 μg/ml). These amphotericin B MICs could be considered WT, and both voriconazole MICs would more likely be considered non-WT, although the final MIC endpoint was not given (voriconazole ECV for the F. solani SC, 32 μg/ml). However, it is important to keep in mind that categorization of an isolate as WT does not indicate that the isolate is susceptible (treatable), given that ECVs do not predict clinical response to therapy. Similarly, other factors preclude correlations of in vitro and clinical responses to therapy in other studies, where cultures, species, and especially MICs are not reported and the response was influenced by the site of infection, the underlying disease, and/or the reversal of immunosuppression. In addition, the molecular mechanisms of resistance have not been evaluated for any Fusarium isolate causing human disease, as they have been for Candida and Aspergillus. As found with the Mucorales, the molecular biology of Fusarium sp. resistance needs to be investigated.
In conclusion, species-specific amphotericin B ECVs (comprising ≥97.5% of the modeled populations) of 4 μg/ml (F. verticillioides) and 8 μg/ml (F. oxysporum SC and F. solani SC), posaconazole ECVs of 2 μg/ml (F. verticillioides), 8 μg/ml (F. oxysporum SC), and 32 μg/ml (F. solani SC), voriconazole ECVs of 4 μg/ml (F. verticillioides), 16 μg/ml (F. oxysporum SC), and 32 μg/ml (F. solani SC), and itraconazole ECVs of 32 μg/ml (F. oxysporum SC and F. solani SC) have been proposed based on CLSI data from multiple laboratories. ECVs were mostly 1 dilution lower when ≥95% of the modeled populations was used, which could be more clinically relevant. Like the ECVs for Candida spp. and Aspergillus spp., the proposed ECVs for the more-prevalent Fusarium spp. may aid in the detection of strains with acquired mechanisms of resistance (non-WT) to the agents evaluated. However, ECVs are not BPs and cannot predict clinical response to therapy, and categorization of an isolate as WT does not mean that it is necessarily treatable or susceptible. Also, as for the Mucorales, knowledge regarding molecular mechanisms of resistance and their relationship with MICs is needed.
ACKNOWLEDGMENTS
We thank M. Castanheira (JMI Laboratories, North Liberty, IA), V. R. de Azevedo Bastos (Universidade Federal de São Paulo, São Paulo, Brazil), and A. Fothergill (University of Texas Health Science Center, San Antonio, TX) for their collaboration.
We declare no conflicts of interest.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
REFERENCES
- 1.Guarro J. 2013. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur J Clin Microbiol Infect Dis 32:1491–1500. doi: 10.1007/s10096-013-1924-7. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell K, Sutton DA, Fothergill A, McCarthy D, Rinaldi MG, Brandt ME, Zhang N, Geiser DM. 2008. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J Clin Microbiol 46:2477–2490. doi: 10.1128/JCM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Donnell K, Sutton DA, Rinaldi MG, Sarver BA, Balajee SA, Schroers HJ, Summerbell RC, Robert VA, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee YH, Kang S, Park B, Geiser DM. 2010. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol 48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tortorano AM, Prigitano A, Esposto MC, Arsic Arsenijevic V, Kolarovic J, Ivanovic D, Paripovic L, Klingspor L, Nordøy I, Hamal P, Arikan Akdagli S, Ossi C, Grancini A, Cavanna C, Lo Cascio G, Scarparo C, Candoni A, Caira M, Drogari Apiranthitou M, ECMM Working Group . 2014. European Confederation of Medical Mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur J Clin Microbiol Infect Dis 33:1623–1630. doi: 10.1007/s10096-014-2111-1. [DOI] [PubMed] [Google Scholar]
- 5.McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, Proud'homme van Reine WF, Smith GF, Wiersema JH, Turland NJ. 2012. International code of nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress, Melbourne, Australia, July 2011. Koeltz Scientific Books, Koenigstein, Germany. [Google Scholar]
