Abstract
Objective: To explore the associations between decreased pulse oximetry values (SpO2) and clinical, laboratory, and demographic variables in general practice patients diagnosed with asthma or chronic obstructive pulmonary disease (COPD), including those with both COPD and asthma in combination.
Design/setting: A cross-sectional study in seven Norwegian general practices of patients aged 40 years or over who were diagnosed by their general practitioner (GP) with asthma and/or COPD. The patients were examined during a stable phase of their disease. Patients diagnosed with COPD (including those with combined COPD/asthma) and those diagnosed with asthma only were analysed separately.
Main outcome measures: Decreased SpO2 values (≤ 95% and ≤ 92%).
Results: Of 372 patients included (mean age 61.5 years, 62% women), 82 (22.0%) had SpO2 ≤ 95%, of which 11 had SpO2 ≤ 92%. In both asthma and COPD patients, SpO2 ≤ 95% was significantly associated with reduced lung function (spirometry), a diagnosis of coronary heart disease and older age (≥ 65 years). In the COPD group, haemoglobin above normal was associated with SpO2 ≤ 95%. These associations were confirmed by multivariable logistic regression, where FEV1% predicted < 50 was the strongest predictor of SpO2 ≤ 95% (odds ratio 6.8, 95% confidence interval 2.8–16.4).
Conclusion. Pulse oximetry represents a useful diagnostic adjunct for assessing the severity of obstructive pulmonary disease. Decreased pulse oximetry values in stable-phase patients with asthma and/or COPD should prompt the GP to consider revising the diagnosis and treatment and to look for co-morbidities.
Key Points
Despite its common use in general practice, the diagnostic benefits of pulse oximetry remain to be established.
Decreased pulse oximetry values are associated with both reduced lung function (spirometry) and with a diagnosis of coronary heart disease.
Decreased pulse oximetry values may reflect suboptimal treatment and/or undiagnosed comorbidity.
Pulse oximetry may therefore be a useful measure in the follow-up of asthma and COPD patients in general practice.
Keywords: Asthma, chronic obstructive pulmonary disease, comorbidity, general practice, Norway, oximetry
Introduction
Pulse oximetry is a non-invasive, simple, inexpensive, and rapid test to estimate haemoglobin oxygen saturation. Hand-held pulse oximeters have become available in general practice and have been reported to be useful diagnostic tools for the assessment of chronic obstructive pulmonary disease (COPD) during both stable phase [1,2] and exacerbations [1–3], and in particular for confirming the need for oxygen therapy [1,4,5]. Pulse oximetry may also be helpful in assessing the severity of asthma exacerbations [1,6]. An oxygen saturation (SpO2) ≤ 92% indicates hypoxaemia, but values between 93% and 95% are lower than normal [1,7,8]. In COPD patients, SpO2 values ≤ 95% predict hypoxia during exercise and air travel [8,9]. Pulse oximetry is regarded as a valid screening test for systemic hypoxia [10], but current guidelines do not inform general practitioners (GPs) how to deal with SpO2 values between 93% and 95%. More evidence of the usefulness of pulse oximetry is urgently needed, because its use is rapidly increasing in general practice [1,11,12].
GPs implementing pulse oximetry should be able to explain to patients the implications of a lower-than-normal SpO2 value. The aim of this study was to describe conditions and patient characteristics associated with decreased pulse oximetry values in primary care patients with stable obstructive lung disease.
Material and methods
The study was carried out at seven Norwegian GP group practices. The practices were not randomly selected, but were chosen based on the availability of spirometry results from the previous five years and the type of electronic medical record system used. Of 43 241 patients listed at the seven practices, 18 931 were adults aged 40 or over, among whom 1784 were identified from the medical records as being diagnosed by the GP with asthma and/or COPD within the previous five years. For reasons of feasibility (e.g. extra workload for the participating GPs), each group practice decided the proportion of registered patients with asthma or COPD that they would invite to participate in the study. A total of 1111 patients were invited to participate, and in all the practices these were randomly selected in alphabetical order among the eligible patients. Invitations were sent by surface mail without additional reminders. Participation required completion of a questionnaire, a meeting during a stable phase of their disease for a clinical examination including spirometry (i.e. baseline assessment), plus examinations during any exacerbations in the subsequent year. This report is based on the baseline examinations, which took place between March 2009 and March 2010. The participants were instructed not to take their regular respiratory medication on the day of the examination.
