Abstract
Background
Propolis is a bee product widely used in folk medicine and possessing many pharmacological properties. In this study we aimed to investigate: i) the antiviral activities of Hatay propolis samples against HSV-1 and HSV-2 in HEp-2 cell line, and ii) the presence of the synergistic effects of propolis with acyclovir against these viruses.
Material/Methods
All experiments were carried out in HEp-2 cell cultures. Proliferation assays were performed in 24-well flat bottom microplates. We inoculated 1×105 cells per ml and RPMI 1640 medium with 10% fetal calf serum into each well. Studies to determine cytotoxic effect were performed. To investigate the presence of antiviral activity of propolis samples, different concentrations of propolis (3200, 1600, 800, 400, 200, 100, 75, 50, and 25 μg/mL) were added into the culture medium. The amplifications of HSV-1 and HSV-2 DNA were performed by real-time PCR method. Acyclovir (Sigma, USA) was chosen as a positive control. Cell morphology was evaluated by scanning electron microscopy (SEM).
Results
The replication of HSV-1 and HSV-2 was significantly suppressed in the presence of 25, 50, and 100 μg/mL of Hatay propolis. We found that propolis began to inhibit HSV-1 replication after 24 h of incubation and propolis activity against HSV-2 was found to start at 48 h following incubation. The activity of propolis against both HSV-1 and HSV-2 was confirmed by a significant decrease in the number of viral copies.
Conclusions
We determined that Hatay propolis samples have important antiviral effects compared with acyclovir. In particular, the synergy produced by antiviral activity of propolis and acyclovir combined had a stronger effect against HSV-1 and HSV-2 than acyclovir alone.
MeSH Keywords: Antiviral Agents; DNA Replication; Herpesvirus 1, Human; Herpesvirus 2, Human; Propolis
Background
Natural herbal products have been used in folk medicine for centuries and have been an active source for modern drug production. Natural products have become more valuable than synthetic products due to their lower cytotoxicity [1]. Thousands of studies on herbal extract activities are carried out in relation with drug production in many countries of the world. Today, the number and variety of available drugs used for the treatment of viral infections is limited compared with antibacterial drugs. New antiviral drug research is rapidly growing due to increasing resistance to current antiviral medications. Most antiviral drug research has focused on natural products [2].
One of these natural products on which intensive drug studies have been carried out is propolis. Propolis is a bee product widely used in folk medicine for many years. It has been reported in numerous studies that propolis has many pharmacological properties and biological activities, such as antiinflammatory, antibacterial, antifungal, antioxidant, anticancer, and antiviral effects [3–10].
The pharmacological properties of propolis are related to its chemical composition, which is highly complex and varies from region to region. Phenolics are well known propolis compounds with numerous pharmacological properties, such as antiviral, antibacterial, and antifungal effects. Hatay propolis is reported to be rich in phenolic compounds such as caffeic acid phenethyl ester (CAPE), galangin, chrysin, dimethoxycinnamic acid, and caffeic acid [3,11]. Propolis consists of complex chemical compounds, the most important group being phenolic acid components, which play a role in antiviral activity. It has been reported that constituents such as caffeic acid, p-coumaric acid, benzoic acid, galangin, pinocembrin, and chrysin may be effective against Herpes Simplex Virus in cell culture [12,13].
Herpes simplex virus (HSV) type 1 usually causes orofacial infections, while HSV type 2 causes genital infections. Recently, HSV-1 and HSV-2 have been reported to cause oral and genital lesions due to autoinoculation. These viruses can spread easily and quickly through interpersonal contact and lead to diseases ranging from superficial infections to life-threatening ones. These virus infections can lead to mortality among immunocompromised patients and stillbirths or congenital malformations [14–18].
Herpes simplex virus infections are one of the most common viral infections all over the world and progress as acute or recurrent herpes infections. Herpes simplex viruses may cause a wide range of infections, ranging from moderate ones to serious life-threatening infections especially among immune-suppressed patients [19–24]. Due to recent advances in medicine, prolongation of life in immune-suppressed patients has led to significant increases in the incidence of viral infection. The most potent antiviral drug currently used in treating herpes viruses is acyclovir, which is a nucleoside derivative. Because frequent use of acyclovir leads to the emergence of resistant virus associated with prolonged survival in immunosuppressed patients, acyclovir-resistant herpes virus infection has become common in recent years. Therefore, research on new active substances for the treatment of herpes viruses is growing rapidly [25–28].
