Abstract
Liver metastatic disease is the main cause of death in colorectal cancer (CRC) patients. During metastatic spread of the disease an imbalance in the oxidative stress and inflammation plays a crucial role in tumor progression. In order to improve the efficacy of current therapies, new complementary therapeutic approaches are being analyzed including biologically active compounds with low side effects. The anti-inflammatory and anti-oxidant properties of Ocoxin® oral solution (OOS) prompt us to analyze its effect on the metastatic development of CRC to the liver. First, in vitro effect of OOS in tumor cell viability and migration was analyzed. Second, in vivo effect of different dosage patterns and concentrations in the development of hepatic metastasis was analyzed by intrasplenic inoculation of C26 colon carcinoma cells in Balb/c mice. Third, the expression of alpha smooth muscle actin, caspase-3 and Ki-67 expression was quantified by immunohistochemistry, then gene expression levels of inflammatory factors were measured by quantitative RT-PCR. According to our results, OOS reduced tumor cell viability and migration in vitro. Moreover, in vivo daily administration of OOS from the 7th day after tumor cell inoculation decreased the total area and size of metastatic foci in the liver. Furthermore, cell proliferation and fibroblast recruitment was decreased in tumor foci while a higher number of apoptotic cells were observed. Finally, RNA levels for the inflammatory mediators COX-2, IFNγ, IL1β, IL6 and TNFα were reduced in total liver. In conclusion, OOS reduced the metastatic development of colorectal cancer to the liver by increasing apoptosis, and decreasing tumor cell proliferation and fibroblast recruitment in the tumor foci, as well as the expression of inflammatory mediators in total liver. These results point out OOS as a potential supplement to be applied as complementary therapy for the treatment of liver metastasis from colorectal cancer.
Keywords: ocoxin oral solution, nutrient mixture, colorectal cancer, liver, metastasis
Introduction
Colorectal cancer (CRC) is one of the most aggressive cancers in the western countries. Despite the development of new treatments, patients suffering from this disease die of the spread of the primary cancer to other organs, mainly the liver, rather than from the primary tumor. To date, the only effective treatment is hepatic resection, and thus, a novel and effective therapies are urgently needed to treat metastatic disease, improving life expectancy and quality in CRC patients. Several studies have included biologically active compounds due to their low toxicity and excellent potential for cancer treatment.
The relationship between inflammation and tumor development is widely accepted (1). During the progression of cancer inflammatory cells are recruited into the tumor stroma by signals derived from the tumor, as well as from the cells residing in the organ that is being colonized. This inflammatory milieu results in the trigger for oxidative stress. In this scenery, the presence of oxidative and inflammatory factors play a crucial role regulating the expression of genes involved in tumor progression and metastasis (2).
This off balance in the microenvironment of the developing tumor affects the major cell types of the host organ in the tumor, namely, the cancer associated fibroblasts (CAFs) (3). The CAFs contribute to this pro-tumoral microenvironment by further producing pro-inflammatory and pro-angiogenic factors favoring the growth of the tumor, invasion of the targeted organ, angiogenesis and finally metastasis (3).
This inflammatory and oxidative millieu has encouraged the validation of several nutritional and biological components with anti-inflammatory and anti-oxidative properties, as complements for antitumor therapies. Among others, extracts from green tea and licorice, and vitamins has been reported to possess immunomodulatory, anti-inflammatory and anti-oxidant properties which make them excellent candidates for the validation of their anti-tumorigenic and anti-metastatic effects in different types of cancer (4–7). Moreover, the administration of several of these compounds as nutrient mixtures has shown greater efficacy than their administration as individual treatments (8,9).
OOS is a nutritional supplement with recognized anti-oxidant, anti-inflammatory and immunomodulatory properties. The solution is composed, among others, of green tea extract, glyzyrrhicic acid, vitamin C, B6 and B12, minerals and aminoacids. It was synthesized by combining two products, Viusid® and Ocoxin®. The Viusid component has been tested in different clinical trials showing beneficial effects in patients suffering from chronic hepatitis C and cirrhosis (10,11). Moreover, OOS antitumor effects have validated in vitro and in vivo preclinical breast cancer models (12). Thus, we aim to study the effects of OOS in the metastatic progression of CRC to the liver.
In the present study, we demonstrate the inhibitory effect OOS in vitro and an in vivo pre-clinical model of metastatic development of colorectal carcinoma to the liver. We show that OOS exerts its effect by reducing tumor cell proliferation and by increasing apoptosis in vivo. Furthermore, this nutritional supplement shows not only a reduction in the fibroblast recruitment to the tumor stroma, which has been related to angiogenesis and tumor progression (13), but also to a modulation of the inflammatory signature in the liver. Thus, OOS might be a novel and effective complementary treatment which properties might help to increase the efficacy of actual anti-metastatic therapies as well as to improve the life-quality of patients suffering from this disease.
Materials and methods
Animals
Balb/c mice (male, 8-weeks old) were obtained from Charles River Laboratories España S.A. (Barcelona, Spain). The animals were fed a standard chow and had access to water ad libitum. All the proceedings were approved by the Basque Country university ethics Committee for experimental Animal with the reference number CEEA/357/2014/ARTETA RUIZ, in accordance with the institutional, national and international guidelines regarding the protection and care of animal use for scientific purposes.
Cell line
The murine colon cancer C26 cells, syngenic with Balb/c mice (ATCC, LGC Standards S.L.U. Barcelona, Spain) were used. Cells were grown under standard conditions in RPMI-1640 medium (Life Technologies, Madrid, Spain) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (100 µg/ml) and amphotericin B (0.25 µg/ml) all purchased from Life Technologies.
Viability and cell cycle assays
PrestoBlue® reagent (Life Technologies) was used for quantification of tumor cell viability following the manufacturer's instructions. The C26 cells were cultured on collagen (Sigma-Aldrich, St. Louis, MO, USA) precoated culture plates at a concentration of 50,000 cells/ml in RPMI-1640 supplemented with 0.5% FBS and antibiotics-antimycotics. After 24-h incubation in the presence of different OOS concentrations ranging from 0 to a maximum of 1:200 (V/Vf) PrestoBlue was added for 2 h. Finally, absorbance was measured with Fluoroskan Ascen (Thermo Labsystems, Waltham, MA, USA). Results were calculated as the average of three independent experiments. For cell cycle analyses, cells were fixed after 72-h incubation in the presence of OOS (1/100; V/Vf) and DNA stained with propidium iodide (PI; Life Technologies) for 30 min. Then, cells were analyzed by flow cytometry in a Beckman Coulter Gallios™ (Beckman Coulter Inc., Barcelona, Spain). The number of cells in each cell cycle phase was quantified by means of weasel software.