Measurements and instruments
The GPs recorded comorbidities including cardiovascular diseases on a computerized questionnaire linked to the patient’s medical record. On a separate questionnaire, patients recorded their smoking habits. The patient’s height and weight were recorded to calculate their body mass index (BMI).
Oxygen saturation was measured with a digital hand-held pulse oximeter, Onyx II model 0550 (Nonin Medical, Inc., Plymouth, MN, USA). The highest value obtained from three measurements was recorded.
The HemoCue Haemoglobin system (Quest Diagnostics, Madison NJ, USA) was used for haemoglobin measurements. The thresholds for raised values were based on the reference values used at the University Hospital of North Norway. The upper normal limit was 16.0 g/dL for women and 17.0 g/dL for men. C-reactive protein (CRP) was analysed using an Afinion AS100 Analyser (Axis-Shield, Oslo, Norway), Orion Quickread CRP (Orion Diagnostica, Espoo, Finland) or ABX Micros CRP (Horbia ABX SAS, Montpellier, France), all of which could display values down to 8 mg/L.
Spirometry was carried out after the pulse oximetry test, following European Respiratory Society/American Thoracic Society guidelines [13], using a Spirare SPS310 spirometer (Diagnostica AS, Oslo, Norway). During spirometry, the patients were seated and a nose clip was not used. Post-bronchodilator spirometry was carried out 20 min after inhalation of 0.4 mg salbutamol. The post-bronchodilator forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were used in the analyses. Norwegian reference values for spirometry were applied [14].
Statistical analyses
Two thresholds of abnormal SpO2 values were used as outcome measures, ≤ 95% and ≤ 92%. The frequencies of reduced SpO2 associated with each patient characteristic (Table I) were analysed separately in asthma and COPD patients. The division into these two groups was based on the GPs’ diagnosis coded as R96 (COPD) and R95 (asthma) according to the International Classification for Primary Care [15]. Patients who were given both diagnoses were allocated to the COPD group. Continuous variables (age, BMI, FEV1, and CRP) were categorized. BMI was categorized as underweight (< 20 kg/m2), normal weight/overweight (20–30 kg/m2), and obese (≥ 30 kg/m2) [16]. The FEV1% predicted was categorized as severely reduced (< 50%), moderately reduced (50–80%), and normal (≥ 80%), in line with the Global Initiative for Chronic Obstructive Lung Disease grading of COPD [5]. CRP values were dichotomized into ≥8 mg/mL and < 8 mg/L. The significance of associations between reduced SpO2 and patient characteristics was analysed using the chi-square test. Age, sex, and variables significantly associated with a decreased SpO2 (p < 0.05) in univariable analysis were entered into a binomial multivariable logistic regression with SpO2 ≤ 95% as the outcome variable. The multivariable analysis was also performed without categorizing the continuous variables. SPSS version 18.0 (IBM, Armonk, NY, USA) was used in all analyses.
Table 1.
Frequency of SpO2 values ≤ 95% in 372 patients aged 40 years or more diagnosed with asthma or COPD.
