Abstract
Objective: To compare the appropriateness of antibiotic prescribing for upper respiratory tract infections (URTIs) in two countries with different prevalence of antimicrobial resistance: Denmark and Iceland.
Design: A cross-sectional study. Settings and subjects. General practitioners (GPs) in Denmark (n = 78) and Iceland (n = 21) registered all patients with URTI according to the Audit Project Odense (APO) method during a three-week period in the winter months of 2008 and 2009.
Main outcome measures: Appropriateness of antibiotic prescribing in patients with URTI in Denmark and Iceland.
Results: A total of 1428 patients were registered (Denmark: n = 1208; Iceland: n = 220). A majority of patients in both countries were prescribed antibiotics, and only a minority of the prescriptions could be classified as appropriate prescribing. In general, Icelandic GPs more often prescribed antibiotics (Iceland = 75.8% vs. Denmark = 59.3%), but Danish GPs had a higher percentage of inappropriate antibiotic prescribing for sinusitis, and Icelandic GPs for pharyngotonsillitis. No differences were found for acute otitis media (AOM). The different antibiotic prescribing patterns between Denmark and Iceland could not fully be explained by different symptoms and signs among patients.
Conclusion: Icelandic GPs have a higher antibiotic prescribing rate compared with Danish GPs, but the percentage of inappropriate antibiotic prescribing is highest in Denmark for sinusitis, and in Iceland for pharyngotonsillitis.
Key points
Within the Nordic countries there are marked differences in antimicrobial resistance and antibiotic use.
Iceland differs from Denmark by a higher antibiotic prescribing rate and a higher prevalence of antimicrobial resistance.
The majority of antibiotics are prescribed in primary care and most often for upper respiratory infections (URTIs).
Only a minor amount of antibiotic prescriptions for URTIs can be classified as appropriate; inappropriate antibiotic prescribing is higher in Denmark than in Iceland for sinusitis and the opposite for pharyngotonsillitis.
The different antibiotic prescribing patterns between Denmark and Iceland cannot be fully explained by different clinical criteria among patients.
Keywords: Antibiotics, Denmark, diagnostic criteria, general practice, upper respiratory tract infection
Background
Antimicrobial resistance is an increasing problem worldwide [1,2], causing increased morbidity and mortality [3]. Inappropriate consumption of antibiotics is one of the most important reasons for the development of antimicrobial resistance [2].
Antimicrobial resistance varies between the European countries: the highest prevalence is found in the Southern and Eastern European countries, while a lower prevalence is seen in the Northern European countries [4,5]. However, since 2004 the prevalence of antimicrobial resistance has been up to five times higher in Iceland than in Denmark [4,6]. This difference may reflect variation in the use of antibiotics: Between 2004 and 2008 Iceland had the highest consumption of antibiotics in the Nordic countries with a number of defined daily doses per 1000 inhabitants per day (DID) in the range of 21.6–23.4, whereas the use of antibiotics in Denmark at the same time was 15.2–16.7 DID [7–9].
The majority of antibiotics are prescribed in general practice, and about two-thirds of all prescriptions are for respiratory tract infections (RTI). However, the majority of RTIs in general practice are harmless and self-limiting [10–12]. Even when the aetiology is bacterial, the duration and severity of symptoms are only slightly influenced by the use of antibiotics, particular in patients with upper respiratory tract infections (URTIs) [12,13].
General practitioners’ (GPs’) antibiotic prescribing pattern differs considerably for RTIs [14–16]. In general practice, the decision to prescribe antibiotics for URTIs is mainly based on the presence of signs and symptoms. There is a lack of simple and accurate microbiological tests that can help the GP to make the right decision concerning antibiotic prescribing [16]. Uncertainty and doubt regarding microbiological aetiology may increase the influence of patient demand and other non-clinical factors leading to an increased risk of inappropriate antibiotic prescribing
In order to explore potential differences in the appropriateness of antibiotic prescribing between Iceland and Denmark more knowledge is needed concerning the diagnoses and clinical criteria for antibiotic prescribing, i.e. the symptoms and signs behind the doctor’s decision to prescribe.