In vitro migration assay
The migration assay was carried out on modified Boyden chambers. Briefly, tumor cells, at a concentration of 2×105 cells/ml were cultured onto 8 µm-diameter pore membrane (Greiner Bio-One, La Jolla, CA, USA) precoated with collagen type I 10 µg/ml (Sigma-Aldrich). Tumor cells were allowed to migrate for 18 h in the presence of OOS (1:100 V/Vf) in culture medium supplemented with 0.5% FBS. Migrated cells were quantified after 4% formalin fixation and crystal violet staining (Sigma-Aldrich). Results were calculated from three independent experiments and data were expressed as the mean of total migrated cells per membrane.
Experimental development of hepatic metastasis
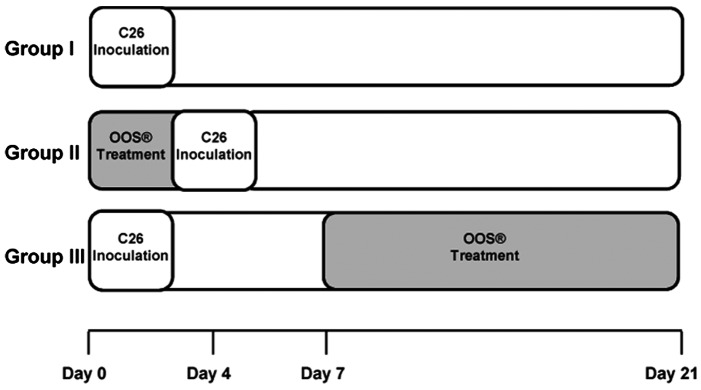
In vivo metastasis assay was carried out by intrasplenical (i.s.) inoculation of tumor cells. The cells (2×105) were inoculated in the inferior pole of the spleen under anesthesia. The animals were treated with 100 µl of OOS and divided into 3 groups based on administration pattern as follows: in group I, animals received no treatment; in group II, mice were treated with OOS 4 days prior to tumor cell inoculation; and in group III, mice were treated with OOS from the 7th day after tumor cell inoculation until the day of sacrifice (Fig. 1). As control for tumor development at the time of first doses of OOS in group III three untreated animals were sacrificed the 7th day after tumor cell inoculation. Animals from groups I, II and III were sacrificed 21 days after tumor cell inoculation, and livers were collected, fixed in zinc solution (Sigma-Aldrich) and paraffin embedded for histological analyses after H&E staining. Tumor occupied area was quantified in three 7 µm-thick sections per liver, separated 500 µm from each other and calculated as the area occupied by tumor foci per section of liver tissue. At least 6 mice per group were used per each experiment. Once the most effective administration pattern was chosen by treating mice with 100 µl of OOS, the effectiveness of a lower OOS concentration (50 µl) was tested under the same experimental conditions.
Figure 1.
Diagram of the experimental animal groups according to OOS dosage pattern. Animals were divided into 3 experimental groups as follows: group I, OOS untreated group; group II, mice treated with OOS 4 days prior to tumor cell inoculation; group III, mice treated with OOS from the 7th day after tumor cell inoculation. All treated groups were administered a daily dose during the period of treatment.
Immunohistological analyses of liver tissue
Liver tissue was analyzed for the expression of Ki-67 (ab16667; 1:100; Abcam, Cambridge, UK) and caspase-3 (ab4051; 1:100; Abcam) by immunohistology. Mice from group I and III were sacrificed and livers were collected for immunohistological analyses in 7 µm tissue sections by staining with specific antibodies. Additionally, the expression of α-smooth muscle actin (ASMA) (MCA5781GA; 1:100; AbD Serotec, Raeigh, NC, USA) was also quantified. Antigen retrieval was carried out in citrate buffer pH 6.0, then endogenous peroxidase and inspecific proteins were blocked by incubating for 40 min with 3% of H2O2 and 40 min with 3% FBS. Finally, liver tissue was incubated with specific antibodies and antigen expression was revealed by horseradish peroxidase (HRP)-conjugated streptavidin (Life Technologies) and 2-Solution DAB kit (Life Technologies) following the manufacturer's instructions. Antigen expression levels were quantified by ImageJ software (NIH, Bethesda, MD, USA). Results were expressed as the mean of at least 6 liver sections for each treatment.
RT-qPCR
Total RNA was extracted from paraffin embedded liver tissues collected from mice treated with 100 µl of OOS according to group I and III dosage pattern using Norgen FFPE RNA Purification kit (Norgen Biotek Corp., Thorold, ON, Canada) following the manufacturer's instructions. RNA concentration and quality was assessed by NanoDrop spectrophotometer (ND-1000; Thermo Fisher Scientific Inc., Rockford, IL, USA), and 2 µg RNA was reverse transcribed into cDNA with recombinant moloney murine leukemia virus reverse transcriptase and random primers (Life Technologies). Quantification of cDNA template was performed with real-time polymerase chain reaction (PCR) using SYBR-Green (Life Technologies) as a fluorophore in ABI 7900HT (Life Technologies). PCR primers (Life Technologies) were as follows: GAPDH (housekeepeing) F, GTATGACTCCACTCACGGCAA and R, CTTCCCATTCTCGGCCTTG; IL1β F, CTGTGACTCATGGGATGATGATG and R, GCCTGTAGTGCAGTTGTCTAAT; INFγ F, TTCTTCAGCAACAGCAAGGC and R, TGTGGGTTGTTGACCCTCAAA; COX2 F, TGCACTATGGTTACAAAAGCTGG and R, TCGGAAGCTCCTTATTTCCCTT; TNFα F, CCAGTGTGGGAAGTGTCTT and R, AAGCAAAAGAGGAGGCAACA; IL6 F, TCTATACCACTTCACAAGTCGGA and R, GAATTGCCATTGCACAACTCTTT. Relative expression of target genes was normalized to the internal control gene GAPDH by the ΔΔCt method. Data were generated by the use of specific software (ABI Prism, SDS2.0; life Technologies) after normalization. Results were calculated at least from 6 livers per treatment and from two independent experiments.