| COPD | Asthma | |||||
| SpO2 ≤ 95% | SpO2 ≤ 95% | |||||
| Patient characteristics | Patients, n | n (%) | p-value | Patients, n | n (%) | p-value |
| All | 166 | 51 (31.7) | 206 | 31 (15.1) | ||
| Age (years) | ||||||
| ≥ 65 | 96 | 37 (38.5) | 0.01 | 55 | 14 | 0.01 |
| < 65 | 70 | 14 (20.0) | 151 | (25.5) 17 (11.3) | ||
| Sex | ||||||
| Men | 67 | 21 (31.3) | 0.9 | 75 | 13 | 0.5 |
| Women | 99 | 30 (30.3) | 131 | (17.3) 18 (13.7) | ||
| Smoking habits | ||||||
| Never | 22 | 5 (22.7) | 0.7 | 72 | 13 | 0.7 |
| Previous | 90 | 28 (31.1) | 83 | (18.1) | ||
| Current | 54 | 18 (33.3) | 51 | 11 (13.3) 7 (13.7) | ||
| Coronary heart disease | ||||||
| Yes | 42 | 18 (42.9) | 0.05 | 22 | 7 (31.8) | 0.02 |
| No | 124 | 33 (26.6) | 184 | 24 (13.1) | ||
| Other cardiovascular disease | ||||||
| Yes | 49 | 18 (36.7) | 0.3 | 28 | 4 (14.3) | 0.9 |
| No | 117 | 33 (28.2) | 178 | 27 (15.2) | ||
| FEV1% predicted | ||||||
| > 80 | 37 | 6 (16.2) | 0.01 | 122 | 10 (8.2) | < 0.01 |
| 50–80 | 84 | 24 (28.6) | 78 | |||
| < 50 | 45 | 21 (46.7) | 6 | 17 (21.8) 4 (66.7) | ||
| BMI (kg/m2)a | ||||||
| < 20 | 17 | 9 (52.9) | 0.06 | 4 | 0 (0.0) | 0.3 |
| 20–30 | 103 | 27 (6.2) | 142 | 24 | ||
| ≥ 30 | 34 | 13 (38.2) | 51 | (16.9) 5 (9.8) | ||
| CRP (mg/L) | ||||||
| ≥ 8 | 29 | 12 (41.4) | 0.2 | 29 | 6 (20.7) | 0.4 |
| < 8 | 137 | 39 (28.5) | 177 | 25 (14.1) | ||
| Haemoglobin above normalb | ||||||
| Yes | 16 | 10 (62.5) | < 0.01 | 15 | 2 (13.3) | 0.9 |
| No | 148 | 40 (27.0) | 190 | 29 (15.3) | ||
Notes: p-values denote the significance of differences between categories of patient characteristics assessed by chi-square test. aValue missing for 21 patients. bHb above 16.0 g/dL in women and above 17.0 g/dL in men; Hb values missing in three patients.
Results
Of the 1111 patients invited to participate, 380 (34.2%) accepted and attended the baseline examination. Eight patients were excluded from analysis, two because they were undergoing an acute exacerbation, two because they did not complete post-bronchodilator spirometry, and four because they did not undergo pulse oximetry. For the 372 included patients, the mean age was 61.5 years and 62% were women. A diagnosis of COPD only was registered in 74 patients, asthma only in 206 patients, whereas 92 patients were registered with both diagnoses. Median SpO2 was 97%. In total, 82 patients (22.0%) had SpO2 values ≤ 95%; 11 had an SpO2 ≤ 92%; of these, 10 were COPD patients and two were on long-term oxygen therapy.
