Abstract
Objective: To identify risk factors for becoming an excessive user over time.
Setting: Prescription database study over five years.
Subjects and method: Norwegians between 30 and 60 years with a first dispensation of a benzodiazepine during 2006, encompassing 23 227 individuals. A Cox hazard regression model was defined, initially stratifying on gender, age, county, previous relevant drug dispensations, household income, education level, and vocational rehabilitation support.
Main outcome measure: The time from the first redemption until excessive use was defined as using more than two DDDs per day on average within a three-month period.
Results: Women’s risk was lower than men’s for excessive use (HR = 0.42, CI 0.35–0.51). Initial oxazepam, alprazolam, or nitrazepam/flunitrazepam use indicated higher risk compared with diazepam (HR = 1.51, CI 1.24–1.85, HR = 2.75, CI 1.54–4.91, HR = 1.67, CI 1.29–2.16). Previous antidepressants or lithium, antipsychotics or opioids, anti-alcohol and smoke cessation treatment indicated a higher risk compared with no such use (HR = 1.4, CI 1.16–1.69, HR = 1.92, CI 1.54–2.4, and HR = 2.88, CI 2–4.15). Higher education and average or high household income were associated with a low risk compared with low education and income (HR = 0.68, CI 0.57–0.81, HR = 0.58, CI 0.46–0.73, and HR = 0.37, CI 0.26–0.54). Working in the private or public sector was associated with a low risk compared with no registered work (HR = 0.53, CI 0.4–0.71 and HR = 0.57, CI 0.45–0.74).
Conclusion: The prevalence of excessive use over a five-year observation period was 2.34%. Risk factors were indications of psychiatric illness, first benzodiazepine choice, low income, and education. Excessive users were also characterized by a more severe disease, indicated by having prescription fulfilments by a psychiatrist and by switching benzodiazepines.
Key points
Guidelines state that benzodiazepines should be used for a short time and excessive use indicates drug dependency.
Of all new benzodiazepine users 2.34% became excessive users, defined as consuming above two defined daily doses (DDDs) per day on average over three months, within a five-year period.
Previous use of other psychotropic drugs, opioids and anti-alcohol and smoke cessation drugs, first benzodiazepine prescribed, low household income, and low education were risk factors for excessive use.
Excessive users were characterized by switching benzodiazepines and having prescription fulfilments by a psychiatrist suggesting a more severe disease.
Keywords: Benzodiazepines, Cox regression, excessive use, general practice, Norway
Introduction
Benzodiazepines (BZDs) act on the central nervous system by reinforcing the effect of the GABAA receptor, and reduce anxiety levels and produce sedation and muscle relaxation. Users can develop tolerance and dependence after only short-term use [1]. Guidelines [2,3] state that these drugs should be used for only a short time, and long-term use has no documented effect [4,5]. Chronic use is an indication of drug dependency and is associated with adverse effects such as drowsiness, slowed reaction time, mood swings, violent and impulsive behaviour, depression, altered perception and nausea [6–9]. However, despite these well-known side effects, guidelines are often not followed. We found it important to identify excessive redeeming patterns developing over time. What distinguishes excessive users from other users? Such knowledge could aid doctors in more rational prescribing. With data from the Norwegian prescription database (NorPD)[10] and Statistics Norway (SSB) [11] we followed previously naive users of BZDs for five years on their path to become excessive users. We defined users redeeming more than two defined daily doses (DDDs) – [12] per day on average – over a three-month period to be excessive users. We sought to identify risk factors for becoming excessive users over time. Possible risk factors considered were: gender, age, county, previous dispensations for other drugs as indicators for comorbidity, the first BZD dispensed, household income, education level, and vocational rehabilitation support. To our knowledge few, if any, population-based observational studies of chronic BZD use with a focus on dose escalation over many years have been published. In a previous paper [13] we followed new users for three years, and found an increasing proportion of excessive users over a three-year period. Whether the increase continued after the three years or not was unknown. This present paper will examine new users’ BZD redeeming pattern over five years to identify risk factors for becoming excessive users.