The aim of this study was to compare the appropriateness of antibiotic prescribing for URTI in two countries with a different prevalence of antimicrobial resistance: Denmark and Iceland.
Material and methods
Data for this cross-sectional study were based on comparable data from Danish and Icelandic GPs participating in audits according to the Audit Project Odense (APO) method [17]. The Danish data came from of the EU-funded project “Health Alliance for Prudent Prescribing, Yield And Use of antimicrobial Drugs In the Treatment of respiratory tract infections” (HAPPY AUDIT) [18] and the Icelandic data from a Nordic audit using exactly the same methodology as HAPPY AUDIT. In both audits, GPs were invited by email or personal contact. A total of 21 Icelandic GPs (10.3% of all Icelandic GPs) and 78 Danish GPs (2.2% of all Danish GPs) volunteered to participate.
Population
A total of 1428 patients with URTI were registered in Denmark (n = 1208) and Iceland (n = 220).
We included patients with URTIs that were diagnosed according to the International Classification of Primary Care: acute otitis media (H71, H72), acute sinusitis (R75) and acute pharyngotonsillitis (R72, R74, R76) [19]. Only patients consulting their GP for the first time for the current infection were included. Telephone consultations, home visits, and patients receiving antibiotics prior to the consultation were excluded.
Data collection
Within a three-week period in the winter months of 2008 (Denmark) and of 2009 (Iceland), GPs prospectively registered patients suspected of RTIs. For each patient the GP registered age, sex, symptoms and signs, duration of symptoms (days), diagnostic tests performed, e.g. Streptococcus antigen test (Strep-A) or a C-reactive protein (CRP), assumed microbiological aetiology (virus or bacteria), assumed diagnosis, antibiotic treatment and allergy to penicillin, patient demand for antibiotics, and referral to hospital. The registration chart used in Iceland was translated into Icelandic and back translated to Danish in order to ensure an equal understanding and interpretation of all variables registered in the two countries. See detailed information about the HAPPY AUDIT method in the study protocol, published in BMC Family Practice [18].
Criteria for appropriateness of antibiotic prescriptions
Antibiotic prescriptions following international recommendations for antibiotic prescribing in patients with URTIs (HAPPY AUDIT, EPOS) [20,21] were classified as appropriate prescribing. Prescriptions not fulfilling international criteria for treatment with antibiotics were classified as inappropriate prescribing. Prescriptions fulfilling only some of the agreed criteria for antibiotic prescribing were classified as possibly appropriate prescribing [20–22]. Table 1 gives the criteria used to classify appropriate and inappropriate antibiotic prescribing.
Table 1.
Criteria for classification of appropriate and inappropriate antibiotic prescribing in patients with upper respiratory tract infections.
| Appropriate antibiotic prescribing | Inappropriate antibiotic prescribing | |
| Acute sinusitis [20,21] | Duration of symptoms >5 days AND fever | Duration of symptoms ≤5 days AND no fever |
| Acute otitis media [20,22] | Duration of symptoms >3 days AND purulent ear secretion OR age <2 years | Duration of symptoms ≤3 days AND no purulent ear secretion |
| Acute pharyngotonsillitis [20,22] | Fulfil ≥2 Centor criteria AND a positive Strep-A test OR Fulfil 4 Centor criteria | Fulfil <2 Centor criteria OR a negative Strep-A test |
Notes: 1Patients ≥5 years of age, suspected of acute pharyngotonsillitis. 2The Centor criteria include: fever >38.5c, tonsillar exudates, tender anterior cervical adenopathy, and absence of cough.
Statistical analyses
Data are presented as proportions. Comparisons between the countries were performed by using the Wilcoxon test for continuous variables and chi-square tests for categorical variables. In all analyses we considered a p-value of <0.05 as statistically significant. The data were analysed in the Statistical Analysis Software™ (SAS) (SAS Institute Inc., Cary, IN, USA) version 9.2 and Microsoft Office Excel™ 2007 (Microsoft Corp., Redmond, WA, USA).