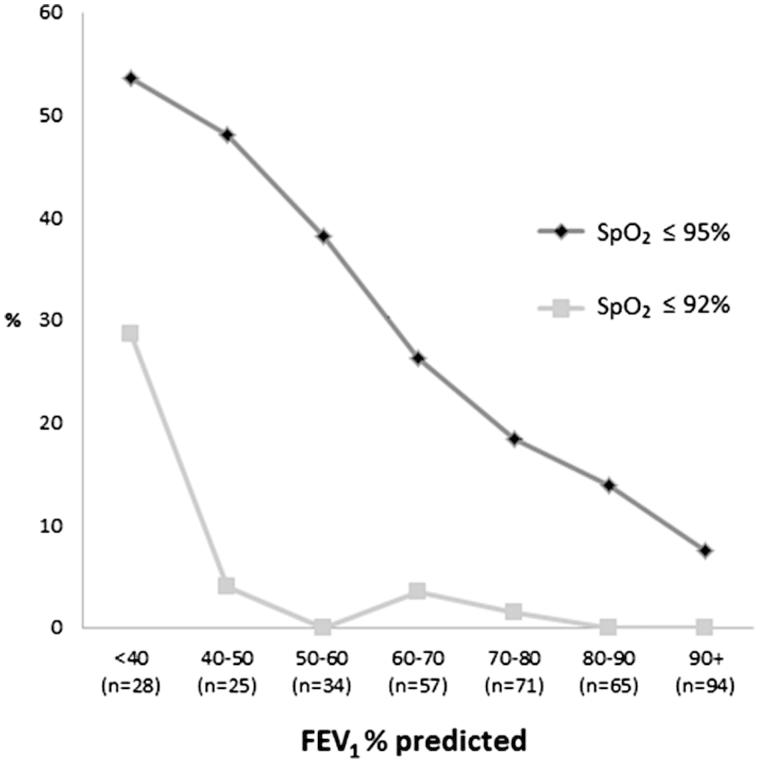
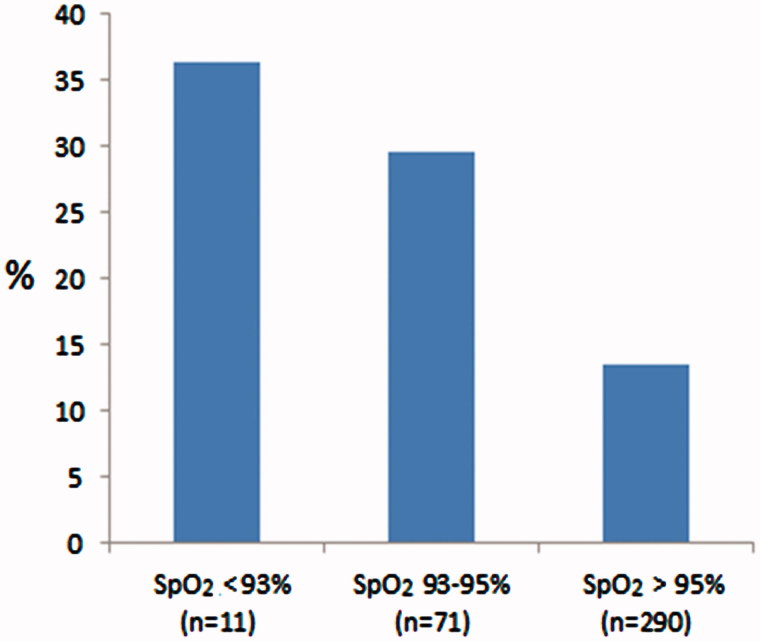
The frequency of both SpO2 ≤ 92% and SpO2 ≤ 95% increased with decreasing levels of FEV1% predicted (Figure 1). When patients with COPD and asthma were analysed separately, the frequency of SpO2 ≤ 95% increased significantly with increasing patient age, decreasing FEV1% predicted, and the presence of coronary heart disease (Table 1). The association of coronary heart disease with decreasing SpO2 values (p < 0.001) is illustrated in Figure 2. Haemoglobin above the threshold value was associated with SpO2 ≤ 95% only in the COPD group (Table 1). Predictors of SpO2 ≤ 92% were evaluated only in the COPD group, for which SpO2 ≤ 92% was associated with decreasing levels of FEV1% predicted, BMI < 20, and raised haemoglobin values (Table 2).
Figure 1.
Frequency of reduced SpO2 by FEV1% predicted in 372 adult patients in general practice with a diagnosis of asthma and/or COPD.
Figure 2.
Frequency of coronary heart disease by pulse oximetry values in 372 adult patients in general practice with a diagnosis of asthma and/or COPD.
Table 2.
Frequency of SpO2 ≤ 92% by patient characteristics in 166 patients aged 40 years or over diagnosed with COPD by a GP.
| SpO2 ≤ 92% | |||
| Patient characteristics | Patients, n | n (%) | p-value |
| All | 166 | 10 (6.0) | |
| Age (years) | |||
| ≥ 65 | 96 | 7 (7.3) | 0.4 |
| < 65 | 70 | 3 (4.3) | |
| Sex | |||
| Men | 67 | 3 (4.5) | 0.5 |
| Women | 99 | 7 (7.1) | |
| Smoking habits | |||
| Never | 22 | 0 | 0.4 |
| Previous | 90 | 7 (7.8) | |
| Current | 54 | 3 (5.6) | |
| Coronary heart disease | |||
| Yes | 42 | 4 (9.5) | 0.3 |
| No | 124 | 6 (4.8) | |
| Other cardiovascular disease | |||
| Yes | 74 | 5(6.8) | 0.7 |
| No | 92 | 5(5.4) | |
| FEV1% predicted | |||
| > 80 | 37 | 0 (0) | |
| 50–80 | 84 | 3 (3.6) | |
| < 50 | 45 | 7 (15.6) | < 0.01 |
| BMI (kg/m2)a | |||
| < 20 | 17 | 5 (29.4) | < 0.01 |
| 20–30 | 103 | 4 (3.9) | |
| ≥ 30 | 34 | 0 (0.0) | |
| CRP (mg/L) | |||
| ≥ 8 | 29 | 3 (10.3) | 0.3 |
| < 8 | 137 | 7 (5.1) | |
| Haemoglobin above normalb | |||
| Yes | 16 | 4 (25.0) | < 0.01 |
| No | 148 | 6 (4.1) |
Notes: aValue missing for 12 patients. bValue missing for two patients.