Material and methods
Design and main outcome
This study is an observational prescription registry study. We extracted data on BZD prescription fulfilments from the NorPD and socioeconomic data were obtained from SSB. The main analysis outcome was to identify risk factors for new BZD users to become excessive users over time, where excessive use was defined as redeeming more than two DDDs per day on average within a three-month period.
Drug users
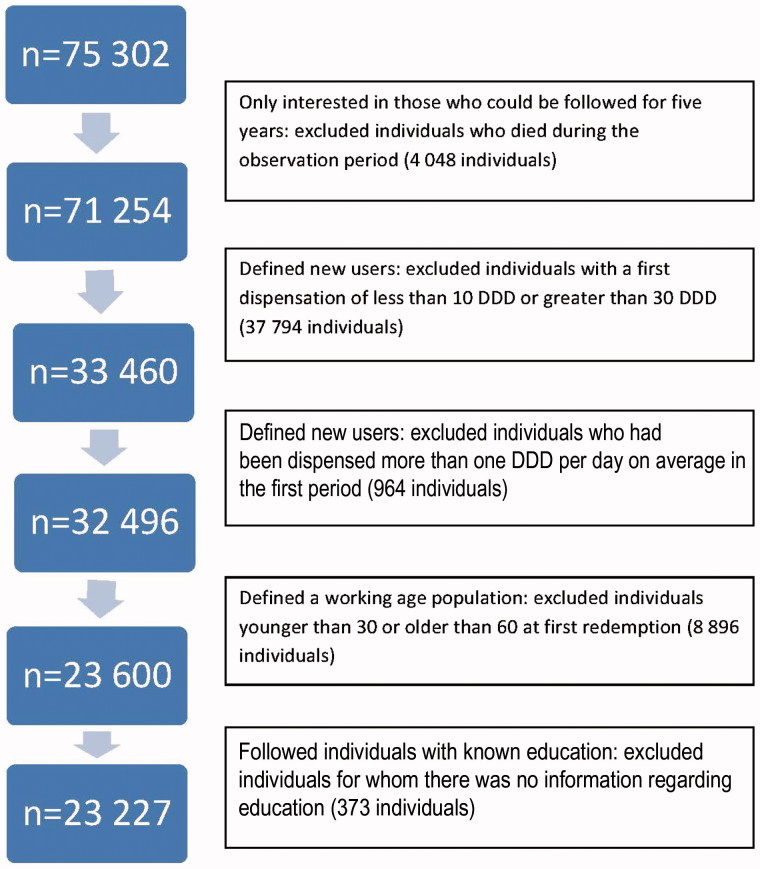
We obtained information on all inhabitants in Norway between 30 and 60 years of age who had a first dispensation of a BZD during 2006. To make the assumption of a new BZD population reasonable we further required that their first dispensation was between 10 and 30 DDDs and that their average DDD per day dispensed in the first three months was less than one DDD. Information from NorPD concerning these prescription fulfilments comprised gender, age, county, and prescribers’ specialty. We excluded individuals who died during the observation period. We included individuals whose education level was registered in SSB. The data-selection procedure is displayed in the flow chart in Figure 1. We followed all individuals for five years from their first dispensation. This encompassed 23 227 new users.
Figure 1.
Flowchart of the data selection procedure.
The five-year follow up time for each individual was divided into three-month periods. For each three-month period everybody was in one of four dispensation levels: 0 = no prescription fulfilments, 1 = less than one DDD per day on average dispensed, 2 = between one and two DDDs per day on average dispensed, and 3 = more than two DDDs per day on average dispensed. The main outcome was the time it took for users to reach level three for the first time. We defined those who reached level three as excessive users.
Data collection
Data from NorPD consisted of prescription fulfilments for the five groups of drugs: (1) diazepam (N05BA01), (2) oxazepam (N05BA04), (3) alprazolam(N05BA12), (4) nitrazepam and flunitrazepam (N05CD02 and N05CD03), and (5) hydroxyzine and buspirone (N05BB01 and N05BE01). The latter group consisted of non-BZD anxiolytics, commonly used as alternatives to BZD. The recommended DDD values for diazepam, oxazepam, alprazolam, nitrazepam/flunitrazepam, and hydroxyzine/buspirone were 10, 50, 1, 5/1, and 75/30 milligrams per day, respectively. All these drugs are hereafter collectively referred to as BZDs. The NorPD was established in 2004 so any prescription fulfilment prior to 2004 was unknown.