We aimed to investigate the effect of antiviral activities of propolis samples (70% ethanol extract of propolis) gathered from Hatay region (southern Turkey) against HSV-1 and HSV-2 in the HEp-2 cell line. We also investigated the synergistic effects of Hatay propolis combined with acyclovir against these viruses.
Material and Methods
Cell culture and viruses
The HEp-2 cell line was obtained from Ankara Refik Saydam Public Hygiene Institute. The cultivation of cells was carried out in RPMI-1640 medium with 10% fetal calf serum. Penicillin (100 IU/ml) and streptomycin (100 g/ml) were added to the medium before use. HSV-1 and HSV-2 virus strains were obtained from Ankara University, Department of Virology, Veterinary Medicine Faculty. We performed experiments using 1, 10, and 100×TCID50 (Tissue Culture of Infectious Dose) concentrations of the viruses. Incubation of the cultures was performed in 5% carbon dioxide atmosphere at 37°C.
Standard drugs
Acyclovir (Sigma, USA) was selected as a standard drug. It was dissolved in bi-distillated water before use (1 mg/ml). Various concentrations of propolis, ranging from 0.5 to 512 μg ml−1, were used in the experiments.
Preparation of propolis extractions
Propolis samples were collected from the south of Turkey (Hatay region). Propolis samples were frozen at −24°C and a grinder was used to break them into small pieces (Delonghi Kg49, Hampshire, UK). Two grams of propolis were shaken with 20 ml 70% ethanol/water (v/v) using a shaker for 1 h, followed by ultra-sonication (Bandelin Sonorex RK100, Berlin, Germany) for 30 min. The mixture was filtered using filter papers (Watman No: 1, Buckinghamshire, UK) to remove wax and bee parts. Finally, the filtrate was placed into glass tubes (tared tubes) and evaporated using a vacuum centrifuge (Jouan, RC 10-10). The tared tubes were weighed after evaporation of propolis solution to determine the amount of the dry propolis extract (DPE), which was stored at −24°C until HPLC-DAD (High-Performance Liquid Chromatography-Diode Array Detector) analysis.
HPLC-DAD analysis
DPE was dissolved at a 1/10 ratio in methanol, filtered through a polyvinyl difluoride (PVDF) syringe filter (Millipore Millex-HV, 0.45 μm), and 5 μL was injected into the HPLC (Shimadzu, Kyoto, Japan) system equipped with an auto-sampler (SIL 20 AC), pump (LC-20AD), diode array detector (SPD-M20A) with 270 nm, and separation using an Intersil ODS column (4.6×150 mm ID) with 5-μm particle size. The column was eluted using a linear gradient as follows: mobile phase A (0.1% formic acid in water) and mobile phase B (acetonitrile) with a flow rate of 1.2 mL/min.
Cell culture
Cell cultures used in the current study were cultured in RPMI 1640 medium containing 10% fetal calf serum, 10 mM of HEPES, 4 mM of glutamine, and 100 IU/ml penicillin/streptomycin. All cultures were incubated at 37°C in a 5% CO2 atmosphere for up to 5 days. Initial density of cells in the cultures was adjusted to 1×105 cells/mL. Cells were incubated to allow the surface coating of the culture dishes for up to 1 week. If there were any changes in pH of the medium, the medium was changed. Cells in the culture dishes were removed through trypsinization solution and then the cells in a 50-ml centrifuge tube were centrifuged for 15 min at 1500 rpm. The cell number and viability of the cells were determined under an inverted microscope by trypan blue exclusion with a hemocytometer.
Proliferation assays were performed in 24-well flat-bottom microplates. We inoculated 1×105 cells per ml and RPMI 1640 medium with 10% fetal calf serum into each well. We used dimethyl sulfoxide (DMSO) (Sigma, USA) to dissolve propolis samples. To determine the non-toxic concentration of DMSO in the cells, HEp-2 cells were inoculated in microplates at a density of approximately 1×105 cells/ml. Different concentrations of DMSO (8%, 4%, 2%, 1%, and 0.5%) were added into the wells of the microplates. After 48 h of incubation, cell counts were performed through using trypan blue exclusion assay for the control group (without DMSO). The dilution of DMSO, which was not significantly different from that in the control group, was determined as the solvent concentration (Figure 1).
Figure 1.
