Abstract
Objective. To determine how often patients with musculoskeletal (MSK) complaints prescribed a non-steroidal anti-inflammatory drug (NSAID) subsequently consult their general practitioner (GP) with a non-serious adverse drug reaction (ADR). Design. Cohort study. Setting. A healthcare database containing the electronic GP medical records of over 1.5 million patients throughout the Netherlands. Patients. A total of 16 626 adult patients with MSK complaints prescribed an NSAID. Main outcome measures. The patients’ medical records were manually assessed for the duration of NSAID use for a maximum of two months, and consultations for complaints predefined as potential ADRs were identified. Subsequently, the likelihood of an association with the NSAID use was assessed and these potential ADRs were categorized as likely, possible, or unlikely ADRs. Results. In total, 961 patients (6%) consulted their GP with 1227 non-serious potential ADRs. In 174 patients (1%) at least one of these was categorized as a likely ADR, and in a further 408 patients (2.5%) at least one was categorized as a possible ADR. Dyspepsia was the most frequent likely ADR, followed by diarrhoea and dyspnoea (respectively 34%, 8%, and 8% of all likely ADRs). Conclusion. Of the patients with MSK complaints prescribed an NSAID, almost one in 30 patients re-consulted their GP with a complaint likely or possibly associated with the use of this drug. The burden of such consultations for non-serious ADRs should be taken into account by GPs when deciding whether treatment with an NSAID is appropriate.
Keywords: Anti-inflammatory agents, drug toxicity, general practice, musculoskeletal/connective tissue, non-steroidal, pharmacoepidemiology, primary health care, The Netherlands
General practitioners (GPs) frequently treat musculoskeletal complaints with non-steroidal anti-inflammatory drugs (NSAIDs).
In this study, it was found that one in 30 patients prescribed an NSAID by their GP for a musculoskeletal complaint subsequently consult their GP with a likely or possible ADR.
In addition to the risk of serious but rare ADRs, GPs should take this occurrence of non-serious ADRs into account when deciding whether treatment with an NSAID is appropriate.
Introduction
Musculoskeletal (MSK) complaints are the most commonly presented complaints in the primary care population [1]. In around one-quarter of consultations for these MSK complaints, the general practitioner (GP) prescribes a non-steroidal anti- inflammatory drug (NSAID) [2]. The use of these is known to be associated with the occurrence of adverse drug reactions (ADRs), ranging from mild complaints to serious complications, particularly of the gastrointestinal (GI), cardiovascular, and renal tract [3–8]. Over the past few decades, many studies have focused on the occurrence of serious ADRs due to NSAIDs, and on related hospitalizations and death [3–9]. However, less is known about the incidence of non-serious ADRs due to NSAIDs in the primary care population and in resulting health care utilization in the form of GP consultations. One previous study that did focus specifically on primary care patients found that 40% of chronic NSAID users suffering from gastrointestinal complaints consulted their GP for these complaints [10]. Whether other types of adverse events also lead to consultation and how frequently such consultations occur in short-term NSAID users is not known. In this cohort study, we aim to determine how often patients with an MSK complaint newly treated with an NSAID by their GP subsequently consult their GP because of an adverse drug reaction.
Materials and methods
Setting
This study was conducted in the Integrated Primary Care Information (IPCI) database. This Dutch primary health care database contains the electronic patient records of more than 1.5 million patients. In the Netherlands, all citizens are registered with one GP, who forms the first point of care for all medical complaints. The electronic medical records contain all journal entries written by the GPs, and coded and anonymous data on patient demographics, diagnoses using the International Classification for Primary Care (ICPC) [11], referrals, laboratory findings, and drug prescriptions. Further details of the database have been described elsewhere [12,13].
Patients
The study population comprised all patients ≥ 18 years newly prescribed an NSAID because of a MSK complaint between 1 January 2010 and 1 July 2010, with at least a 12-month valid database history prior to the date of study entry. Excluded were patients who had been prescribed an NSAID in the six months prior to study entry and patients without sufficient follow-up time in the IPCI database. Diagnoses of MSK complaints were identified based on ICPC coding and were considered new if the patient had not been diagnosed with the same MSK complaint in the six months prior to consultation. Only patients who received an NSAID prescription, identified by Anatomical Therapeutic Chemical (ATC) code [14], on the day of a consultation for the MSK complaint were included.