We also had information from 2004 regarding BZD users’ other dispensations. This information was used as indicators for comorbidity: (1) opioids, anti-alcohol and smoke cessation treatment as indication of tendency to dependency, (2) antidepressants and (3) antipsychotics as indications of psychiatric disease, (4) drugs for chronic obstructive pulmonary disease (COPD), (5) drugs for cardiac diseases, and (6) drugs for rheumatic diseases. We gathered information on the patient’s: (1) household income, (2) education level, (3) type of work (if any), and (4) whether they had received vocational rehabilitation support (all in 2004). Vocational rehabilitation support could be given to individuals who for medical reasons have difficulties in re-entering the labour market. With information on the prescriber’s specialty we considered prescriptions written by a psychiatrist throughout the observation period as a marker for mental illness severity.
Education was categorized as (1) low (no or primary school) or (2) high (senior high school, university, or college). Household income was categorized as (1) low (from 0 to 3G (176 364 Norwegian Kroner (NKr))), (2) medium (from 3G to 6G (352 728 NKr)) and (3) high (from 6G (352 728 NKr)). Within the public social security system in Norway G is the National Insurance basic amount, forming the basis for estimating social benefits and pension schemes [14], and is hence a key measure. An income below 3G is low and an income above 6G is regarded as high. Work type was categorized as (1) private sector (farming/industry/commodity trade), (2) public sector, or (3) no registered work. The last category encompasses a variety of individuals: unemployed, work at home, sick, disabled, etc. Students would also be part of this latter group, but since we focused on 30- to 60-year-old users there were probably few students in the study.
Table 1 shows how many (percentage) became excessive users (reached level three) and how many who did not, stratified on the various background variables.
Table 1.
Baseline characteristics for those (in percentages) who became excessive users and not.
| Not excessive user |
Excessive user |
||||
| Variable | Description | n | % | n | % |
| Gender | Men | 8762 | 0.386 | 319 | 0.586 |
| Women | 13921 | 0.614 | 225 | 0.414 | |
| Age | 47 years1 | 43 years1 | |||
| First BZD | diazepam | 15626 | 0.689 | 301 | 0.553 |
| oxazepam | 3678 | 0.162 | 142 | 0.261 | |
| alprazolam | 175 | 0.008 | 12 | 0.022 | |
| hydroxyzine, buspirone | 996 | 0.044 | 17 | 0.031 | |
| nitrazepam, flunitrazepam | 2208 | 0.097 | 72 | 0.132 | |
| Previous drugs used | Drugs for cardiac diseases | 4898 | 0.216 | 97 | 0.178 |
| Antidepressants and lithium | 4609 | 0.203 | 185 | 0.34 | |
| Drugs for COPD | 2348 | 0.104 | 163 | 0.116 | |
| Antipsychotics | 1321 | 0.058 | 108 | 0.199 | |
| Drugs for rheumatic diseases | 1231 | 0.054 | 35 | 0.064 | |
| Opioids, anti-alcohol and smoke cessation drugs | 151 | 0.007 | 33 | 0.061 | |
| Vocational rehabilitation support | 2147 | 0.095 | 99 | 0.182 | |
| Education | No or low | 6994 | 0.308 | 278 | 0.511 |
| Higher | 15689 | 0.692 | 266 | 0.489 | |
| Household income | No or low | 8443 | 0.372 | 354 | 0.651 |
| Average | 9434 | 0.416 | 144 | 0.265 | |
| High | 4806 | 0.212 | 46 | 0.085 | |
| Type of work | Private sector | 8428 | 0.372 | 130 | 0.239 |
| Public sector | 7488 | 0.330 | 79 | 0.145 | |
| Not given | 6767 | 0.298 | 335 | 0.616 |
1Mean.