Statistical analysis
Statistical analysis was performed with the Student's two-tailed unpaired t-test. Data are expressed as the mean ± SD. The criterion for significance was P<0.05 for all comparisons.
Results
OOS reduced the viability of C26 cell line in vitro
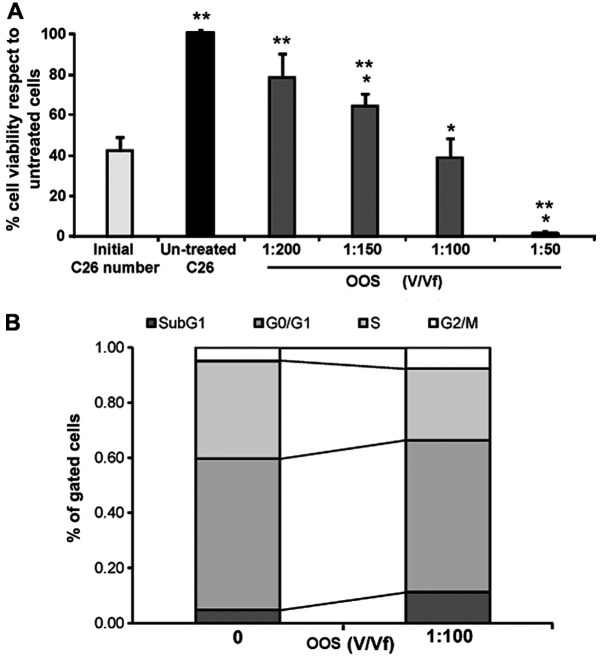
In order to test the effect of OOS in the viability of colon carcinoma cells, C26 cells were treated with increasing concentrations from 0 to 1:200 (V/Vf) of OOS for 24 h. As shown in Fig. 2A, the viability of tumor cells decreased in a dose-dependent manner from 20% at 1:200 (V/Vf) of OOS to >90% at 1:50 (V/Vf) (Fig. 2A) in respect to untreated C26 cells. However, when compared to the initial cell number, control untreated cells and every treatment shows viability differences but 1:100 (V/Vf) resulted in a significant non-proliferation.
Figure 2.
OOS reduces in vitro viability of C26 cells. (A) The viability of C26 cells was tested after 24-h incubation in the presence of OOS. C26 cells were treated with increasing concentrations of OOS ranging from 1:200 to 1:50 during 24 h before viability quantification and compared to the viability of initially cultures cells (light grey) and to that of untreated cells (black). Differences in the viability of treated cells vs. Untreated cells (*) and vs. initially cultured cells (**) were considered to be statistically significant at P<0.05. (B) The cell number in each cell phase respect to total gated cell number was analyzed after PI staining by flow cytometry after 72-h incubation with 1:100 OOS (V/Vf). Differences between treated and untreated cells were considered statistically significant at *P<0.05.
Since the only concentration which inhibited completely the proliferation of C26 cells was 1:100 OOS (V/Vf), we next, analyzed the amount of cells in each phase of the cell cycle by means of PI staining after the cuture for 72 h in the presence of OOS. Then, the cells were analyzed by flow cytometry. As seen in Fig. 2B, the number of cells in phase G2/M and in the peak Sub G1 were increased while the cell number ongoing DNA replication in phase S showed a significant decrease compared to those cells cultured under basal conditions.
In vitro migratory potential of C26 cells is reduced by OOS
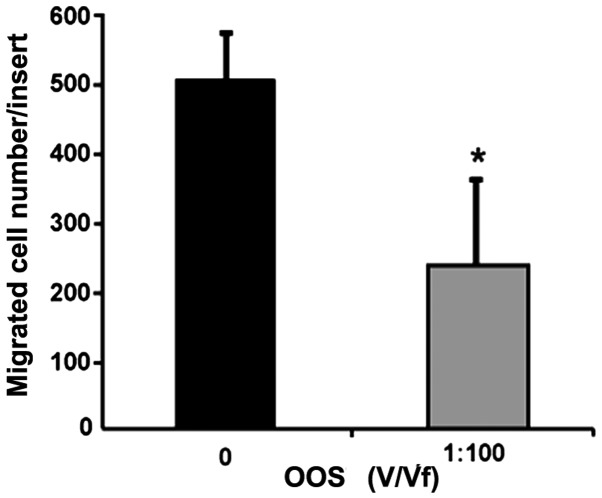
To assess the effect of OOS in the migratory potential of C26 cells through a collagen type I layer, we incubated C26 cells on top of collagen type I-covered 8 µm-diameter pore membranes. The C26 cells were allowed to migrate for 18-h in the presence of 1:100 of OOS (V/Vf). The amount of migrated tumor cells was decreased by 50% in presence of 1:100 of OOS (V/Vf) (Fig. 3).
Figure 3.
OOS reduces in vitro migration of C26 cells. C26 cells were cultured on top of 8 µm-pore membrane modified Boyden chambers. The number of migrated C26 cells were quantified after 18-h incubation in the presence of 1:100 OOS (V/Vf) Data are mean values ± SD from three different experiments. Differences were considered statistically significant at *P<0.05.