Manual assessment of electronic medical records
The electronic medical record of all included patients was manually assessed for the duration of continuous NSAID use for a maximum of two months. Continuous use was defined as use of NSAIDs with treatment gaps of no more than 10% of the previous prescription duration. This means that the medical records were assessed for the duration of the first NSAID prescription, as well as any follow-up prescriptions (if issued), provided the treatment gap between these prescriptions was no more than 10%, with an overall maximum of two months.
Consultations for adverse drug reactions
The aim of this manual assessment was to determine whether the patient consulted his/her GP with an ADR likely or possibly associated with the NSAID treatment. To achieve this, the following stepwise approach was used:
Step 1: Identification of potential ADRs. First, any consultations for complaints that may potentially be an ADR were identified. We predefined the complaints that should be considered a “potential ADR” based on listed common NSAID-related adverse drug reactions. All clinical complaints listed as common ADRs for at least two types of NSAID were included [15]. In addition to these, angio-oedema and signs of gastrointestinal bleeding were also included because, although rare, they are listed for all types of NSAIDs. As a result, the following 13 complaints were predefined as potential ADRs: skin reactions, dyspepsia, diarrhoea, constipation, peripheral oedema, dyspnoea (including wheezing), headache, dizziness, drowsiness, angio-oedema, hematemesis, black stool, and rectal bleeding. If a patient consulted his/her GP with a complaint that was not predefined as a potential ADR but which the patient attributed to the use of NSAIDs, this was also recorded. If the GP recorded the occurrence of an ADR, without specifying the type of complaint presented, these unspecified ADRs were also recorded.
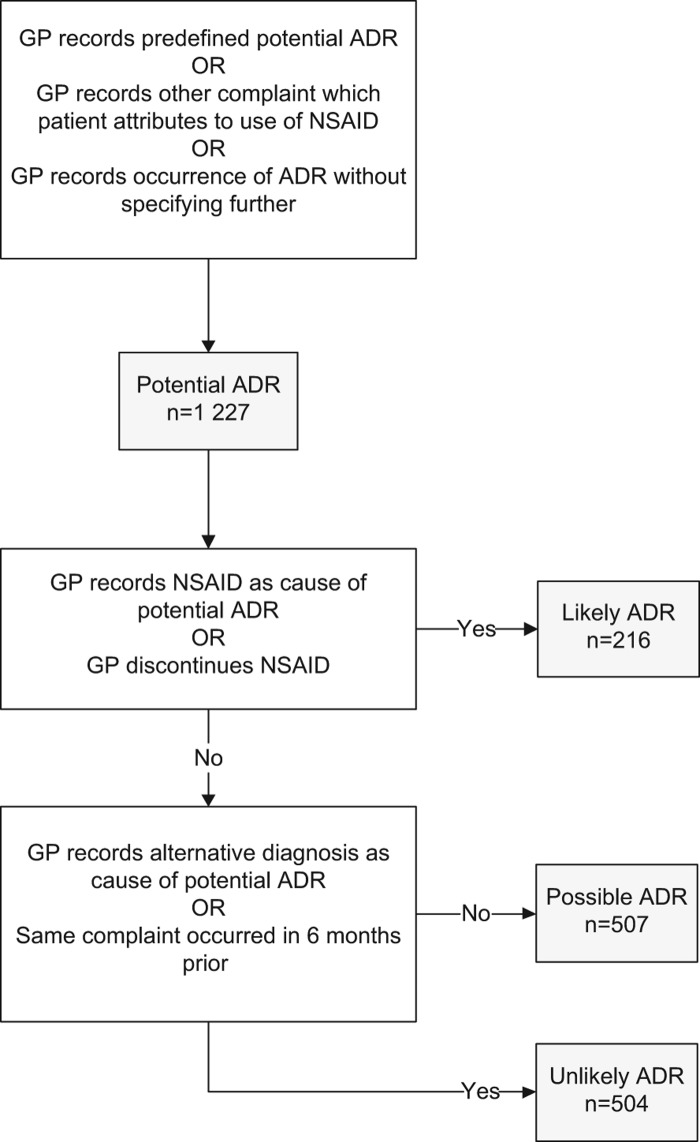
Step 2: Categorization of potential ADRs as likely, possibly or unlikely to be associated with the NSAID use. We then estimated the likelihood that a potential ADR which led to GP consultation was indeed associated with the use of the NSAID. In order to assess this, we read the GP's journal entry and recorded whether: (1) the GP explicitly recorded the NSAID as the cause of the potential ADR; (2) the GP explicitly discontinued the NSAID; (3) the GP recorded an alternative diagnosis as the cause of the potential ADR; and (4) the patient had consulted his/her GP for the same complaint as the potential ADR in the six months prior to study entry. Based on these four criteria, potential ADRs were subsequently categorized as likely, possible, or unlikely ADR according to the algorithm shown in Figure 1.