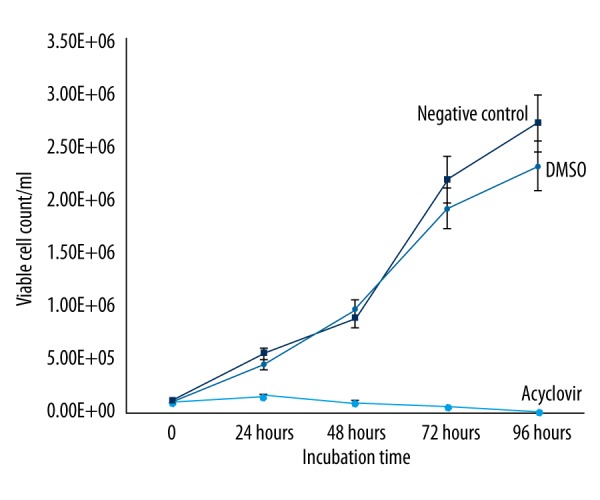
The effects on cell viability of DMSO and acyclovir compared with the control group.
Activity studies
Preparation of cell culture
Primarily, non-toxic concentrations of propolis samples were determined in the study. Antiviral activity studies were performed with these predetermined non-toxic concentrations. Studies to determine cytotoxic effect were performed by MTT (3- (4.5-Dimethylthiazol-2-yl) -2.5-diphenyltetrazolium bromide) method. To investigate the presence of antiviral activity of propolis samples, cell cultures were prepared to contain 1×105 cells per ml. For cell adhesion, following 6-h incubation, those amounts containing different concentrations of propolis (3200; 1600; 800; 400; 200; 100; 75; 50 and 25 μg/mL) were added into the culture medium.
As the control group, the cultures containing DMSO with the same amount of solvent were cultivated at the same time as the experiment group. Cultures not including propolis were selected as a negative control and different concentrations (0.05, 0.1, 0.5, 1, 5, 10, 25, 50, and 100 nm) of acyclovir (Sigma, USA) were used as a positive control. All experiments were performed 2 times in 3 copies.
After incubation, the cells were removed from culture dishes with 0.25% of trypsinization solution and placed into centrifuge tubes. Cells were collected by centrifuging for 10 min at 1500 rpm in a refrigerated centrifuge (at +4°C) and determination of cell viability was done.
Cytotoxicity assay
Determination of cell viability with trypan blue
The cells were inoculated into culture dishes at a concentration of 1×105 cells/ml. The cells in medium containing different concentrations of propolis samples and cells of the control group without propolis were incubated for 24 h under the same conditions. Then, cells were removed by trypsinization solution, collected into centrifuge tubes, and centrifuged. After centrifugation, cell viability was determined by mixing the cell suspension 1:1 with trypan blue exclusion (Sigma) with 0.9% NaCl solution.
MTT assay 3- (4.5-Dimethylthiazol-2-yl) -2.5-diphenyltetrazolium bromide
Viable cells cultured by MTT method can be determined colorimetrically and quantitatively. This method is based on the principle that active mitochondria cleave the MTT tetrazolium ring [29]. The 3- (4.5-dimethyltriazole-2-yl) -2.5-diphenyltetrazolium bromide (MTT) method first described by Mosmann [30] is a practical method commonly used to determine cell viability. MTT is a substance that actively absorbs to cells; it is colored by a reaction due to mitochondria and is reduced to insoluble formazan. MTT reduction into formazan of cells is used as a measure of cell viability, and viable cell count has a correlation with intensity of the dye obtained in the MTT assay. We evaluated effects on cell proliferation by MTT cell proliferation method in cell cultures treated with propolis samples in different concentrations (3200, 1600, 800, 400, 200, 100, 75, 50, and 25 mg/ml).
With MTT method, after adding propolis samples to the cultures, the cells were incubated in a 5% carbon dioxide incubator at 37°C for 96 h. Then, 10 ml of MTT was added to each well and plates were incubated under the same conditions for 4 h. Absorbance measurements were made at 570 nm. Proliferation rate was expressed as the ratio of cells in the wells treated with propolis samples to that in the control group. IC50 values were calculated using SPSS (SPSS, Inc., Chicago).
Viral quantification by real-time PCR
Viral DNA isolation was performed according to the commercial test procedure using the MagNA Pure extraction kit from Roche.