Effects of administration pattern in OOS efficacy during the development of metastasis to the liver in vivo
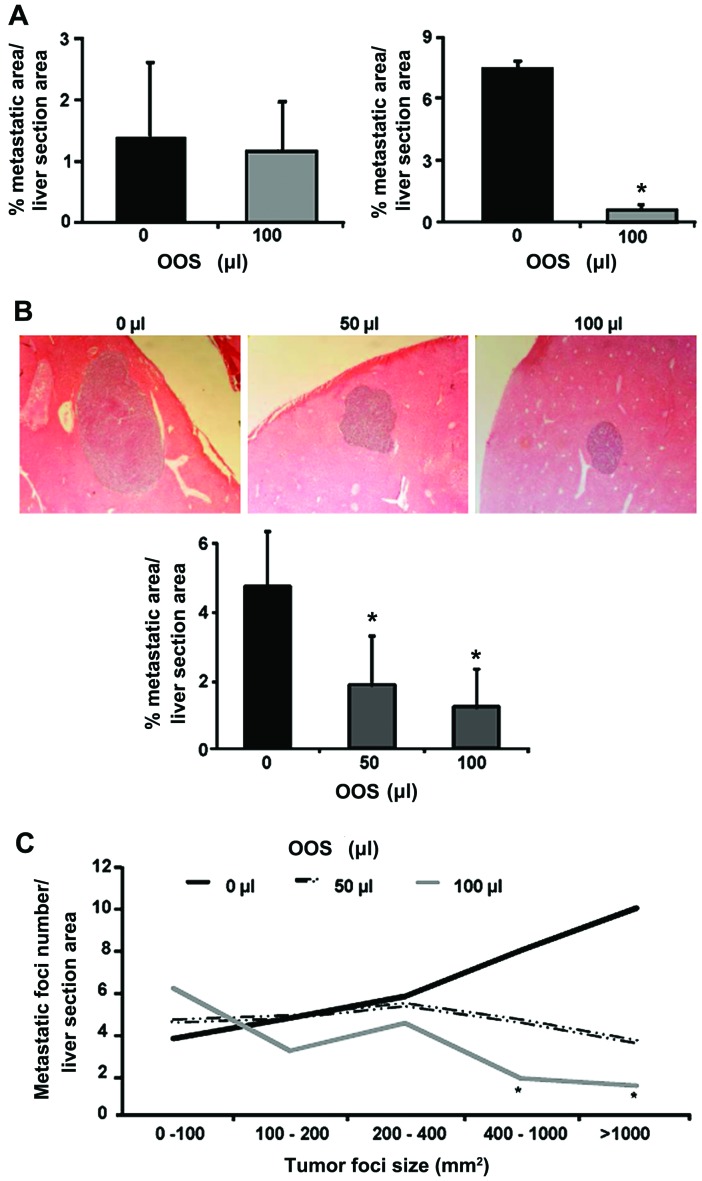
Next, the more efficient dosage pattern of OOS was assessed. The tumor cells were inoculated i.s. and 100 µl of OOS was administered once a day under the schedule described in Materials and methods (Fig. 1), until sacrifice. Under these dosage protocols, the weight of liver and spleen did not show any change (data not shown) among the experimental groups. The nutrient mixture only exerted significant effects in those animals included in group III (Fig. 4A), while no effect was detected in the preventive administration of OOS (group II) on the tumor burden in the liver when compared to untreated group I (Fig. 4A). Even though, the metastatic development was decreased at day 21 after tumor cell inoculation in mice treated with OOS from day 7, the progression was not inhibited but slowed down, since at day 7 after tumor cell inoculation only few micrometastasis could be observed (data not shown).
Figure 4.
OOS slows down in vivo tumor growth. (A) C26 cells were i.s. inoculated and mice were treated with 100 µl of OOS under the administration patterns described in Materials and methods. Group I, untreated; group II, OOS administered four days prior to tumor cell inoculation; group III, OOS administered from day 7th after tumor cell inoculation until sacrifice. (B) Mice were treated with 50 and 100 µl of OOS under administration pattern of group III. (C) The amount of liver foci developed in the liver of mice untreated or treated with 50 and 100 µl of OOS was quantified in liver tissue based on tumor foci size. Image original magnification, ×20. Differences were considered statistically significant at *P<0.05.
Dose-dependent effect of OOS in the metastasic development to the liver in vivo
Once the most effective pattern of administration was established, a lower dose of OOS efficacy was tested under these circumstances. Mice were treated with 50 and 100 µl of OOS following the administration pattern of group III. After the collection of livers and processing for histological analyses the quantification of liver area occupied by tumor foci were carried out. The results show, a reduction of >50% in the area of liver tissue occupied by the tumor in those livers collected from 50 and 100 µl-treated mice (Fig. 4B). Even more, after the classification and quantification of the tumor foci by their size, an decrease in the number of metastatic foci larger than 400 mm2 of diameter were detect in mice treated with 50 and 100 µl OOS compared to those observed in the livers of untreated mice. Indeed, this difference was only significant in livers collected from the animals treated with 100 µl of OOS (Fig. 4C). However, no significant difference was observed between the treatments.
OOS affects tumor proliferation and apoptosis in vivo
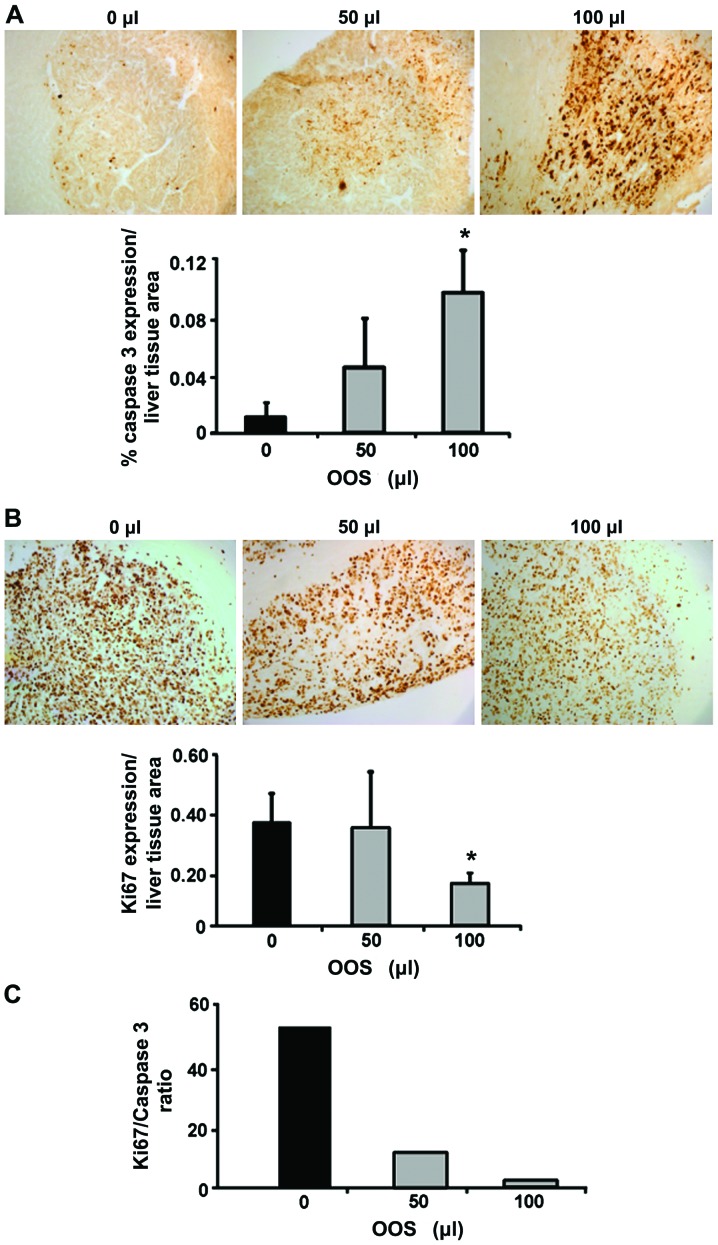
To quantify the expression levels for Ki-67 and caspase-3, livers were collected either from untreated C26-bearing mice and from C26-bearing mice treated with 50 or 100 µl of OOS from day 7 since this dosage pattern was the only one showing any kind of effectiveness. The livers were fixed and embedded in paraffin and the expression levels of Ki-67 and caspase-3 were analyzed by immunohistochemistry. As shown in Fig. 5A while the levels of caspase-3 expression were increased 10-fold after 100 µl OOS administration compared to the untreated group. No significant changes were observed in the animals treated with 50 µl of OOS. Furthermore, the levels of Ki-67 showed a decrease in the tumor foci developed in the livers of mice treated with 100 µl OOS compared to those observed in the livers from untreated mice (Fig. 5B). However, the difference was not observed in the livers collected from mice treated with 50 µl of OOS. Even more, the ratio between Ki-67 and caspase-3 expression was reduced in mice injected with C26 cells and treated with both concentrations of OOS vs. Untreated mice (Fig. 5C).