Figure 1.
Assessment algorithm. Notes: GP = general practitioner; NSAID = non-steroidal anti-inflammatory drug; ADR = adverse drug reaction. *Skin reaction, angio-oedema, dyspepsia, diarrhoea, constipation, peripheral oedema, dyspnoea, chest pain, headache, dizziness, tinnitus, drowsiness, hematemesis, black stool, or rectal bleeding.
Predictors of consultation for a likely ADR
To determine whether any predictive factors for consultation with a likely ADE could be identified, we compared patients who consulted their GP because of a likely ADR with those without a consultation. In addition to age and gender, concomitant prescription of a gastroprotective agent (GPA) was determined. This was defined as concomitant use of a proton pump inhibitor (PPI), double-dosed histamine-2 receptor antagonist (H2RA), or misoprostol, on the day of first NSAID prescription. In addition, the NSAID prescribed was classified as a non-selective NSAID (nsNSAID) or a selective cox-2 inhibitor (coxib) based on ATC coding.
Statistical analyses
The incidence rate of consultations for potential ADRs was calculated by dividing the number of consultations for potential ADRs by the total number of person-days of NSAID use in the entire cohort. This was then multiplied by 1000 to present the number of consultations for potential ADRs per 1000 person-days of NSAID use. The same method was used to determine the incidence rate of consultations for likely and possible ADRs. Univariate analyses of potential predictors of a likely ADR such as age, gender, and type of NSAID were conducted and unadjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated by performing logistic regression analyses. In order to determine ORs adjusted for age and gender, multivariate analyses were also performed for the predictor type of NSAID and concomitant prescription of GPA. All analyses were performed using SPSS™ version 20 (IBM, Armonk, NY, USA).
Results
Patients
In total, 16 626 adults were newly prescribed an NSAID for a MSK complaint and were included in this study (Table I). Symptomatic diagnoses of the back or neck were the most common indication for NSAID treatment. The median duration of continuous NSAID treatment was 11 days, with an interquartile range of seven days. The most commonly prescribed NSAIDs were diclofenac (65%), ibuprofen (11%), and naproxen (8%). Coxibs were prescribed in 4% of patients. In total, 36% of patients were prescribed a concomitant GPA or were already using a GPA on the date of NSAID prescription.
Table I.
Baseline characteristics of the study population.
| No. of patients n = 16 626 |
|
|---|---|
| Age in years, mean (± SD) | 50.9 (± 15.7) |
| Age category, n (%): | |
| 18–35 36–50 51–65 > 65 |
2 918 (17.6) 5 670 (34.1) 5 108 (30.7) 2 930 (17.6) |
| Female, n (%) | 8 950 (53.8) |
| Musculoskeletal complaint diagnosed, n (%): | |
| Symptomatic diagnosis | 11 397 (68.5) |
| Back or neck Upper extremity Lower extremity Generalized/other |
4 155 (25.0) 3 540 (21.3) 2 107 (12.7) 1 595 (9.6) |
| Arthritis | 1 283 (7.7) |
| Inflammatory arthritis Osteoarthritis Gout |
201 (1.2) 442 (2.7) 640 (3.8) |
| Radiculopathy Trauma Other |
1 165 (7.0) 765 (4.6) 2 016 (12.1) |
| Type of NSAID prescribed, n (%): | |
| Non-selective NSAID | 16 041 (96.5) |
| Diclofenac Ibuprofen Naproxen Other |
10 799 (65.0) 1 800 (10.8) 1 382 (8.3) 2 060 (12.4) |
| Coxib | 585 (3.5) |
| Celecoxib Etoricoxib |
170 (1.0) 415 (2.5) |
| Duration of NSAID prescription in days, median (IQR) | 11.0 (7.0) |
| Concomitant GPA prescribed, n (%) | 6 032 (36.3) |
Notes: NSAID = non-steroidal anti-inflammatory drug; coxib = selective cox-2 inhibitor;
IQR = interquartile range; GPA = gastroprotective agent.