The following primer sequences for HSV-1 and HSV-2 were selected.
| Gene | Oligonucleotide sequence (5′-3′) | Size of amplified products (bp) |
|---|---|---|
| HSV-1 | TGGGACACATGCCTTCTTGG | 147 |
| ACCCTTAGTCAGACTCTGTTACTTACCC | ||
| HSV-2 | CTACGACGCGTACCGGTC CGATG | 637 |
| GTGGTGCACGAACAGCGTGGTGA |
The amplifications of HSV-1 and HSV-2 DNA were performed by using LightCycler (Roche Molecular Biochemicals) by real-time PCR method according to the study of Kaneko et al. The study of PCR was carried out as described by Readand et al. so that the total volume could be 20 μl [31].
Preparation of cells for SEM
The morphology and structure of the HEp-2 cells infected with virus suspension containing 100 TCID50 and those treated for 72 h with the selected propolis concentrations (100; 75; 50 and 25 μg/mL) were analyzed by scanning electron microscopy (SEM) using a JEOL JSM 5510 LV [32]. On the 3rd, 5th, and 7th day after inoculation, infected HEp-2 cells were collected by centrifugation and washed in warm (37°C) phosphate-buffered saline (NaCl, 8 g/L; KCl, 0.2 g/L; Na2HPO4, 1.15 g/L; KH2PO4, 0.2 g/L; CaCl2, 0.1 g/L; and MgCl, 0.1 g/L). All the processes in electron microscopy were performed according to the method described by McFall et al. [33].
Evaluation of drug synergy
To evaluate the occurrence of synergistic effects of propolis and acyclovir on HSV-1 and -2, infections were evaluated. Propolis and acyclovir effects were evaluated alone and in combination. The synergistic effect of propolis and acyclovir was calculated using a combination index (CI) as described by Chou et al. in 2006 [34].
Results
Component analysis of propolis
We identified and quantified the composition of propolis (Table 1). We determined that propolis samples showed cytotoxic effects on cells at levels of 200 μg/mL. Therefore, antiviral activity investigations of propolis were performed by selecting the 100 μg/ml/ml concentration of propolis (Figure 2).
Table 1.
The analysed of propolis components.
| Phenolic compounds | Content % (μg/g) |
|---|---|
| Gallic acid | 8.5 |
| Protocatheuic acid | 42.5 |
| (±)-Catechin | 80.7 |
| Caffeic acid | 5756.8 |
| Syringic acid | 84.7 |
| Epigallokatechin | 200.8 |
| P-coumaric acid | 1207.3 |
| Trans ferulic acid | 1792.9 |
| Benzoic acid | 3588.9 |
| M-coumaric acid | 35.2 |
| Trans isoferulic acid | 3391.9 |
| Viteksin | ND |
| Ellagic asid | ND |
| Rutin | ND |
| Methyl syringate | ND |
| Naringin | ND |
| 3-4 dimethoxycinnamic acid | 6646.0 |
| Quercetin | ND |
| Myricetin | 538.3 |
| Rosomarinic acid | ND |
| Trans-Cinnamic acid | 2665.7 |
| Daidzein | 462.8 |
| Luteolin | 2164.4 |
| Pinobanksin | 6043.5 |
| (±)-Naringenin | 563.6 |
| Apigenin | 3270.9 |
| Kaempherol | 2767.6 |
| Isorhamnetin | ND |
| Chrysin | 22480.0 |
| Pinocembrin | 8265.1 |
| Galangin | 28772.8 |
| Caffeic acid phenethyl ester (CAPE) | 20072.9 |
| Emodin | ND |
| Trans-Chalcon | ND |
ND – not determined.
Figure 2.
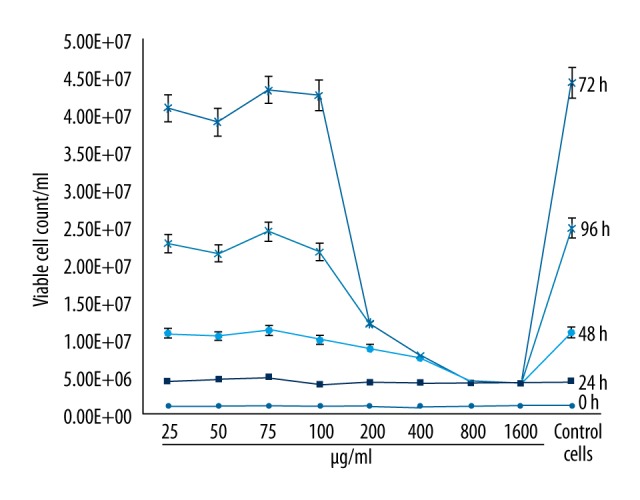
The effects of Hatay propolis on cell viability of HEp-2 cell culture as compared to the cell control.