Results
A total of 1428 patients with acute otitis media, acute sinusitis, and acute pharyngotonsillitis were included in the study (Denmark = 1208 and Iceland = 220). Acute pharyngotonsillitis was most frequent in Denmark, while acute sinusitis and acute otitis media were more frequent in Iceland (Table II). No significant differences were found for age and sex distribution.
Table 2.
Number and characteristics of patients with upper respiratory tract infections in Iceland and Denmark.
| Iceland (n = 220) | Denmark (n = 1208) | p-value | |
| Males (%) | 106 (48.2) | 505 (41.8) | 0.08 |
| Median age (years) (IQR1) | 25 (6-42) | 23 (7-40) | 0.91 |
| Acute otitis media (%) | 72 (32.7) | 286 (23.7) | <0.005 |
| Acute pharyngotonsillitis (%) | 75 (34.1) | 650 (53.8) | <0.0001 |
| Acute sinusitis (%) | 73 (33.2) | 272 (22.5) | <0.005 |
| Antibiotic prescribing2 (%) | 166 (75.8) | 712 (59.3) | <0.0001 |
Notes: 1IQR = interquartile range. 2Nine missing values (Iceland = 1, Denmark = 8).
In both countries, the majority of patients with URTI were prescribed antibiotics (Denmark = 59.3%, Iceland = 75.8%). Considerable variations in antibiotic prescribing rates were found (Table III). The highest antibiotic prescribing rate was found in patients with suspected acute sinusitis (Iceland = 98.6%, Denmark = 75.5%), and the lowest prescribing rate in patients with acute pharyngotonsillitis (Iceland = 58.7%, Denmark = 46.3%).
Table 3.
Distribution of symptoms and signs and antibiotic prescribing in patients with upper respiratory tract infections in Iceland and Denmark.
| Iceland | Denmark | p-value | |
| Acute otitis media, no. (%) | 72 (32.7) | 286 (23.7) | <0.05 |
| Males, no. (%) | 44 (61.1) | 139 (47.6) | 0.06 |
| Median age, years (IQR) | 3 (1–8) | 3 (1–7) | 0.92 |
| Duration of symptoms, median days, (IQR) | 4 (2–7) | 3 (2–5) | <0.01 |
| Fever1 no. (%) | 28 (38.9) | 152 (53.2) | <0.05 |
| Purulent ear secretion no. (%) | 18 (25.0) | 69 (24.1) | 0.88 |
| Antibiotic prescribing no. (%) | 50 (70.4) | 210 (73.7) | 0.58 |
| Pharyngotonsillitis, no. (%) | 75 (34.1) | 650 (53.8) | <0.0001 |
| Males, no. (%) | 35 (46.7) | 281 (43.2) | 0.57 |
| Age, median years (IQR) | 28 (18–41) | 24 (12–38) | <0.05 |
| Duration of symptoms, median days (IQR) | 4 (3–6) | 3 (2–5) | <0.05 |
| No. of patients (%) with ≥ 2 Centor criteria | 41 (54.7) | 336 (51.7) | 0.63 |
| No of patients (%) with a Strep-A2 test performed | 50 (66.7) | 543 (83.5) | <0.05 |
| Antibiotics prescribed, no. (%) | 44 (58.7) | 299 (46.3) | <0.05 |
| Sinusitis, no. (%) | 73 (33.2) | 272 (22.5) | <0.05 |
| Males, no. (%) | 27 (37.0) | 85 (31.3) | 0.35 |
| Median age, years (IQR) | 39 (27–55) | 41 (32–55) | 0.41 |
| Duration of symptoms, median days (IQR) | 11 (8–20) | 7 (4–12) | <0.0001 |
| Fever, no. (%) | 17 (23.3) | 83 (30.5) | 0.23 |
| No. of patients (%) with CRP3 performed | 2 (2.7) | 159 (58.5) | <0.0001 |
| Antibiotics prescribed, no. (%) | 72 (98.6) | 203 (75.5) | <0.0001 |
Notes: 1Fever = >38.5°c. 2Rapid Streptococcus A antigen detection test. 3C-reactive protein measurement.