Consultation for a potential adverse drug reaction and likelihood of an association with NSAID use
In total, 961 patients (6%) consulted the GP for at least one potential ADR (Table II). As 224 patients consulted their GP for more than one potential ADR, a total of 1227 potential ADRs were reported by these 961 patients. The median duration between the start of the NSAID and GP consultation for a potential ADR was seven days, with an interquartile range of eight days. The incidence rate was four consultations for a potential ADR per 1000 person-days of NSAID prescription.
Table II.
Consultation for a potential ADR.
| No. of patients n = 16 626 |
|
|---|---|
| At least one consultation for a potential ADR, n (%) | 961 (5.8) |
| Duration since start NSAID in days, median (IQR) | 7.0 (8.0) |
| Number of potential ADR reported per patient, n (%): | |
| 1 2 3 4 5 |
743 (4.5) 184 (1.1) 34 (0.2) 4 (0.02) 2 (0.01) |
Notes: ADR = adverse drug reaction; NSAID = non-steroidal anti-inflammatory drug; IQR = interquartile range.
Table III shows the type of potential ADRs presented in more detail. The most frequently presented potential ADRs were dyspepsia (32%), dyspnoea (13%) and skin reactions (12%). As previously described and shown in Figure 1, we then assessed the likelihood that these potential ADRs were associated with the use of the NSAID:
Table III.
Types of potential ADRs presented to GP and likelihood of an association with NSAID use.
| Potential ADR | Assessment criteria† | Likelihood of an association with NSAIDs‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Type | Number n = 1 227 n (%) |
GP records NSAID as cause n = 146 n (%) |
GP discontinues NSAID n = 121 n (%) |
GP records alternate diagnosis n = 452 n (%) |
Same complaint in 6 months prior n = 248 n (%) |
Likely ADR n = 216 n (%) |
Possible ADR n = 507 n (%) |
Unlikely ADR n = 504 n (%) |
| Not specified | 39 (3.2) | 39 (26.7) | 6 (5.0) | 0 (0) | 0 (0) | 39 (18.1) | 0 (0) | 0 (0) |
| Not predefined | 38 (3.1) | 5 (3.4) | 7 (5.8) | 7 (1.5) | 4 (1.6) | 9 (4.2) | 21 (4.1) | 8 (1.6) |
| Skin reaction | 142 (11.6) | 10 (6.8) | 6 (5.0) | 100 (22.1) | 31 (12.5) | 13 (6.0) | 26 (5.1) | 103 (20.4) |
| Angio-oedema | 28 (2.3) | 8 (5.5) | 7 (5.8) | 12 (2.7) | 6 (2.4) | 10 (4.6) | 5 (1.0) | 13 (2.6) |
| Dyspepsia | 398 (32.4) | 39 (26.7) | 51 (42.1) | 83 (18.4) | 78 (31.5) | 73 (33.8) | 208 (41.0) | 117 (23.2) |
| Diarrhoea | 104 (8.5) | 13 (8.9) | 10 (8.3) | 22 (4.9) | 14 (5.6) | 17 (7.9) | 60 (11.8) | 27 (5.4) |
| Constipation | 53 (4.3) | 1 (0.7) | 0 (0) | 12 (2.7) | 13 (5.2) | 1 (0.5) | 32 (6.3) | 20 (4.0) |
| GI blood loss* | 37 (3.0) | 9 (6.2) | 8 (6.6) | 13 (2.9) | 4 (1.6) | 14 (6.5) | 10 (2.0) | 13 (2.6) |
| Oedema | 57 (4.6) | 5 (3.4) | 8 (6.6) | 16 (3.5) | 15 (6.0) | 10 (4.6) | 26 (5.1) | 21 (4.2) |
| Dyspnoea | 153 (12.5) | 12 (8.2) | 6 (5.0) | 90 (19.9) | 49 (19.8) | 17 (7.9) | 32 (6.3) | 106 (21.0) |
| Headache | 57 (4.6) | 1 (0.7) | 2 (1.7) | 34 (7.5) | 14 (5.6) | 3 (1.4) | 19 (3.7) | 35 (6.9) |
| Dizziness | 82 (6.7) | 2 (1.4) | 7 (5.8) | 29 (6.4) | 20 (8.1) | 9 (4.2) | 36 (7.1) | 37 (7.3) |
| Drowsiness | 39 (3.2) | 2 (1.4) | 3 (2.5) | 5 (1.1) | 0 (0) | 3 (1.4) | 32 (6.3) | 4 (0.8) |