Effect of propolis on viral replication
Effects of propolis were studied against both HSV-1 and HSV-2 at the concentrations of 25, 50, 100, 200, and 400 μg/ml. Because propolis at 200 and 400 μg/ml concentrations showed toxic effects on HEp-2 cells, antiviral activity studies were carried out on the concentrations that did not produce any cytopathological effects. We determined that propolis did not show any toxic effects at concentrations of 100, 50, and 25 μg/ml on HEp-2 cells. Morphological determination of non-toxic concentrations of propolis on cells was made by examining them microscopically through an inverted microscope for 96 h. Cell viability was determined with trypan blue method and toxicity tests were performed by MTT assay.
Microscopic examinations did not show any cytopathological changes, such as aggregation, nuclear enlargement, or cell rounding, at propolis concentrations of ≤100 μg/ml.
In the experiments, the activity of propolis against both HSV-1 and HSV-2 was investigated in comparison with acyclovir on 3 different titrations of virus (1, 10, and 100 TCID50). No cytopathological changes were observed in cell cultures that contained 100 μg/ml of acyclovir and propolis, while all cell cultures without acyclovir or propolis were determined to demonstrate ++++CPE presence. CPE level was detected as ++ in cultures that contained 50 μg/ml of propolis, whereas it was +++ CPE in cultures containing 25 μg/ml of propolis (Figures 3, 4).
Figure 3.
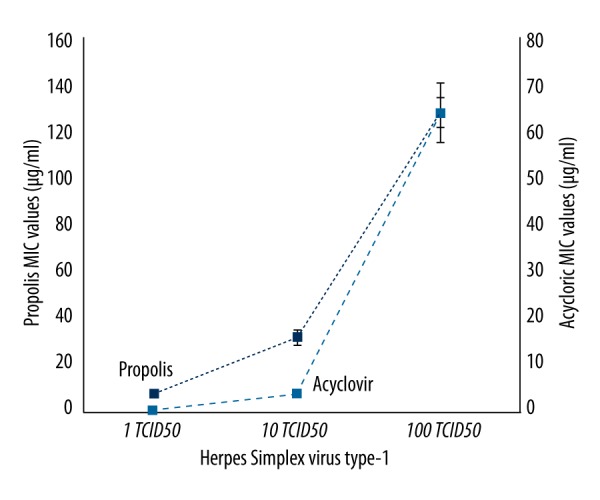
MIC values of Hatay propolis and acyclovir against some HSV-1.
Figure 4.
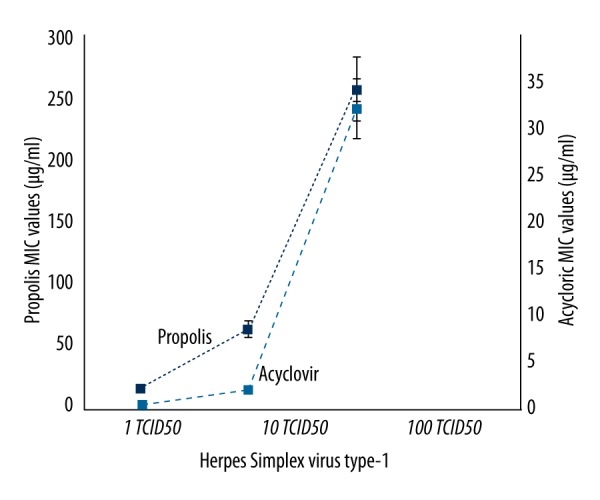
The effects of Hatay propolis against to the replication of HSV-2.
The effects of viral replications of propolis against HSV-1 and HSV-2 were evaluated in comparison with the control group. Cytopathologic changes, such as aggregation, rounding of the cells, and nuclear enlargement, were detected in the cell control group culture (acyclovir- and propolis-free), while in cultures containing propolis, viral replications of both HSV-1 and HSV-2 were inhibited and pathological changes in cells related to viral growth did not occur. We also observed that cells in these cultures had morphologically typical form and coated the surface of the cell culture dishes. However, we found that in comparison with the control cells, there was no significant difference in terms of cell viability.