Statistical methods
The statistical analysis was conducted in the open source statistical software R [15]. We first drew Kaplan–Meier plots to explore the background variables’ impact on the time it took to become excessive users. We defined a Cox proportional hazard regression model, initially taking into consideration gender, age (scaled by subtracting the average age), county, previous relevant drug dispensations, household income, education level, and vocational rehabilitation support. We first specified a “full” model, a model with all the initial background variables, and then used an automatic model selection procedure (the “step function”), based on the Akaike (AIC) information criterion used for model evaluation, to find an optimal model. From the analysis, we present the hazard ratios for different levels of the background variables.
Age is the only continuous background variable. We checked the statistical reasonability of a linear functional form in the regression term (by also applying the pspline function in the R-package smoothHR [16]), and also for an interaction term with age.
In addition, to the background variables listed above it was interesting to allow for time-varying variables in the model. We suggested two: (1) two or more than two different BZDs dispensed during the observation time versus just one and (2) whether users had prescription fulfilments by a psychiatrist in the previous period or not.
Results
The percentage of users in the two highest dispensation levels increased over the four first observation years, then remained at a steady level for the last year. Of all the new BZD redeemers who started on a regular dose (less than one DDD per day on average over a three-month period), 2.34% (544 individuals) reached dispensation level three within a five-year period. The mean time it took to reach level three for the first time was 2.75 years (11 periods). Of all new BZD redeemers 38% did not redeem after the first period. At any time 75% of the initial users did not redeem any BZD prescriptions.
Kaplan–Meier plots
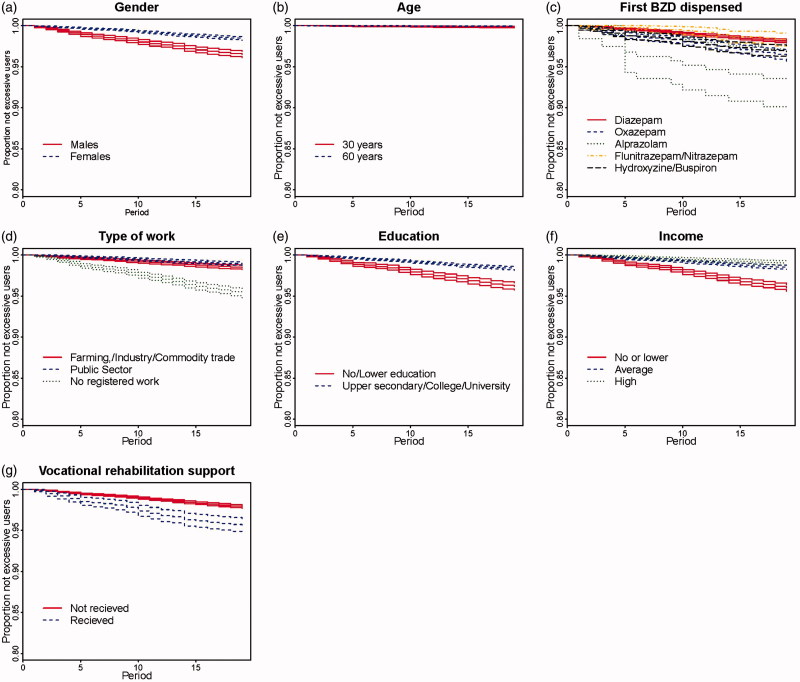
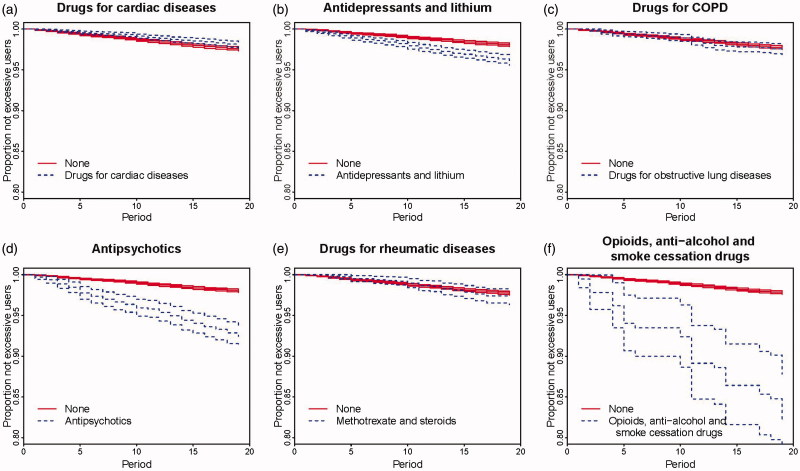
Kaplan–Meier plots are presented in Figures 2 and 3. More men than women became excessive users and this difference increased over time (Figure 2a). Figure 2b shows no clear difference in percentages becoming excessive users for 30- and 60-year-old individuals. Users without registered work more often became excessive users than those with registered work (Figure 2d). A higher percentage of individuals with no or low education became excessive users compared with higher educated individuals (Figure 2e). Also, a higher fraction of users with no or low household income became excessive users compared with those with medium or high household income (Figure 2f). Users who had previously received vocational rehabilitation support more often became excessive users than those without such a history (Figure 2g). A higher percentage of users with a previous history of antidepressants or lithium, antipsychotics or opioids, anti-alcohol or smoke cessation treatment became excessive users compared with those with no such history (Figure 3b, d, f). In Figure 3c a higher fraction of users who started on alprazolam became excessive users compared with those starting on any other BZD. There was no difference between users with comorbidities for cardiac diseases, COPD, or rheumatic diseases and users with no such history (Figure 3a, c, e).