Notes: ADR = adverse drug reaction; GP = general practitioner; NSAID = non-steroidal anti-inflammatory drug; GI = gastrointestinal. *Hematemesis, melena or rectal bleeding. †Criteria can overlap. ‡According to assessment algorithm.\
Likely ADRs: The GP recorded the NSAID use as the cause of the adverse events in 146 cases, and discontinued the NSAID in 121 cases. Some overlap was present between these two criteria, leading to 216 potential ADRs (18%) being categorized as likely. This corresponds with an incidence rate of one potential ADR presented per 1000 person-days of NSAID prescription.
Unlikely ADRs: In 452 cases, the GP recorded an alternative diagnosis as the cause of the potential ADR and in 248 cases the patient had presented the same complaint as the potential ADR in the six months prior, resulting in 504 potential ADRs being categorized as unlikely ADRs (again, overlap between criteria was present).
Possible ADRs: The remaining 507 adverse events presented (41%) were categorized as possible ADRs.
The incidence rate of likely and possible ADRs combined was three per 1000 person-days of NSAID prescription.
Predictors of consultation for a likely ADR
In total, 174 patients, or 1% of the total cohort, consulted the GP for at least one likely ADR (Table IV). Of those patients in whom no likely ADR occurred, 408 patients (2.5%) presented at least one possible ADR. When compared with patients who did not re-consult the GP for an adverse event, GP consultation for a likely ADR was more frequent in elderly patients, in women, and in those prescribed a coxib or concomitant GPA.
Table IV.
Predictors of consultation for a likely ADR.
| Consultation | ||||||
|---|---|---|---|---|---|---|
| At least one likely ADR n = 174 n (%) |
At least one possible ADR* n = 408 n (%) |
At least one unlikely ADR** n = 379 n (%) |
No consultation n = 15 665 n (%) |
OR (95% CI) Likely ADR vs. no consultation |
Adjusted OR†
(95% CI) Likely ADR vs. no consultation |
|
| Age category: | ||||||
| 18–35 36–50 51–65 > 65 |
37 (21.3) 49 (28.2) 36 (20.7) 52 (29.9) |
53 (13.0) 137 (33.6) 108 (26.5) 110 (27.0) |
54 (14.2) 110 (29.0) 110 (29.0) 105 (27.7) |
2 774 (17.7) 5 374 (34.3) 4 854 (31.0) 2 663 (17.0) |
1 (ref.) 0.7 (0.4–1.1) 0.6 (0.4–0.9) 1.5 (1.0–2.2) |
– – – – |
| Gender: | ||||||
| Male Female |
59 (33.9) 115 (66.1) |
131 (32.1) 277 (67.9) |
137 (36.1) 242 (63.9) |
7 349 (46.9) 8 316 (53.1) |
1 (ref.) 1.7 (1.3–2.4) |
– – |
| Type of NSAID: | ||||||
| Non-selective NSAID | 162 (93.1) | 392 (96.1) | 355 (93.7) | 15 132 (96.6) | 1 (ref.) | 1 (ref.) |
| Coxib | 12 (6.9) | 16 (3.9) | 24 (6.3) | 533 (3.4) | 2.1 (1.2–3.8) | 1.9 (1.1–3.5) |
| Concomitant GPA prescribed: | ||||||
| None PPI H2RA in double dosage Misoprostol |
82 (47.1) 67 (38.5) 0 (0.0) 25 (14.4) |
227 (55.6) 142 (34.8) 0 (0.0) 39 (9.6) |
214 (56.5) 135 (35.6) 0 (0.0) 30 (7.9) |
10 071 (64.3) 4 231 (27.0) 3 (0.0) 1 350 (8.7) |
1 (ref.) 1.9 (1.4–2.7) – 2.3 (1.4–3.5) |
1 (ref.) 1.8 (1.3–2.6) – 2.2 (1.4–3.5) |
Notes: ADR = adverse drug reaction; NSAID = non-steroidal anti-inflammatory drug; coxib = selective cox-2 inhibitor;
GPA = gastroprotective agent; PPI = proton pump inhibitor; H2RA = histamine-2 receptor antagonist. *And no likely ADR. **And no likely or possible ADR. †Adjusted for age and gender.