- 6.Lombard L, van der Merwe NA, Groenewald JZ, Crous PW. 2014. Generic concepts in Nectriaceae. Stud Mycol 80:189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhammed M, Anagnostou T, Desalermos A, Kourkoumpetis TK, Carneiro HA, Glavis-Bloom J, Coleman JJ, Mylonakis E. 2013. Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine 92:305–316. doi: 10.1097/MD.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nir-Paz R, Strahilevitz J, Shapiro M, Keller N, Goldschmied-Reouven A, Yarden O, Block C, Polacheck I. 2004. Clinical and epidemiological aspects of infections caused by Fusarium species: a collaborative study from Israel. J Clin Microbiol 42:3456–3461. doi: 10.1128/JCM.42.8.3456-3461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheel CM, Hurst SF, Barreiros G, Akiti T, Nucci M, Balajee SA. 2013. Molecular analyses of Fusarium isolates recovered from a cluster of invasive mold infections in a Brazilian hospital. BMC Infect Dis 13:49. doi: 10.1186/1471-2334-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Diepeningen AD, Al-Hatmi AMS, Brankovics B, de Hoog GS. 2014. Taxonomy and clinical spectra of Fusarium species: where do we stand in 2014? Curr Clin Microbiol Rep 1:10–18. doi: 10.1007/s40588-014-0003-x. [DOI] [Google Scholar]
- 11.Garnica M, daCunha OM, Portugal R, Maiolino A, Colombo AL, Nucci MM. 2015. Risk factors for invasive fusariosis in patients with acute myeloid leukemia and in hematopoietic cell transplant recipients. Clin Infect Dis 60:875–880. doi: 10.1093/cid/ciu947. [DOI] [PubMed] [Google Scholar]
- 12.Homa M, Shobana CS, Singh YR, Manikandan P, Selvam KP, Kredics L, Narendran V, Vagvolgyi C, Galgoczy L. 2013. Fusarium keratitis in South India: causative agents, their antifungal susceptibilities and a rapid identification method for the Fusarium solani species complex. Mycoses 56:501–511. doi: 10.1111/myc.12062. [DOI] [PubMed] [Google Scholar]
- 13.Chang DC, Grant GB, O'Donnell K, Wannemuehler KA, Noble-Wang J, Rao CY, Jacobson LM, Crowell CS, Sneed RS, Lewis FM, Schaffzin JK, Kainer MA, Genese CA, Alfonso EC, Jones DB, Srinivasan A, Fridkin SK, Park BJ. 2006. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 23:953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez JA, Miceli MH, Alangaden G. 2013. Invasive fungal infections in transplant recipients. Ther Adv Infect Dis 3:85–105. doi: 10.1177/2049936113491936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocchi S, Codeluppi M, Venturelli C, Bedini A, Grottola A, Gennari W, Cavrini F, Di Benedetto F, De Ruvo N, Rumpianesi F, Gerunda GE, Guaraldi G. 2011. Fusarium verticillioides fungemia in a liver transplantation patient: successful treatment with voriconazole. Diagn Microbiol Infect Dis 71:438–441. doi: 10.1016/j.diagmicrobio.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Lortholary O, Obenga G, Biswas P, Caillot D, Chachaty E, Bienvenu AL, Cornet M, Greene J, Herbrecht R, Lacroix C, Genouillet F, Raad I, Sitbon K, Troke P, French Mycoses Study Group . 2010. International retrospective analysis of 73 cases of invasive fusariosis. Antimicrob Agents Chemother 54:4446–4450. doi: 10.1128/AAC.00286-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perfect JR, Marr KA, Walsh TJ, Greenberg RN, DuPont B, de la Torre-Cisneros J, Just-Nübling G, Schlamm HT, Lutsar I, Espinel-Ingroff A, Johnson E. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis 36:1122–1131. doi: 10.1086/374557. [DOI] [PubMed] [Google Scholar]
- 18.Raad I, Hachem R, Herbrech R, Graybill JR, Hare R, Corcoran G, Kontoyannis DP. 2006. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis 42:1398–1403. doi: 10.1086/503425. [DOI] [PubMed] [Google Scholar]
- 19.Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, Munoz P, Johnson E, Meletiadis J, Pana ZD, Lackner M, Verweij P, Freiberger T, Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Guinea J, Guarro J, de Hoog S, Hope W, Kathuria S, Lortholary O, Meis JF, Ullmann AJ, Petrikkos G, Lass-Flörl C, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology . 2014. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect 20:27–46. doi: 10.1111/1469-0691.12465. [DOI] [PubMed] [Google Scholar]
- 20.Nucci M, Anaissie E. 2007. Fusarium infections in immunocompromised patients. Clin Microbiol Rev 20:695–704. doi: 10.1128/CMR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nucci M, Anaissie E, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, Mangini C, Simoes BP, Colombo AL, Vaz J, Levy CE, Costa S, Moreira VA, Oliveira JS, Paraguay N, Duboc G, Voltarelli JC, Maiolino A, Pasquini R, Souza CA. 2003. Outcome predictor of 84 patients with hematologic malignancies and Fusarium infection. Cancer 98:315–319. doi: 10.1002/cncr.11510. [DOI] [PubMed] [Google Scholar]