Multivariable logistic regression revealed that age, comorbidity with coronary heart disease, decreased FEV1% predicted, and haemoglobin above normal were all independent predictors of SpO2 ≤ 95% (Table 3). The strongest predictor of SpO2 ≤ 95% was FEV1% predicted < 50%, recording an odds ratio of 6.8 (Table 3). Using FEV1% predicted as a continuous variable gave similar results and did not improve the model, with the Nagelkerke R2 increasing only from 0.21 to 0.22. In the logistic regression analyses, there was no significant interaction effect between age and the other predictors. Multivariable analysis with SpO2 ≤ 92% as outcome was not performed because of the low number of patients with such values (n = 11).
Table 3.
Predictors of SpO2 ≤ 95% in 369 patients aged 40 years or over diagnosed in general practice with asthma and/or COPD.
| Patient characteristics | Odds ratio (95% CI) | p-value |
| FEV1% predicted | ||
| > 80 | Reference | 0.002 |
| 50–80 | 2.9 (1.5–5.8) | <0.001 |
| < 50 | 6.8 (2.8–16.4) | |
| Coronary heart disease | 2.0 (1.1–3.8) | 0.03 |
| Age | 1.0 (1.0–1.1) | 0.004 |
| Sex male | 0.8 (0.4–1.4) | 0.43 |
| Asthma-only diagnosis | 1.1 (0.6–2.1) | 0.75 |
| Haemoglobin above normal | 2.5 (1.0–6.0) | 0.04 |
Notes: CI = confidence interval; SpO2 = arterial oxygen saturation measured by pulse oximetry; FEV1 = forced expiratory volume in one second.
Discussion
Principal findings
SpO2 ≤ 95% was strongly associated with reduced lung function, coronary heart disease, greater age, and above-normal haemoglobin. Among the COPD patients, SpO2 ≤ 92% was also more frequently found in patients with BMI < 20.
Strengths and weaknesses
In clinical practice, asthma and COPD may sometimes be hard to differentiate [17]. The inclusion of patients diagnosed with either or both of these conditions may make our results relevant for the population of patients in primary care who have obstructive lung disease. More than half the patients in the COPD group had also been diagnosed with asthma within the previous five years. COPD was most frequently the later diagnosis [17], and this may represent the trend to a change in diagnosis from asthma to COPD [18]. Asthma can develop into COPD [19], but the tendency to choose an asthma diagnosis even if COPD would be more appropriate may partly reflect the changed reimbursement regulations for respiratory medication introduced in Norway in 2006. At the time that this study was performed, the costs of inhaled corticosteroids and of inhaled corticosteroids combined with long-acting β2-agonists could generally only be reimbursed in patients with a diagnosis of asthma. Mixed diagnoses may also reflect consultations with more than one doctor during the five-year period.
In this study, current smoking was not associated with decreased pulse oximetry readings. The oxygen saturation in current smokers may have been overestimated, because the presence of carboxyhaemoglobin may falsely increase SpO2 readings [20]. Pulse oximeters also have other limitations [7]. Oxygen saturation may be overestimated in patients with darkly pigmented skin and anaemia [7]. Nail polish, dirt, and artificial nails may cause falsely low readings [21]. Poor perfusion (cold digits) may also result in uncertain results [7]. Analysis of arterial blood obtained by arterial puncture remains the gold standard for measurement of oxygen saturation [7].
The low participation rate (34%) increases the risk of including a study sample that is not representative of the patients in primary care with asthma or COPD. It is likely that the most severely impaired COPD patients, who had been followed up closely in secondary care, were less interested in taking part in the study. The same would apply to patients at the other end of the spectrum, namely those who felt healthy. This is discussed in more detail in a previous report from the study [17]. Although the prevalence of low SpO2 values in our population might have been affected by a somewhat skewed selection of participants, it is not likely that the associations between decreased SpO2 values and lung function were substantially affected.
Findings in relation to other studies
The prevalence of SpO2 ≤ 95% (22%) in the participants in our study was considerably higher than that found in the general population in a Norwegian study of 6317 adults of similar age [22]. In that study, the overall prevalence of SpO2 ≤ 95% was 6.3%, and 15.5% among individuals with self-reported COPD [22]. In a retrospective analysis of 81 COPD patients attending a US university medical centre and tested for desaturation during exercise, the prevalence of SpO2 ≤ 95% was 46% [8]. In our study, 32% of the COPD patients had an SpO2 ≤ 95% (see Table 1).