Discussion
Statement of principal findings
In this study, we aimed to provide an insight into the incidence of GP consultation for non-serious ADRs, among primary care patients prescribed an NSAID by their GP because of MSK complaints. We found that almost one in 30 patients treated with NSAIDs for a median duration of 11 days consulted their GP with a complaint that was likely or possibly an ADR. The incidence rate of consultations for such likely and possible ADRs combined was three per 1000 person-days of NSAID prescription. Elderly and female patients were more likely to consult their GP because of a likely ADR, which may reflect the fact that such patients are generally more likely to consult their GP [1]. Patients prescribed coxibs or an nsNSAID with concomitant GPA were also most likely to present a likely ADR, which is probably due to confounding by indication. These results do indicate, however, that such gastroprotective strategies do not fully protect against the occurrence of non-serious ADRs in patients prescribed NSAIDs.
Strengths and weaknesses of the study
When interpreting the results, there are some limitations to be taken into account. First, as the data were obtained from the medical journals, some information relevant to this study, such as verbal advice given to the patient regarding a complaint, may have been missed. This would result in an underestimation of the occurrence of adverse drug reactions reported to the GP. In addition, we were unable to apply existing causality assessment methods, such as the algorithms by Kramer or Naranjo, or the WHO-UMC causality assessment system [16–18]. Our assessment of the likelihood that an ADR was associated with the NSAID use was based on the opinion of the treating GP as documented in the medical journal, or, if no information was recorded, on the GP's active discontinuation of the NSAID as a proxy. Again, the true number of ADRs is likely to be higher, as GPs may fail to take the use of the NSAID into account when assessing the cause of a complaint, or refrain from recording their considerations. Second, we had no information on the use of over-the-counter NSAIDs. Included patients were prescribed an NSAID by their GP, those patients not prescribed an NSAID but using NSAIDs over-the-counter were therefore not included in this study.
Strengths and weaknesses in relation to other studies
One previous study focusing on gastrointestinal complaints in primary care patients found that, of 1014 included chronic NSAID users, 185 had consulted their GP because of a gastrointestinal complaint in the past year (incidence rate 0.5 per 1000 person-days) [10]. As this study included only chronic NSAID users who had used NSAIDs for at least nine months, patients particularly prone to gastrointestinal symptoms while taking NSAIDs would have been “selected out” and not formed part of the study population. The strength of our study is that we included and followed up all new NSAID users, which may explain the higher consultation rate for gastrointestinal and other adverse events found. Another study performed in Italy included 1842 patients who were treated by their GP with ibuprofen [19]. During the follow-up of 30 days, ADRs were found to occur in 14% of patients (incidence rate 4.7 per 1000 person-days). Although these results are of interest, the generalizability to the total primary care population remains unclear, as the methods for recruiting patients and the inclusion criteria were not reported.
Meaning of the study
The majority of patients in our study were treated with NSAIDs for symptomatic complaints of the musculoskeletal system, which are often self-limiting in nature. Indeed, the median duration of NSAID prescription in these patients was only 11 days and almost 60% of patients consulted their GP only once for their musculoskeletal complaint. In light of this, we feel that GPs should address not only the risk of serious ADRs when discussing treatment options for MSK complaints with their patients, but also our finding of re-consultation for non-serious ADRs. Although these non-serious ADRs are less harmful to the patient, they lead to an increase in primary health care utilization and may outweigh the benefits of NSAID treatment for many patients.
Conclusion
Of the patients with MSK complaints prescribed an NSAID, almost one in 30 patients consulted their GP with a complaint likely or possibly associated with the use of this drug. The burden of such non-serious ADRs should be taken into account by GPs when deciding whether treatment with an NSAID is appropriate.