Figure 2.
Kaplan–Meier plots showing risk of excessive use, with 95% uncertainty bands for background variables: gender, age, first BZD dispensed, type of work, education, household income, vocational rehabilitation support.
Figure 3.
Kaplan–Meier plots showing risk of excessive use, with 95% uncertainty bands for background variables: previous redemptions for relevant drugs.
Cox proportional regression model
We considered a nonlinear relationship for age, and this relationship revealed that a linear term was reasonable. We also tried a model with the background variables gender, age, and an interaction term between gender and age, and the latter term was not significant.
Table 2 displays the fitted model resulting from an automatic model selection procedure (step function in R). The final model does not contain the following background variables: county, previous treatment for cardiac diseases, drugs for obstructive lung diseases, and methotrexate and steroids. All intervals in the following text are 95% uncertainty intervals (CI) and hazard ratios are denoted by HR.
Table 2.
A fitted Cox proportional hazard regression model taking into consideration gender, age (scaled by subtracting the average age), first BZD, previous relevant drug dispensations, vocational rehabilitation support, education level, household income and type of work.
| Variable Group | Hazard ratio | 95% uncertainty interval | p Value | |
| Gender | women vs men | 0.42 | (0.35,0.51) | <0.001 |
| Age | 0.96 | (0.95,0.97) | <0.001 | |
| First BZD | oxazepam vs. diazepam | 1.51 | (1.24,1.85) | <0.001 |
| alprazolam vs. diazepam | 2.75 | (1.54,4.91) | <0.001 | |
| hydroxyzine/buspirone vs. diazepam | 0.77 | (0.47,1.26) | 0.3031 | |
| nitrazepam/flunitrazepam vs. diazepam | 1.67 | (1.29,2.16) | <0.001 | |
| Previous drugs | Antidepressants and lithium | 1.4 | (1.16,1.69) | <0.001 |
| Antipsychotics | 1.92 | (1.54,2.4) | <0.001 | |
| Opioids, anti-alcohol and smoke cessation drugs | 2.88 | (2,4.15) | <0.001 | |
| Vocational rehabilitation support | 1.18 | (0.94,1.49) | 0.1487 | |
| Education | High vs. no/low | 0.68 | (0.57,0.81) | <0.001 |
| Income | Average vs. no/low | 0.58 | (0.46,0.73) | <0.001 |
| High vs. no/low | 0.37 | (0.26,0.54) | <0.001 | |
| Type of work | Primary sector/industry vs. not given | 0.53 | (0.4,0.71) | <0.001 |
| Public sector vs. not given | 0.57 | (0.45,0.74) | <0.001 |
Table 2 shows that women had a lower risk for becoming excessive users than men (HR = 0.42, CI 0.35–0.51). A higher age indicated a lower risk (HR = 0.96, CI 0.95–0.97), and this was true for both genders. Those who first used oxazepam had a higher risk compared with those who first used diazepam (HR = 1.51, CI 1.24–1.85). Those who started on alprazolam also had a higher risk compared with those starting on diazepam (HR = 2.75, CI 1.54–4.91). To start on hydroxyzine or buspirone was associated with a lower risk compared with having started on diazepam, but this difference was not significant (HR = 0.77, CI 0.47–1.26). To start on nitrazepam or flunitrazepam was associated with a higher risk compared with having started on diazepam (HR = 1.67, CI 1.29–2.16).