The appropriateness of antibiotic prescribing for the different types of URTI is shown in Figure 1. In patients with acute sinusitis the percentage of inappropriate antibiotic prescribing was higher in Denmark than in Iceland (16.4% vs. 4.1%, p < 0.0001), and for acute otitis media the corresponding figures were 19.7% and 8.3% (p > 0.05). In patients with acute pharyngotonsillitis we found a higher percentage of inappropriate antibiotics in Iceland compared with Denmark (14.7% vs. 5.7%, p = 0.003).
Figure 1.
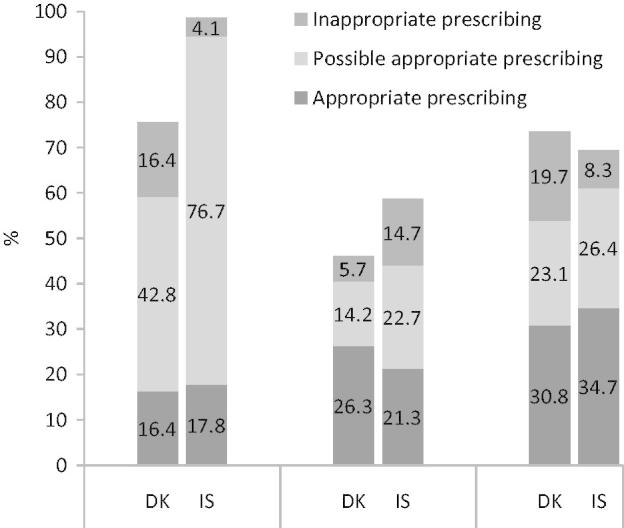
Appropriateness of antibiotic prescribing in patients with upper respiratory tract infections in Iceland (IS) and Denmark (DK).
Note: The bars show the proportion of patients prescribed antibiotics.
For all types of URTI the median duration of symptoms before GP contact was higher in Iceland than in Denmark (see Table III). For acute sinusitis more than 90% of Icelandic patients compared with 60% of Danish patients had symptoms > 5 days before they contacted a GP. A greater proportion of patients with acute otitis media in Denmark than in Iceland had fever (53.2% vs. 38.9%), but no difference was found in the occurrence of purulent ear secretion. In both countries more than 50% of patients with suspected acute pharyngotonsillitis fulfilled two or more Centor criteria. However, no significant difference was found in the distribution of Centor criteria between Denmark and Iceland.
A higher proportion of GPs in Denmark than Iceland used CRP (58.5% vs. 2.7%) and Strep-A test (83.5% vs. 66.7%).
Discussion
Our results showed that antibiotic prescribing for URTI was high in both countries. Only a minority of all antibiotic prescriptions could be classified as appropriate. In general, Icelandic GPs showed the highest antibiotic prescribing rate. However, in patients with sinusitis, Danish GPs had the highest proportion of inappropriate antibiotic prescribing and in patients with pharyngotonsillitis the highest proportion of inappropriate prescribing was found among Icelandic GPs. We found no significant difference in appropriate prescribing in patients with AOM. Several studies have shown that the majority of URTIs are caused by a virus, and most antibiotic treatments for URTI are therefore not associated with any benefit. On the contrary, overuse of antibiotics may increase the risk of adverse effects and enhance the risk of antibiotic resistance.
Weaknesses and strengths
GPs participated on a voluntary basis and this may have introduced a selection bias; prescribing habits may not represent the average antibiotic prescribing in the two countries. Furthermore, participating GPs may be more interested in rational antibiotic treatment than GPs in general. Our results may therefore be an underestimate of potential overprescribing of antibiotics. Second, all data were self-reported by the GPs, and we are thereby not able to explore the accuracy of the diagnoses or the symptoms reported.