- 22.Stempel JM, Hammond SP, Sutton DA, Weiser LM, Marty FM. 2015. Invasive fusariosis in the voriconazole era: single-center 13-year experience. Open Forum Infect Dis 2:ofv099. doi: 10.1093/ofid/ofv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maertens J, Cornely OA, Ullmann AJ, Heinz WJ, Krishna G, Patino H, Caceres M, Kartsonis N, Waskin H, Robertson MN. 2014. Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease. Antimicrob Agents Chemother 58:3610–3617. doi: 10.1128/AAC.02686-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abuhelwa AY, Foster DJR, Mudge S, Hayes D, Upton RN. 2015. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and Sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother 59:5681–5696. doi: 10.1128/AAC.00973-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Espinel-Ingroff A, Chakrabarti A, Chowdhary A, Cordoba S, Dannaoui E, Dufresne P, Fothergill A, Ghannoum M, Gonzalez GM, Guarro J, Kidd S, Lass-Flörl C, Meis JF, Pelaez T, Tortorano AM, Turnidge J. 2015. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales. Antimicrob Agents Chemother 59:1745–1750. doi: 10.1128/AAC.04435-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espinel-Ingroff A, Alvarez-Fernandez M, Cantón E, Carver PL, Chen SC-A, Eschenauer G, Getsinger DL, Gonzalez GM, Govender NP, Grancini A, Hanson KE, Kidd SE, Klinker K, Kubin CJ, Kus JV, Lockhart SR, Meletiadis J, Morris AJ, Pelaez T, Quindós G, Rodriguez-Iglesias M, Sánchez-Reus F, Shoham S, Wengenack NL, Borrell Solé N, Echeverria J, Esperalba J, Gómez-García de la Pedrosa E, García García I, Linares MJ, Marco F, Merino P, Pemán J, Pérez del Molino L, Roselló Mayans E, Rubio Calvo C, Ruiz Pérez de Pipaon M, Yagüe G, Garcia-Effron G, Guinea J, Perlin DS, Sanguinetti M, Shields R, Turnidge J. 2015. Multicenter study of epidemiological cutoff values and detection of resistance in Candida spp. to anidulafungin, caspofungin, and micafungin using the Sensititre YeastOne colorimetric method. Antimicrob Agents Chemother 59:6725–6732. doi: 10.1128/AAC.01250-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alastruey-Izquierdo A, Cuenca-Estrella M, Monzon A, Mellado E, Rodrıguez-Tudela JL. 2008. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother 61:805–809. doi: 10.1093/jac/dkn022. [DOI] [PubMed] [Google Scholar]
- 29.Arikan S, Lozano-Chiu M, Paetznick V, Nangia S, Rex JH. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J Clin Microbiol 37:3946–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castanheira M, Duncanson FP, Diekema DJ, Guarro J, Jones RN, Pfaller MA. 2012. Activities of E1210 and comparator agents tested by CLSI and EUCAST broth microdilution methods against Fusarium and Scedosporium species identified using molecular methods. Antimicrob Agents Chemother 56:352–357. doi: 10.1128/AAC.05414-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espinel-Ingroff A, Johnson E, Hockey H, Troke P. 2008. Activities of voriconazole, itraconazole and amphotericin B in vitro against 590 moulds from 323 patients in the voriconazole phase III clinical studies. J Antimicrob Chemother 61:616–620. doi: 10.1093/jac/dkm518. [DOI] [PubMed] [Google Scholar]
- 32.Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Blackwell Publishing, Ames, IA. [Google Scholar]
- 33.Al-Hatmi AM, van Diepeningen AD, Curfs-Breuker I, de Hoog GS, Meis JF. 2015. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J Antimicrob Chemother 70:1068–1071. doi: 10.1093/jac/dku505. [DOI] [PubMed] [Google Scholar]
- 34.O'Donnell K, Sutton DA, Rinaldi MG, Gueidan C, Crous PW, Geiser DM. 2009. Novel multilocus sequence typing scheme reveals high genetic diversity of human-pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J Clin Microbiol 47:3851–3861. doi: 10.1128/JCM.01616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turnidge J, Kahmeter G, Kronvall G. 2006. Statistical characterization of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 36.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalhoff A, Ambrose PG, Mouton JW. 2009. A long journey from minimum inhibitory concentration testing to clinically predictive breakpoints: deterministic and probabilistic approaches in deriving breakpoints. Infection 37:296–305. doi: 10.1007/s15010-009-7108-9. [DOI] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; 4th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A, Fuller J, Ghannoum M, Johnson E, Pelaez T, Pfaller MA, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob Agents Chemother 55:5150–5154. doi: 10.1128/AAC.00686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 53:24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