Those who had previously used antidepressants or lithium, antipsychotics or opioids, anti-alcohol and smoke cessation treatment had a higher risk compared with those without such drug experience (HR = 1.4, CI 1.16–1.69, HR = 1.92, CI 1.54–2.4, and HR = 2.88, CI 2–4.15, respectively).
Users who had previously received vocational rehabilitation support had a higher risk compared with those who had not previously received such support, but this difference was not significant (HR = 0.18, CI 0.94–1.49). Users with higher education had a lower risk compared with those with lower education (HR = 0.68, CI 0.57–0.81). Users with average or high household income had a lower risk compared with those with no or low income (HR = 0.58, CI 0.46–0.73 and HR = 0.37, CI 0.26–0.54, respectively). Users who had worked in the primary or public sector had a lower risk compared with those without any registered work (HR = 0.53, CI 0.4–0.71 and HR = 0.57, CI 0.45–0.74).
Regarding the number of different BZDs dispensed during the observation period, among the 544 individuals who became excessive users there were 41.4% (225 users) who had dispensations for two different BZDs and there were 18.2% (99 users) who had dispensations for more than two different BZDs. The corresponding numbers for those who did not become excessive users were 19.1% (4332 users) and 3.87% (877 users) respectively. Among patients who had prescription(s) from a psychiatrist during the previous period there was an increased frequency of excessive users. Among the 544 individuals who became excessive users 75 (13.79%) had at least one prescription fulfilment by a psychiatrist, while the corresponding number among the 22 683 individuals who did not become excessive users was 598 (2.64%). We considered a model with the two time-dependent variables: multiple BZDs dispensed and psychiatrist prescriber. We again started out with a full model and applied the model reduction procedure (step function in R). The resulting model did not contain the vocational rehabilitation support variable. By including time-dependent variables these were given importance, and hence reduced the estimated effect of (some of) the background variables. Such process variables measured steps on the way to becoming excessive users. Hence, these variables described the process, but could not be interpreted in the same way as the background variables.
Discussion
Mainly, BZDs were used for a short time, and 38% of the users stopped after three months or less. About 75% of the users did not redeem BZDs in any other period, indicating a high prevalence for short-term treatment, which is in accordance with guidelines [2,3]. Many users had intermittent use, in line with Nelson and Chouinard [17]. In a previous paper we followed individuals for three years, and found an increasing proportion of users moving to dispensation levels two and three over time [13]. In this present paper we found that the percentage of users in the two highest dispensation groups increased over the first four years, then remained at a steady level for the last year. However, a small percentage redeemed far beyond guidelines, and this group is different from other users with regard to several background variables, as discussed below.
Although it is known that BZDs are more frequently used among women than among men, we found that men had a higher risk of becoming excessive users compared with women. One simple explanation might be that men need higher doses than women to achieve the same effect [18] but it might also indicate that women and men use BZDs differently.
Starting on oxazepam gave a higher risk for ending up with excessive BZD use compared with starting on diazepam. This is interesting given the guidelines’ suggestions for oxazepam initiation when uncertain about a patient’s proneness to dependency. Oxazepam is classified as a low potency BZD [17] with its slow absorption and accordingly slow onset of CNS effects. Together with its one-compartment distribution (lack of fast decline in plasma concentration due to distribution) and medium long elimination half-life, oxazepam has repeatedly been suggested as a candidate drug when possible drug dependency is suspected in an individual in need of antianxiety treatment. On second thoughts, however, this finding might not be surprising at all as the first group might need higher doses (more DDDs) of oxazepam compared with other BZDs to achieve the same (initial kick) effect [19,20]. Starting on oxazepam at the recommended dosage (or even lower dosage due to cautiousness) might give an inadequate antianxiety effect initially and drive some individuals to seek a stronger antianxiety effect by increased dosage.