We found greater age to be an independent predictor of SpO2 ≤ 95%. This was also the case in the Norwegian population-based study [22], and we know from previous studies that lower oxygen saturation can be found in the healthy elderly compared with that in younger healthy adults [23].
In a Dutch study of primary care patients, Schermer et al. [1] showed a positive association between FEV1% predicted < 50 and SpO2 in COPD patients with acute exacerbation or worsening dyspnoea, whereas the association observed in patients with stable COPD did not reach significance. In the Norwegian population-based study referred to above [22], both age ≥ 65 years and FEV1% predicted < 50 were strong predictors of SpO2 ≤ 95%, as in our study, but increased BMI was also an independent predictor. This latter association was not found in our study. A reason for this discrepancy may be that obesity is proportionally a more common reason for breathing problems in the general population than among patients diagnosed with asthma or COPD. In our study, BMI < 20 was strongly associated in COPD patients with SpO2 ≤ 92%. The increased occurrence of decreased oxygen saturation in underweight COPD patients fits with the increased COPD mortality that has been found in patients with very low BMI [16]. In a COPD population from primary care in the Netherlands [24], 11.7% had a BMI < 18.5, similar to the 11.5% in our COPD patients with BMI < 20.
In both univariable and multivariable analyses, coronary heart disease was a strong predictor of SpO2 ≤ 95% in both asthma and COPD patients. An association between coronary heart disease and decreased oxygen saturation was also found in a study from Spain, which concluded that pulse oximetry is useful in establishing the diagnosis and severity of heart failure in acute myocardial infarction [25]. Cardiovascular disease (including ischaemic heart disease, heart failure, atrial fibrillation, and hypertension) is probably both the most frequent and the most important comorbidity coexisting with COPD [18, -26], because they share similar aetiology (i.e. smoking) [27]. Cardiovascular comorbidity is probably underdiagnosed in COPD patients [28].
Implications for primary care practice
We could not find any particular recommendations in the guidelines for the follow-up of stable COPD and asthma patients with SpO2 values ≤ 95% [2,5,7]. Our study shows that patients with moderately decreased oxygen saturation have an increased risk of severely reduced lung function and comorbid coronary heart disease. Some may have received suboptimal pharmacological treatment. Although old age may sometimes be the only explanation, the increased risk of comorbidity and of suboptimal treatment in patients with SpO2 ≤ 95% indicate that these patients should be given special attention and followed up more closely than patients with normal oxygen saturation. The pulse oximeter may be a particularly helpful tool in primary care, because it is tolerable for the patients and is easy to use, and is thus acceptable within the time constraints of a busy practice.
Acknowledgements
The authors would like to thank all patients, GPs, and health secretaries who participated in the study.
Ethical approval
Participating patients signed a written consent form and the study was approved by the Regional Committee for Medical and Health Research Ethics in North Norway.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
LGD were supported by a grant from the Norwegian Research Fund for General Practice.
References
- [1].Schermer T, Leenders J, in 't Veen H, van den Bosch W, Wissink A, Smeele I, Chavannes N. Pulse oximetry in family practice: Indications and clinical observations in patients with COPD. Fam Pract 2009;26:524–531. [DOI] [PubMed] [Google Scholar]
- [2].National Clinical Guideline Centre (2010) Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre. Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English.
- [3].Network. BTSSIG: British guideline on the management of asthma. In; 2014. [Google Scholar]
- [4].Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–55. [DOI] [PubMed] [Google Scholar]
- [5].Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of COPD. Updated 2010. http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf. Accessed April 7, 2011.