Figure 5.
OOS affects proliferation and apoptosis in vivo. The effect of OOS on Ki-67 and caspase-3 expression in livers from tumor-bearing mice was analyzed by immunohistochemistry. (A) Caspase-3 expression (brown) was quantified in livers collected from untreated, 50 and 100 µl OOS treated C26-bearing mice as the percentage of the area positive for caspase-3 expression respect to total liver section area. (B) Ki-67 expression (brown) was quantified in livers collected from untreated, 50 and 100 µl OOS treated C26-bearing mice as the percentage of the area positive for Ki-67 expression in respect to total liver section area. (C) The ratio between Ki-67 and caspase-3 was calculated as the expression of Ki-67 relative to caspase-3 expression. Image original magnification, ×20. Differences were considered statistically significant at *P<0.05.
OOS treatment limits the infiltration of myofibroblasts in the metastatic liver
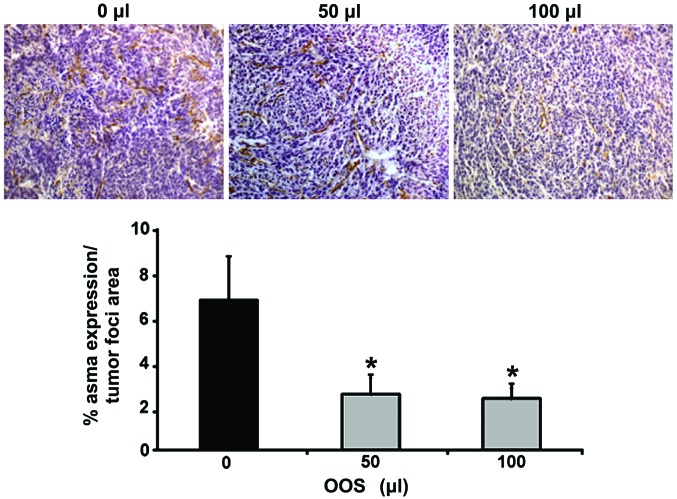
Finally, the expression of ASMA was quantified in the metastatic liver tissue in order to analyze the infiltration by liver cancer-associated fibroblasts within the tumor foci. Sections from those livers collected either from untreated C26-bearing mice (group I) or from C26-bearing mice treated with 50 or 100 µl of OOS following the dosage pattern for group III were stained with specific antibodies for ASMA, an antigen expressed in CAFs. The intratumoral levels of ASMA expression in liver tissue collected from mice treated with 50 and 100 µl of OOS was reduced by 50% compared to the liver tumors obtained from untreated mice (Fig. 6).
Figure 6.
In vivo HSC infiltration in the tumor is reduced by OOS. Expression levels of ASMA were analyzed in liver tissue by immunohistochemistry. ASMA was stained with specific antibodies in liver tissue collected from untreated, 50 and 100 µl OOS treated mice. Data are calculated as % of ASMA expression per tumor foci area. Image original magnification, ×20. Differences were considered statistically significant at *P<0.05.
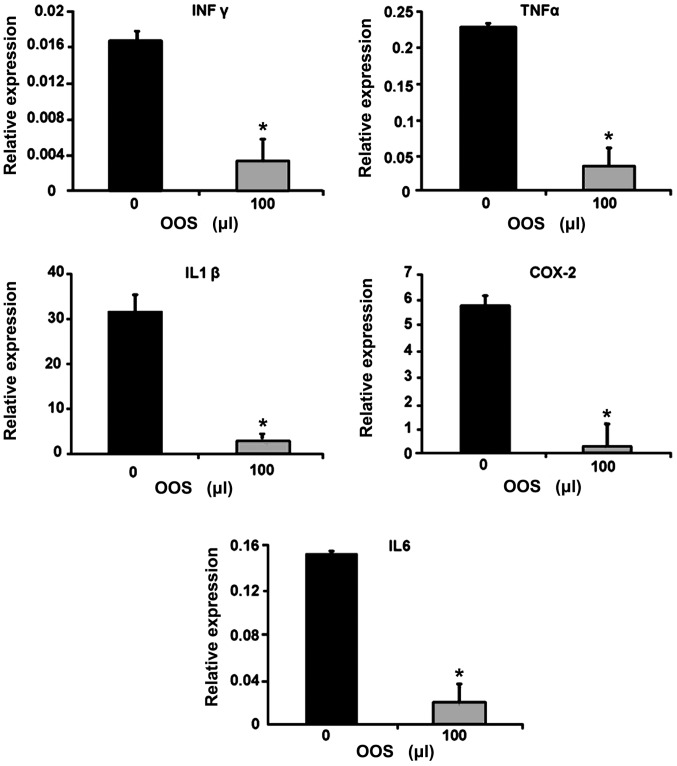
OOS modulates the expression of inflammatory genes in metastatic livers
Since the tumor microenvironment is characterized by an inflammatory signature, the gene expression of the inflammatory molecules IFNγ, TNFα, COX-2, IL6 and IL1β was quantified in total liver tissue by qPCR. As shown in Fig. 7, the expression of genes coding for the aforementioned inflammatory molecules was significantly reduced in the livers collected from mice treated with 100 µl of OOS according to group III compared to those obtained from untreated mice (Fig. 7).