The data in the present study gave an actual picture of the prescription pattern in a population. This was in contrast to the guidelines, which give advice probably on a more theoretical basis. The present finding could encourage a warning against starting on oxazepam, but further analyses are warranted to explore this finding.
Individuals starting on alprazolam also had a higher risk of ending up as high BZD users compared with those who started on diazepam. It must, however, be noted that although the estimated hazard ratio was high, this concerned few people. Only 187 individuals started on alprazolam, and 12 of these ended up as excessive users. Doctors are warned against prescribing alprazolam as it is known to be especially addictive, and our analysis thus confirms well-established knowledge [17].
The finding of a higher risk of becoming an excessive user for first-time users of nitrazepam/flunitrazepam compared with diazepam was as expected given the plasma profiles with their “hit and run” pattern. Also, the finding of a non-significant but nominally increased risk for first-time users of hydroxyzine/buspirone compared with first-time diazepam users was unsurprising as the antianxiety effect of these drugs appears very slowly compared with, for example, diazepam [20,21].
Comorbidities as indicated by use of antidepressants and lithium, antipsychotics and opioids, anti-alcohol and smoke cessation drugs were all associated with a higher risk of becoming an excessive user compared with those who had not previously redeemed such drugs. These findings were unsurprising as combination treatments of antidepressants or antipsychotics and BZDs frequently occur [22,23]. Smoking could be said to indicate a propensity towards dependency, and this might also be the case for opioids and alcohol use [24,25].
Having previously received vocational rehabilitation support was not a significant risk factor for becoming an excessive user in our final model, but in a preliminary analysis with this variable alone it was clearly significant. In general, when one includes several background variables, one or more previously significant variable(s) might become non-significant as the feature(s) might be explained by one or more of the other (significant) variables. Presumably, users who received vocational rehabilitation support are described through other variables such as low income and work status. No or low income and no or low education were risk factors. Such users might experience a socio-economically stressful situation, resulting in higher BZD consumption compared with others.
To have prescriptions written by a psychiatrist or dispensed for different BZDs cannot in themselves be called risk factors. But in the process of becoming excessive users these users tended to visit a psychiatrist and switch BZDs to a larger degree than users who did not end up on a high-dose treatment regime. To have been referred to a psychiatrist was regarded as a marker for more severe mental illnesses.
This analysis was retrospective, but being a population-based analysis there is no observational bias. As an indication of drug dependency we focused on those who became excessive users. We have not discussed drug dependency without dose escalation, but of course long-term use could be a marker for dependency as well.
The NorPD was established in 2004, and hence there were no redemptions registered prior to 2004. We defined previous BZD naive users based on two years without redemptions. We further assumed that the first prescription redeemed contained between 10 and 30 DDDs, and also considered users who redeemed prescriptions of less than one DDD per day on average in the first period. This made it reasonable to assume that the study population consisted of new BZD users.
As for all register-based studies, it was the amount dispensed that was analysed, and not the amount consumed. If the amount dispensed did not correspond to the amount used the results would be partly flawed. There was unfortunately no way to estimate a possible discrepancy.
Most users redeemed BZDs for only a short period, and hence seemed to use BZDs according to guidelines. Nevertheless, a small percentage ended up as excessive users. We believe our findings of risk factors associated with excessive BZD can aid doctors to identify individuals prone to excessive BZD use.
Acknowledgements
The authors acknowledge the helpful services of NorPD and SSB. They thank Professor Ørnulf Borgan for helpful comments. They also thank four anonymous reviewers for their valuable comments and feedback improving the paper.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was done under the Norwegian Research council project number 190420/V50.
References
- [1].Lader M. Benzodiazepines revisited: Will we ever learn? Addiction 2011;106:2086–109. [DOI] [PubMed] [Google Scholar]
- [2].Available from: http://helsedirektoratet.no/sites/vanedannende-legemidler/Sider/default.aspx, accessed January 2015.