- [6].Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008;31:143–78. [DOI] [PubMed] [Google Scholar]
- [7].Holmes S, Peffers SJ. PCRS-UK opinion sheet No. 28: Pulse Oximetry in Primary Care. www.pcrs-uk.org. [Google Scholar]
- [8].Knower MT, Dunagan DP, Adair NE, Chin R Jr.. Baseline oxygen saturation predicts exercise desaturation below prescription threshold in patients with chronic obstructive pulmonary disease. Arch Intern Med 2001;161:732–6. [DOI] [PubMed] [Google Scholar]
- [9].Edvardsen A, Akero A, Christensen CC, Ryg M, Skjonsberg OH.. Air travel and chronic obstructive pulmonary disease: A new algorithm for pre-flight evaluation. Thorax 2012;67:964–9. [DOI] [PubMed] [Google Scholar]
- [10].Hanning CD, Alexander-Williams JM.. Pulse oximetry: A practical review. BMJ 1995;311:367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cunningham S, McMurray A.. The availability and use of oxygen saturation monitoring in primary care in order to assess asthma severity. Prim Care Respir J 2006;15:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parkes G, Pluddemann A, Heneghan C, Price CP, Wolstenholme J, Thompson M.. Spirometry in primary care for case finding and management of chronic obstructive pulmonary disease: Primary care diagnostic technology update. Br J Gen Pract 2011;61:698–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- [14].Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L.. Forced spirometry reference values for Norwegian adults: The Bronchial Obstruction in Nord-Trondelag Study. Eur Respir J 2001;18:770–9. [DOI] [PubMed] [Google Scholar]
- [15].Lamberts H, Wood M.. The birth of the International Classification of Primary Care (ICPC): Serendipity at the border of Lac Leman. Fam Pract 2002;19:433–5. [DOI] [PubMed] [Google Scholar]
- [16].Yang L, Zhou M, Smith M, Yang G, Peto R, Wang J, et al. Body mass index and chronic obstructive pulmonary disease-related mortality: A nationally representative prospective study of 220,000 men in China. Int J Epidemiol 2010;39:1027–36. [DOI] [PubMed] [Google Scholar]
- [17].Melbye H, Drivenes E, Dalbak LG, Leinan T, Hoegh-Henrichsen S, Ostrem A.. Asthma, chronic obstructive pulmonary disease, or both? Diagnostic labeling and spirometry in primary care patients aged 40 years or more. Int J Chron Obstruct Pulmon Dis 2011;6:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haugan T, Bakken IJ, Storro O, Oien T, Langhammer A.. Utvikling i diagnostisering og helsetjenesteforbruk ved obstruktiv lungesykdom [Utilization of diagnostic tools and health care services for obstructive lung disease]. Tidsskr Nor Laegeforen 2008;128:2431–4. [PubMed] [Google Scholar]
- [19].Guerra S. Asthma and chronic obstructive pulmonary disease. Curr Opin Allergy Clin Immunol 2009;9:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jensen LA, Onyskiw JE, Prasad NGN.. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart & Lung: J Acute Crit Care 1998;27:387–408. [DOI] [PubMed] [Google Scholar]
- [21].Hakverdioglu Yont G, Akin Korhan E, Dizer B.. The effect of nail polish on pulse oximetry readings. Intensive Crit Care Nurs 2014;30:111–15. [DOI] [PubMed] [Google Scholar]
- [22].Vold ML, Aasebo U, Hjalmarsen A, Melbye H.. Predictors of oxygen saturation </=95% in a cross-sectional population based survey. Respir Med 2012;106:1551–8. [DOI] [PubMed] [Google Scholar]
- [23].Hardie JA, Vollmer WM, Buist AS, Ellingsen I, Morkve O.. Reference values for arterial blood gases in the elderly. Chest 2004;125:2053–60. [DOI] [PubMed] [Google Scholar]
- [24].Van den Bemt L, Smeele IJ, Kolkman M, Grol R, van Weel C, Schermer TR.. Low body mass index, airflow obstruction, and dyspnoea in a primary care COPD patient population. Prim Care Respir J 2010;19:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Masip J, Gaya M, Paez J, Betbese A, Vecilla F, Manresa R, et al. Pulse oximetry in the diagnosis of acute heart failure. Revista espanola de cardiologia (English ed) 2012; 65:879–84. [DOI] [PubMed] [Google Scholar]
- [26].Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA.. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax 2008;63:599–605. [DOI] [PubMed] [Google Scholar]
- [27].Fabbri LM, Luppi F, Beghe B, Rabe KF.. Complex chronic comorbidities of COPD. Eur Respir J 2008;31:204–12. [DOI] [PubMed] [Google Scholar]
- [28].Brekke PH, Omland T, Smith P, Soyseth V.. Underdiagnosis of myocardial infarction in COPD: Cardiac Infarction Injury Score (CIIS) in patients hospitalised for COPD exacerbation. Respir Med 2008;102:1243–7. [DOI] [PubMed] [Google Scholar]