It is a strength that we used exactly the same method to register patients with URTI in Denmark and Iceland. The APO audit method is primarily a quality improvement method that measures performance on two occasions, before and after an intervention. Data from both countries came from the first registration, i.e. before intervention, and therefore reflect the quality of antibiotic prescribing among GPs who were not exposed to any intervention. Neither of the two countries had official national guidelines for diagnosis and treatment of URTI during the study period. According to national statistics, overall prescribing of antibiotics in Iceland reduced slightly from 2008 to 2009, but we do not believe that the quality of antibiotic prescribing by Icelandic GPs changed markedly between 2008 and 2009 [7]. Since there were no official guidelines for diagnosis and treatment of URTI during the study period we do not believe that difference in data collection year, 2008 and 2009, respectively, has had an impact on the results in our study.
It is a limitation that GPs had only a short time to register characteristics of patients during the consultation and only the most typical signs and symptoms of RTIs according to the medical literature were recorded. The sample of patients from Iceland was small compared with the Danish sample size. This reflects, to some extent, the fact that Iceland is a small country compared with Denmark. If we look at the percentage of participating patients in relation to the country population it was more than three times higher in Iceland (0.07%) than in Denmark (0.02%). The participating GPs in Iceland represented more than 10% of all GPs in the country and, in spite of the limited absolute numbers of GPs and patients involved in Iceland, we believe that our data represent a true picture of the antibiotic prescribing pattern in both countries.
Most patients with URTI are diagnosed in general practice, but there is no general agreement regarding the specific diagnostic criteria to be used, and this may have induced a risk of diagnostic misclassification. Often, GPs’ decisions concerning diagnosis and treatment are made simultaneously, or the prescribing decision may even be taken before a clear diagnosis is established. The antibiotic prescribing may therefore have influenced the GP’s choice of diagnosis in order to fit the decision regarding treatment. In both countries, a substantial number of antibiotic prescriptions were classified as possible appropriate prescriptions and, for all types of URTI, the percentages of possible appropriate prescriptions were highest in Iceland. Possible appropriate prescribing thus represents a large “grey zone” in both countries. The potential uncertainty regarding how to interpret possible appropriate prescriptions may have induced a misclassification bias. If a higher number of possible appropriate prescriptions from Iceland were in reality inappropriate it might have changed our conclusion. Due to the potential uncertainty concerning interpretation of possible appropriate prescribing we compared only inappropriate antibiotic prescriptions in the two countries.
Finally, the study is cross-sectional and any causal interpretation should be made with caution.
One of the strengths of our study is that our results are based on a pragmatic study design reflecting the presentation and daily management of patients with URTI in general practice. Therefore, it is most likely that our results reflect symptoms and signs among patients in the two countries included. Furthermore, our classification of antibiotic prescribing as appropriate, inappropriate, and possibly appropriate was based on widely accepted and approved international recommendations and guidelines [20–22].
Comparison between Denmark and Iceland
Our results showed that inappropriate antibiotic prescribing was higher in Denmark than in Iceland for sinusitis and the opposite for pharyngotonsillitis and no significant differences were found for AOM. The larger proportion of inappropriate antibiotic prescribing in Denmark than in Iceland for acute sinusitis may partially be explained by the fact that over 90% of the Icelandic patients had a symptom duration of >5 days, as compared with two-thirds of the Danish patients. According to international guidelines, appropriate prescribing of antibiotics in patients with acute sinusitis implies that the duration of symptoms is >5 days and fever should be present [21,22].
In Denmark, CRP is recommended in patients with suspected sinusitis to help the GP make the right decision regarding antibiotic prescribing. In Iceland, however, CRP testing was practically not performed. CRP testing has been shown to reduce antibiotic overprescribing in patients with acute sinusitis [23,24], and it is therefore surprising that the percentage of inappropriate antibiotic prescribing was higher in Denmark than in Iceland. One reason for this may be that Danish GPs rely more on the CRP test result instead of using clinical criteria (e.g. duration of symptoms) when making a decision as to antibiotic treatment [20,21].
For acute pharyngotonsillitis the inappropriate prescribing was more pronounced in Iceland than in Denmark. An equal proportion of patients with acute pharyngotonsillitis in Iceland and Denmark fulfilled two or more Centor criteria, but the Icelandic GPs less often used the Strep-A testing compared with their Danish colleagues. This is in agreement with studies that have shown that implementation of Strep-A testing in primary care may lead to a reduction in antibiotic overprescribing [13].