Figure 7.
OOS affects inflammatory gene expression in total liver. Gene expression of IFNγ, TNFα, IL1β, IL6 and COX-2 was analyzed by RT-PCR. Total RNA was extracted from paraffin-embedded liver tissue collected from untreated or 100 µl OOS treated C26-bearing mice. Data are expressed as the mean values ± SD from six livers from two independent experiments. Differences were considered statistically significant at *P<0.05.
Discussion
CRC is one of the leading causes of cancer-related deaths in the world, due mainly to the metastatic spread to distant organs, specially the liver. Even though great advances have been made in the development of therapies to treat CRC, they are often aggressive and with limited efficacy. Thus, new complementary therapies are being developed consisting in biological compound mixtures. Certain nutrient mixtures have been proved to be effective in several preclinical in vivo and in vitro models such as pulmonary metastasis of melanoma and cervical cancer (8,14). However, there is no report describing the efficacy of these nutrient mixtures in the in vivo metastatic spread of CRC to the liver. Thus, in the present study, we aimed to investigate the effectiveness of OOS, a complex mixture containing licorice extract, vitamins and minerals which have proven anti-oxidant and anti-inflammatory properties in other diseases (15,16) in the metastatic development of colon carcinoma cells. Here, we highlight the anti-metastatic effect of this nutrient mixture in vivo by means of reducing tumor cell proliferation, migration and recruitment of CAFs, along with an increase in apoptosis. Additionally, the mixture induced a decrease in the expression of RNA levels for pro-inflammatory and pro-angiogenic factors in metastasized liver provoking a slow down of the metastatic development of colorectal cancer C26 cells to the liver. Thus, these results support the need for further studies for the use of OOS as a nutritional complement during the treatment of colorectal cancer liver metastatic disease.
According to our results, the effect of OOS on the metastatic progression of C26 cells to the liver depends on the dosage pattern. Because a significant reduction in the tumor burden of the liver was observed only in the mice treated with OOS 7 days after tumor cell inoculation, but not when the solution was administered days before the tumor inoculation. Lode et al (17) have reported the inefficacy of a nutrient mixture against metastasis in a neuroblastoma model. However, as stated by Niedzwiecki (18) these observations might be due to several reasons. On the one hand, the excessive amount of cells inoculated by the authors or the need for longer and continuous periods of treatment may account for this lack of effect. On the other hand, antioxidants have been proved to exert their functions mainly in the presence of chronic inflammation and in a highly hypoxic environment (1). In the absence of a highly inflammatory and pro-oxidant microenvironment biologically active compounds might lack their anti-tumoral activity (19), such as the one in mice before the tumor inoculation. Thus, this could explain the lack of effect of OOS in the early stage treatment. Even so, further studies are needed to fully establish the mechanisms of OOS when it is administered at early stages.
Dietary agents are believed to suppress, among others, the hyper-proliferative processes during the development of a tumor. Several phytochemicals have been shown to exert suppressive effects on AP-1 and NF-κB interfering with growth and proliferative signals (20,21). In accordance to this, supplementation with dietary nutrient mixture OOS significantly slows down murine colon carcinoma C26 tumor growth in vivo in immune competent mice related to a decrease in the intratumoral expression of Ki-67, an antigen strictly associated with cell proliferation. The in vitro studies supported these findings since the viability of tumor cells was significantly decreased and a slight reduction in the number of cells in phase S was induced by OOS treatment. Along with these results, an increase in the amount of cells in phase G2/M was observed. In fact, cathechins and licorice components cause cell cycle arrest by downregulating cyclin D1 and E, key molecules in G1 to S transition and completion of the latter one (22,23). Ultimately, the cell cycle arrest will trigger cell cycle deregulation and apoptotic processes via upregulation of p21 and p27, both well-known tumor suppressor proteins downregulated in numerous cancers (24). In addition to the effect in the cell cycle progression, OOS breaks the characteristic resistance of tumor cells to enter apoptosis. Consistent with these observations, an increase in cell number in subG1 in vitro and the intratumoral increase of caspase-3 in vivo after treatment with the complex nutrient mixture points to the induction of programmed cell death within tumor foci by this compound. The hypoxic and inflammatory tumor microenvironment induces the activation of anti-apoptotic pathways supporting the effect of anti-inflammatory or anti-oxidative drugs which have been shown to increase the sensitivity of tumor cells to pro-apoptotic signals (25). In addition to its effects in tumor cell proliferation and apoptosis, OOS reduced the migratory potential of C26 colon carcinoma cells in vitro. Migration of tumor cells is a key step not only for invasion of adjacent tissue in the primary organ but also for extravasation and colonization of the secondary target organ during the metastasic process. It is well-known that chronic inflammation is linked to oxidative stress and both of them are related to the invasive and migratory potential of cancer cells during tumor progression (1). Thus, the migratory capacity of tumor cells is also a potential target for nutrient mixtures with anti-inflammatory and anti-oxidant properties. Roomi et al (9,14) have shown that in vitro, a nutrient mixture consisting in lysine, ascorbic acid, proline and green tea, among others, significantly reduced the migratory potential of a selected set of different tumor cell types related to a decrease in the levels of pro-migratory and pro-angiogenic factors such as proteases and VEGF (9,14).