- [3].Available from: http://www.rcpsych.ac.uk/healthadvice/treatmentswellbeing/benzodiazepines.aspx, accessed January 2015.
- [4].Barker MJ, Greenwood KM, Jackson M, Crowe SF.. Cognitive effects of long-term benzodiazepine use: A meta-analysis. CNS Drugs 2004;18:37–48. [DOI] [PubMed] [Google Scholar]
- [5].Lader M. Effectiveness of benzodiazepines: Do they work or not? Expert Rev Neurother 2008; 8:1189–91. [DOI] [PubMed] [Google Scholar]
- [6].Ashton H. Adverse effects of prolonged benzodiazepine use. Adverse Drug React Bull 1986;118:1–7. [Google Scholar]
- [7].Smith RG. Fall-contributing adverse effects of the most frequently prescribed drugs. J Am Podiatr Med Assoc 2003;93:42–50. [DOI] [PubMed] [Google Scholar]
- [8].Barbone F, McMahon AD, Davey PG, et al. Association of road-traffic accidents with benzodiazepine use. Lancet 1998;24:1331–6. [DOI] [PubMed] [Google Scholar]
- [9].Oster G, Huse DM, Adams SF, et al. Benzodiazepine tranquilizers and the risk of accidental injury. Am J Public Health 1990;80:1467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].The Norwegian Prescription Database. Available from: http://www.norpd.no, accessed June 2014.
- [11].Statistics Norway http://www.ssb.no/, accessed June 2014.
- [12].DDD. Available from: http://www.whocc.no/ddd/definition_and_general_considera/, accessed June 2014.
- [13].Tvete I, Bjørner T, Skomedal T.. A 3-year survey quantifying the risk of dose escalation of benzodiazepines and congeners to identify risk factors to aid doctors to more rationale prescribing BMJ Open. Available from: http://dx.doi.org/10.1136/bmjopen-2013-003296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Available from: http://www.regjeringen.no/upload/AD/publikasjoner/veiledninger_brosjyrer/2012/Trygdesysteme_2012_engelsk.pdf, accessed January 2015.
- [15].R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/, accessed June 2014.
- [16].Meira-Machado L, Cadarso-Suárez C, Gude F, and Araújo A. An R Package for Pointwise Nonparametric Estimation of Hazard Ratio Curves of Continuous Predictors. Computational and Mathematical Methods in Medicine, 2013, 1-11. [DOI] [PMC free article] [PubMed]
- [17].Nelson J, Chouinard G.. Guidelines for the clinical use of benzodiazepines: Pharmacokinetics, dependency, rebound and withdrawal. Can J Clin Pharmacol 1999;6:69–83. [PubMed] [Google Scholar]
- [18].Greenblatt DJ, Allen MD, Shader RI.. Diazepam disposition determinants. Clin Pharmacol Ther 1980;27:301–12. [DOI] [PubMed] [Google Scholar]
- [19].Breimer DD. Pharmacokinetics and metabolism of various benzodiazepines used as hypnotics. Br J Clin Pharmacol 1979;8:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Amrein R, Eckert M, Haefeli H, Leishman B.. Pharmacokinetic and clinical considerations in the choice of a hypnotic. Br J Clin Pharmacol 1983;16:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hofman EJ, Mathew SJ.. Anxiety disorders: A comprehensive review of pharmacotherapies. Mt Sinai J Med 2008;75: 248–62. [DOI] [PubMed] [Google Scholar]
- [22].Dunlop BW, Davis PG.. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: A review. Prim Care Companion J Clin Psychiatry 2008;10:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cochrane Database of Systematic Review. Benzodiazepines alone or in combination with antipsychotic drugs for acute psychosis. Available from: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0011800/, accessed June 2014. [DOI] [PubMed]
- [24].Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M.. Nicotinic acetylcholine receptors in the mesolimbic pathway: Primary role of ventral tegmental area receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci 2010;30:5311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Govind AP, Vezina P, Green WN.. Nicotine-induced upregulation of nicotinic receptors: Underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 2009;78:756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]