Implications for clinicians and future research
Iceland and Denmark, both Nordic countries, have partially the same historical background and the cultural and social factors are very similar. It is therefore relevant to explore the factors behind the difference in antibiotic prescribing rates in these two countries. By understanding the difference in the prescribing pattern it may be easier to improve prescribing habits and reduce overprescribing. In this study we focused on URTI because most infections are due to a virus, and the majority of patients do not benefit from antibiotic treatment. Other studies have identified patient demand as the most important non-clinical factor for antibiotic overprescribing in general practice [25,26]. We did not examine patient expectation, since this was not within the scope of the present study.
Generally, GPs in Iceland showed a higher total prescribing of antibiotics but, interestingly, GPs in Denmark had the highest rate of inappropriate antibiotic prescribing for sinusitis, i.e. prescriptions where patients did not fulfil the clinical criteria for antibiotic treatment. The difference in prescribing pattern in Iceland and Denmark can only partially be explained by clinical symptoms and signs, and other factors known to influence the GP’s decision should be addressed in future research.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors would like to thank Dr Jon Bjarni Thorsteinsson for his contribution to data registration and documentation in Iceland. They also wish to thank the study patients and practitioners in the HAPPY AUDIT study for their time and cooperation.
References
- [1].Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD.. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Aystematic review and meta-analysis. BMJ 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
- [2].Sande-Bruinsma N, Grundmann H, Verloo D, Tiemersma E, Monen J, Goossens H, Ferech M.. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis 2008. 14:1722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Garau J. Treatment of drug-resistant pneumococcal pneumonia. Lancet Infect Dis 2002. 2:404–15. [DOI] [PubMed] [Google Scholar]
- [4].European Antimicrobial Resistance Surveillance Network (EARS-Net) European Centre for Disease Prevention and Control 2012 [cited 2012 Jun 18]. Available from: http://ecdc.europa.eu/en/activities/surveillance/EARS-Net/database/Pages/table_reports.aspx [Google Scholar]
- [5].Cars O, Molstad S, Melander A.. Variation in antibiotic use in the European Union. Lancet 2001. 357:1851–3. [DOI] [PubMed] [Google Scholar]
- [6].Registration of susceptibility testing in Iceland. Department of Clinical Microbiology and Infection, Landspítali University Hospital 2011 September 23 [cited 2012 Apr 26]. Available from: http://www.lsh.is/pages/14378
- [7].Statistics for use of antimicrobials in Iceland 2007–2011. Iceland Medicine Agency 2012 [cited 2012 Apr 26]. Available from: http://www.lyfjastofnun.is/media/Tolfraedi/Syklalyf_09_11_I.swf
- [8].Poulsen J, Mortensen I, Mørkøre H, Voipio T, Paakkari P, Arnórsson M, Litleskae I, Ericsson Ö, Nielsen J. The Nordic Medico-Statistical Committee. NOMESCO 2009 January 1 [cited 2012 Jun 18];80–6. Available from: http://nomesco.-eng.nom-nos.dk
- [9].Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. DANMAP 2011 [cited 2012 Oct 1]. Available from: http://www.danmap.org
- [10].Arroll B. Antibiotics for upper respiratory tract infections: An overview of Cochrane reviews. Respir Med 2005. 99:255–61. [DOI] [PubMed] [Google Scholar]
- [11].Molstad S. Reduction in antibiotic prescribing for respiratory tract infections is needed! Scand J Prim Health Care 2003. 21:196–8. [DOI] [PubMed] [Google Scholar]
- [12].National Institute for Health and Clinical Excellence (NICE) Prescribing of antibiotics for self limiting respiratory tract infections in adults and children in primary care. NICE 2008 July [cited 2012 Sep 18]. Available from: http://www.nice.org.uk/guidance/CG69 [PubMed]