In the hepatic tumor microenvironment, myofibroblasts are the most prominent CAFs. In the liver, CAFs are mainly originated from transdifferentiated hepatic stellate cells in the tumor stroma, and such tumor activated-hepatic stellate cells promote tumor growth and invasiveness (26). Additionally, they contribute not only to create a pro-tumoral stroma but also to trigger the angiogenic switch allowing one step forward from an avascular to a vascular state during the metastasic tumor growth (26). Furthermore, these tumor associated fibroblasts participate actively in the production of pro-inflammatory and pro-oxidant factors taking active part in tumor expansion by inducing, among others, the trigering of an angiogenic response. Moreover, recruited fibroblasts within a tumor have been positively correlated with tumor progression and poor prognosis in colorectal cancer (27). In our model, the nutrient mixture OOS reduced the recruitment of myofibroblasts within the tumor foci, as shown by a decreased amount of ASMA expressing cells within the tumor foci which might also account for the reduced angiogenesis. This reduction in myofibroblast infiltration in vivo was related with an impaired migratory potential of 3T3 fibroblast in vitro by OOS (data not shown). The impaired recruitment of CAFs might account as an additional mechanism by which the nutrient mixture might exert its antitumoral effects. Indeed, several compounds with anti-oxidant and anti-inflammatory properties reduce the angiogenic response in different types of tumors and show a significant impact in the tumor stroma formation (28).
The antioxidant properties of several components of OOS, such as polyphenols, cannot fully explain their antitumoral activity. Cathechins modulate inflammatory pathways since they act as signaling agents by interfering with NF-κB and AP-1 which, in turn, results in inhibition of pro-inflammatory and pro-angiogenic factors, including IL6, IL-1β, TNF-α and VEGF (29). Among them, TNFα or IL-1β induce the expression of growth factors, stimulate epithelial tumor motility and tumor angiogenesis (30,31). Another proinflammatory molecule commonly upregulated in colon cancer, COX-2, has been implicated in the growth and progression of colorectal cancer via multiple pathways (32–34) including the acquisition of resistance to apoptosis and promoting angiogenesis through VEGF production (35,36). Moreover, a decrease in COX-2 expression at RNA levels in total liver caused by OOS administration might be related to a reduction in VEGF levels (36). Additionally, the reduction in Il-1β, and TNFα RNA levels after OOS administration is consistent with the downregulation in the levels of COX-2 RNA which is commonly stimulated by cytokines such as Il-1β and TNFα as a result of the interaction between colon carcinoma cells and the cells of the liver microenvironment (36,37).
In summary, OOS slows down the metastatic progression of CRC to the liver. Thus, the anti-oxidant and anti-inflammatory properties of this nutrient mixture induced an inhibition of the proliferative and migratory potential of tumor cells which together with an increase in the sensitivity to apoptotic signals might modulate the metastatic development of colorectal cancer cells to the liver. Besides, OOS limits tumor infiltration by CAFs and inhibits the production of inflammatory and angiogenic factors within the tumor microenvironment. Collectively, this creates an unfavorable and non-permissive microenvironment for tumor growth suppressing the final steps of tumor progression. Therefore, OOS may constitute a pharmacologically safe complementary compound for the treatment of cancer and its metastasis slowing down the tumor growth, and, thus, increasing the life time and quality for patients suffering from CRC liver metastasis. The above justifies further characterization and validation of OOS in the metastatic development and their combination with actual therapies.
Acknowledgments
We thank Evangelina Garcia and Maria Jesus Fernandez for the excellent technical assistance. Additionally, we greatly appreciate the support of the Genomics and Proteomics Unit, the Analytical and High-Resolution Microscopy Unit and the Animal Facilities from the Advance Research Facilities (SGIker) of the University of Basque Country.
References
- 1.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamlouk S, Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer. 2013;132:2721–2729. doi: 10.1002/ijc.27901. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, Wang Z. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303:47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Shankar S, Suthakar G, Srivastava RK. Epigallocatechin-3-gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Front Biosci. 2007;12:5039–5051. doi: 10.2741/2446. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg ED, Amosova EN, Zueva EP, Razina TG, Krylova SG, Zorikov PS. Licorice preparations improve efficiency of chemotherapy and surgical treatment of transplanted tumors. Bull Exp Biol Med. 2008;145:252–255. doi: 10.1007/s10517-008-0063-0. [DOI] [PubMed] [Google Scholar]
- 6.Ma YC, Li C, Gao F, Xu Y, Jiang ZB, Liu JX, Jin LY. Epigallocatechin gallate inhibits the growth of human lung cancer by directly targeting the EGFR signaling pathway. Oncol Rep. 2014;31:1343–1349. doi: 10.3892/or.2013.2933. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama T, Murata S, Nakayama K, Sano N, Ogawa K, Nowatari T, Tamura T, Nozaki R, Fukunaga K, Ohkohchi N. (−)-Epigallocatechin-3-gallate suppresses liver metastasis of human colorectal cancer. Oncol Rep. 2014;31:625–633. doi: 10.3892/or.2013.2925. [DOI] [PubMed] [Google Scholar]
- 8.Roomi MW, Roomi N, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Inhibition of pulmonary metastasis of melanoma b16fo cells in C57BL/6 mice by a nutrient mixture consisting of ascorbic Acid, lysine, proline, arginine, and green tea extract. Exp Lung Res. 2006;32:517–530. doi: 10.1080/01902140601098552. [DOI] [PubMed] [Google Scholar]
- 9.Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Modulation of uPA, MMPs and their inhibitors by a novel nutrient mixture in human colorectal, pancreatic and hepatic carcinoma cell lines. Int J Oncol. 2015;47:370–376. doi: 10.3892/ijo.2015.3008. [DOI] [PubMed] [Google Scholar]