Funding
The study was supported by a grant from the Dutch Arthritis Foundation. The funding body had no involvement in the design of the study, data collection, management, analysis, interpretation of data, drafting of the report, or decision to submit the report for publication.
Competing interests
VV conducted research for AstraZeneca in the past as employee of the Erasmus MC University Medical Center; MS coordinates a research group that occasionally performs research for pharmaceutical industries; none of the grants were related to the submitted work. The other authors declare no other relationships or activities that could appear to have influenced the submitted work.
Declaration of interest
There are no conflicts of interest in connection with the paper. The authors alone are responsible for the content and writing of the paper.
References
- Van der Linden MW, Westert GP, De Bakker DH, Schellevis FG Second Dutch national survey of general practice: Morbidity in the population and in general practice. Utrecht/Bilthoven: NIVEL/RIVM; 2004. [Google Scholar]
- Cardol M, Van Dijk L, De Jong JD, De Bakker DH, Westert GP. Second Dutch national survey of general practice. GP care: What does the gatekeeper do? Utrecht/Bilthoven: NIVEL/RIVM; 2004. [Google Scholar]
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of non-steroidal anti-inflammatory drugs: A meta-analysis. Ann Intern Med 1991;115:787–96. [DOI] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Rodriguez LA. Association between non-steroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: An overview of epidemiologic studies published in the 1990s. Arch Intern Med 2000; 160:2093–99. [DOI] [PubMed] [Google Scholar]
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and non-selective inhibitors of cyclooxygenase 2. JAMA 2006;296:1633–44. [DOI] [PubMed] [Google Scholar]
- Varas-Lorenzo C, Riera-Guardia N, Calingaert B, Castellsague J, Pariente A, Scotti L, et al. Stroke risk and NSAIDs: A systematic review of observational studies. Pharmacoepidemiol Drug Saf 2011;20:1225–36. [DOI] [PubMed] [Google Scholar]
- Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis 2005;45:531–9. [DOI] [PubMed] [Google Scholar]
- Warlé-Van Herwaarden MF, Kramers C, Sturkenboom MC, Van den Bemt PMLA, De Smet PAGM. Targeting outpatient drug safety: Recommendations of the Dutch Harm-Wrestling Task Force. Drug Saf 2012;35:245–59. [DOI] [PubMed] [Google Scholar]
- Jones RH, Tait CL. Gastrointestinal side-effects of NSAIDs in the community. BJCP 1995;49:67–70. [PubMed] [Google Scholar]
- Lamberts H WM, Hofmans-Okkens IM. International primary care classifications: The effect of fifteen years of evolution. Fam Pract 1992;9:330–9. [DOI] [PubMed] [Google Scholar]
- Van der Lei J, Duisterhout JS, Westerhof HP, van der Does E, Cromme PV, Boon WM. The introduction of computer-based patient records in The Netherlands. Ann Intern Med 1993;119:1036–41. [DOI] [PubMed] [Google Scholar]
- Vlug AE, van der Lei J, Mosseveld BM, van Wijk MA, van der Linden PD, Sturkenboom MC, et al. Postmarketing surveillance based on electronic patient records: The IPCI project. Methods Inf Med 1999;38:339–44. [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology Guidelines for ATC Classification and DDD Assignment. Available at: http://www.whocc.no/ (accessed 1 May 2014).
- Dutch College for Health Insurances Pharmacotherapeutic compass. 2013. Available at: http://www.farmacotherapeutischkompas.nl/ (accessed 1 May 2014).
- Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions, I: Background, description, and instructions for use. JAMA 1979:242;623–32. [PubMed] [Google Scholar]
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981:30; 239–45. [DOI] [PubMed] [Google Scholar]
- World Health Organisation – Uppsala Monitoring Centre The use of the WHO-UMC system for standardised case causality assessment. Available at: http://who-umc.org/Graphics/24734.pdf (accessed 1 May 2014).
- Benvenuti C, Beretta A, Longoni A, Pickvance N. A multi-centre general practice study evaluating the efficacy and tolerance of ibuprofen in common painful conditions. Pharmatherapeutica 1984:4:9–12. [PubMed] [Google Scholar]