- [13].Andre M, Odenholt I, Schwan A, Axelsson I, Eriksson M, Hoffman M, Molstad S, Runehagen A, Lundborg CS, Wahlstrom R. Upper respiratory tract infections in general practice: Diagnosis, antibiotic prescribing, duration of symptoms and use of diagnostic tests. Scand J Infect Dis 2002;34:880–6. [DOI] [PubMed] [Google Scholar]
- [14].Bjerrum L, Munck A, Gahrn-Hansen B, Hansen MP, Jarbol DE, Cordoba G, Llor C, Cots JM, Hernandez S, Lopez-Valcarcel BG, et al. Health alliance for prudent antibiotic prescribing in patients with respiratory tract infections (HAPPY AUDIT): Impact of a non-randomised multifaceted intervention programme. BMC Fam Pract 2011;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goossens H, Ferech M, vander SR, Elseviers M.. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005. 365:579–87. [DOI] [PubMed] [Google Scholar]
- [16].Hansen MP, Bjerrum L, Gahrn-Hansen B, Jarbol DE.. Quality indicators for diagnosis and treatment of respiratory tract infections in general practice: A modified Delphi study. Scand J Prim Health Care 2010. 28:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Munck AP, Hansen DG, Lindman A, Ovhed I, Forre S, Torsteinsson JB.. A Nordic collaboration on medical audit: The APO method for quality development and continuous medical education (CME) in primary health care. Scand J Prim Health Care 1998. 16:2–6. [DOI] [PubMed] [Google Scholar]
- [18].Bjerrum L, Munck A, Gahrn-Hansen B, Hansen MP, Jarboel D, Llor C, Cots JM, Hernandez S, Lopez-Valcarcel BG, Perez A, et al. Health Alliance for Prudent Prescribing, Yield and Use of Antimicrobial Drugs in the Treatment of Respiratory Tract Infections (HAPPY AUDIT) Study protocol. BMC Fam Pract 2010;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hasvold T. A new classification system for primary health care. ICPC – International Classification of Primary Care. Tidsskr Nor Laegeforen 1991. 111:2830–1. [PubMed] [Google Scholar]
- [20].Vejledning i diagnostik og behandling af luftvejsinfektioner i almen praksis – Danish recommendations [Danish guidelines on rational diagnosis and treatment of respiratory tract infections in general practice]. HAPPY AUDIT 2008 [cited 2012 Sep 5]. Available from: http://www.happyaudit.org/files/pub/3490.pdf
- [21].Thomas M, Yawn BP, Price D, Lund V, Mullol J, Fokkens W.. EPOS primary care guidelines: European position paper on the primary care diagnosis and management of rhinosinusitis and nasal polyps 2. Prim Care Respir J 2008. 17:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guidelines för diagnos och behandling av luftvägsinfektioner i primärvärden 2008– Swedish recommendations [Swedish guidelines on rational diagnosis and treatment of respiratory tract infections in general practice]. HAPPY AUDIT 2012 [cited 2012 Oct 7]. Available from: http://www.happyaudit.org/files/pub/3531.pdf
- [23].Bjerrum L, Gahrn-Hansen B, Munck AP.. C-reactive protein measurement in general practice may lead to lower antibiotic prescribing for sinusitis. Br J Gen Pract 2004. 54:659–62. [PMC free article] [PubMed] [Google Scholar]
- [24].Cals JW, Schot MJ, de Jong SA, Dinant GJ, Hopstaken RM.. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: A randomized controlled trial. Ann Fam Med 2010. 8:124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fischer T, Fischer S, Kochen MM, Hummers-Pradier E.. Influence of patient symptoms and physical findings on general practitioners’ treatment of respiratory tract infections: A direct observation study. BMC Fam Pract 2005. 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Varonen H, Rautakorpi UM, Huikko S, Honkanen PO, Klaukka T, Laippala P, Palva E, Roine R, Sarkkinen H, Makela M, et al. Management of acute maxillary sinusitis in Finnish primary care: Results from the nationwide MIKSTRA study. Scand J Prim Health Care 2004. 22:122–7. [DOI] [PubMed] [Google Scholar]