- 10.Gomez EV, Perez YM, Sanchez HV, Forment GR, Soler EA, Bertot LC, Garcia AY, del Rosario Abreu vazquez M, Fabian LG. Antioxidant and immunomodulatory effects of viusid in patients with chronic hepatitis C. World J Gastroenterol. 2010;16:2638–2647. doi: 10.3748/wjg.v16.i21.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilar Gomez E, Sanchez Rodriguez Y, Torres Gonzalez A, Calzadilla Bertot L, Arus Soler E, Martinez Perez Y, Yasells Garcia A, Abreu Vazquez MR. Viusid, a nutritional supplement, increases survival and reduces disease progression in HCV-related decompensated cirrhosis: A randomised and controlled trial. BMJ Open. 2011;1:e000140. doi: 10.1136/bmjopen-2011-000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández-García S, González V, Sanz E, Pandiella A. Effect of oncoxin oral solution in HER2-overexpressing breast cancer. Nutr Cancer. 2015;67:1159–1169. doi: 10.1080/01635581.2015.1068819. [DOI] [PubMed] [Google Scholar]
- 13.Tommelein J, Verset L, Boterberg T, Demetter P, Bracke M, De Wever O. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front Oncol. 2015;5(63) doi: 10.3389/fonc.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roomi MW, Kalinovsky T, Cha J, Roomi NW, Niedzwiecki A, Rath M. Effects of a nutrient mixture on immunohistochemical localization of cancer markers in human cervical cancer Hela cell tumor xenografts in female nude mice. Exp Ther Med. 2015;9:294–302. doi: 10.3892/etm.2014.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SK, Park KK, Park JH, Lim SS, Chung WY. The inhibitory effect of roasted licorice extract on human metastatic breast cancer cell-induced bone destruction. Phytother Res. 2013;27:1776–1783. doi: 10.1002/ptr.4930. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Yu QY, Zhu ZL, Tang PY, LI K. Vitamin B2 intake and the risk of colorectal cancer: A meta-analysis of observational studies. Asian Pac J Cancer Prev. 2015;16:909–913. doi: 10.7314/APJCP.2015.16.3.909. [DOI] [PubMed] [Google Scholar]
- 17.Lode HN, Huebener N, Strandsby A, Gaedicke G. Nutrient mixture including vitamin C, L-lysine, L-proline, and epigal-locatechin is ineffective against tumor growth and metastasis in a syngeneic neuroblastoma model. Pediatr Blood Cancer. 2008;50:284–288. doi: 10.1002/pbc.21172. [DOI] [PubMed] [Google Scholar]
- 18.Niedzwiecki A. Micronutrient mixture in tumor growth and metastasis. Pediatr Blood Cancer. 2008;50:422–423. 424–425. doi: 10.1002/pbc.21467. author reply. [DOI] [PubMed] [Google Scholar]
- 19.Srividhya R, Jyothilakshmi V, Arulmathi K, Senthilkumaran V, Kalaiselvi P. Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (−)-epigallocatechin-3-gallate. Int J Dev Neurosci. 2008;26:217–223. doi: 10.1016/j.ijdevneu.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Eferl R, Wagner EF. AP-1: A double-edged sword in tumori-genesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 21.Takada Y, Aggarwal BB. Flavopiridol inhibits NF-kappaB activation induced by various carcinogens and inflammatory agents through inhibition of IkappaBalpha kinase and p65 phosphorylation: Abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem. 2004;279:4750–4759. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Hussain T, Mukhtar H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch Biochem Biophys. 2003;410:177–185. doi: 10.1016/S0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 23.Hsia SM, Yu CC, Shih YH, Yuanchien Chen M, Wang TH, Huang YT, Shieh TM. Head Neck. Jan 10, 2015. Isoliquiritigenin as a cause of DNA damage and inhibitor of ataxia-telangiectasia mutated expression leading to G2/M phase arrest and apoptosis in oral squamous cell carcinoma. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Zhang X, Zeng X, Huang Y, Wei JA, Han L, Li CX, Zhang GW. HuR-mediated posttranscriptional regulation of p21 is involved in the effect of Glycyrrhiza uralensis licorice aqueous extract on polyamine-depleted intestinal crypt cells proliferation. J Nutr Biochem. 2012;23:1285–1293. doi: 10.1016/j.jnutbio.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Ahn JH, Cho YJ, Kim HY, Yang YI, Lee KT, Jang DS, Choi JH. α-Terthienylmethanol, isolated from Eclipta prostrata, induces apoptosis by generating reactive oxygen species via NADPH oxidase in human endometrial cancer cells. J Ethnopharmacol. 2015;169:426–434. doi: 10.1016/j.jep.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Olaso E, Salado C, Egilegor E, Gutierrez V, Santisteban A, Sancho-Bru P, Friedman SL, Vidal-Vanaclocha F. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology. 2003;37:674–685. doi: 10.1053/jhep.2003.50068. [DOI] [PubMed] [Google Scholar]
- 27.Berdiel-Acer M, Sanz-Pamplona R, Calon A, Cuadras D, Berenguer A, Sanjuan X, Paules MJ, Salazar R, Moreno V, Batlle E, et al. Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information. Mol Oncol. 2014;8:1290–1305. doi: 10.1016/j.molonc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan LE, Ahamed MB, Majid AS, Baharetha HM, Muslim NS, Nassar ZD, Majid AM. Correlation of antiangiogenic, antioxidant and cytotoxic activities of some Sudanese medicinal plants with phenolic and flavonoid contents. BMC Complement Altern Med. 2014;14(406) doi: 10.1186/1472-6882-14-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fassina G, Venè R, Morini M, Minghelli S, Benelli R, Noonan DM, Albini A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10:4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- 30.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 31.Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, Maemondo M, Hong X, Tazawa R, Kikuchi T, Matsushima K, et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of lewis lung carcinoma by induction of angiogenic factors: In vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469–475. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- 32.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/S0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA. Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res. 2002;62:6323–6328. [PubMed] [Google Scholar]
- 35.Han YD, Hong YK, Kang JG, Choi YJ, Park CH. Relation of the expression of cyclooxygenase-2 in colorectal adenomas and adenocarcinomas to angiogenesis and prognosis. J Korean Soc Coloproctol. 2010;26:339–346. doi: 10.3393/jksc.2010.26.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valcárcel M, Arteta B, Jaureguibeitia A, Lopategi A, Martínez I, Mendoza L, Muruzabal FJ, Salado C, Vidal-Vanaclocha F. Three-dimensional growth as multicellular spheroid activates the proangiogenic phenotype of colorectal carcinoma cells via LFA-1-dependent VEGF: Implications on hepatic micrometastasis. J Transl Med. 2008;6(57) doi: 10.1186/1479-5876-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arteta B, Lasuen N, Lopategi A, Sveinbjörnsson B, Smedsrød B, Vidal-Vanaclocha F. Colon carcinoma cell interaction with liver sinusoidal endothelium inhibits organ-specific antitumor immunity through interleukin-1-induced mannose receptor in mice. Hepatology. 2010;51:2172–2182. doi: 10.1002/hep.23590. [DOI] [PubMed] [Google Scholar]