To evaluate the effects of propolis on the viral replication using the 100TCID50 dilution from the virus stocks, mRNA levels of HEp-2 cells were compared with acyclovir. Comparing the effects of propolis with acyclovir, we found that propolis showed activity similar to that of acyclovir. Both acyclovir and propolis began to inhibit HSV-1 replication after 24 h of incubation. The effect of propolis was found to increase, like acyclovir, in direct proportion with incubation time. Compared with the control group, the number of viral DNA copies was significantly reduced after 24 h (Figure 5).
Figure 5.
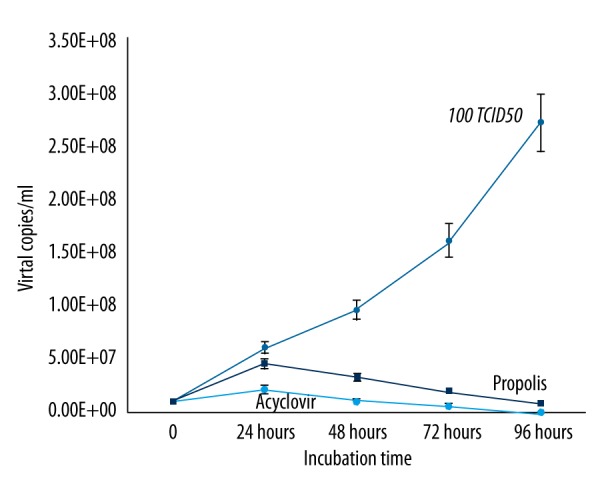
The effects of Hatay propolis on HSV-1 replication as compared to acyclovir.
In contrast to HSV-1, propolis activity against HSV-2 started at 48 h after incubation. The activity of propolis against HSV-2 was confirmed by a significant decrease in the number of viral copies (Figure 6). We found a statistically significantly difference in synergistic effects of Hatay propolis and acyclovir on HSV-1 and HSV-2 replication as compared to acyclovir alone (Figures 7, 8).
Figure 6.
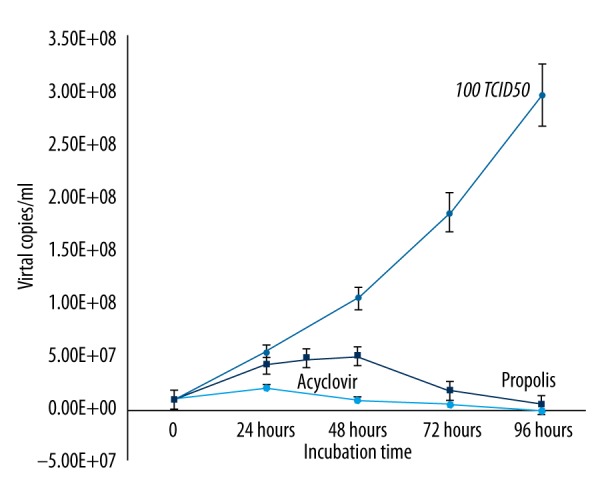
The effects of Hatay propolis on HSV-2 replication as compared to acyclovir.
Figure 7.
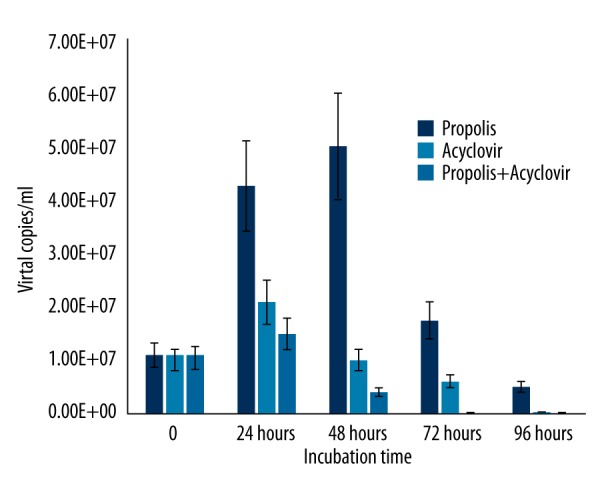
The synergistic effects of Hatay propolis and acyclovir on HSV-1 replication as compared to acyclovir.
Figure 8.
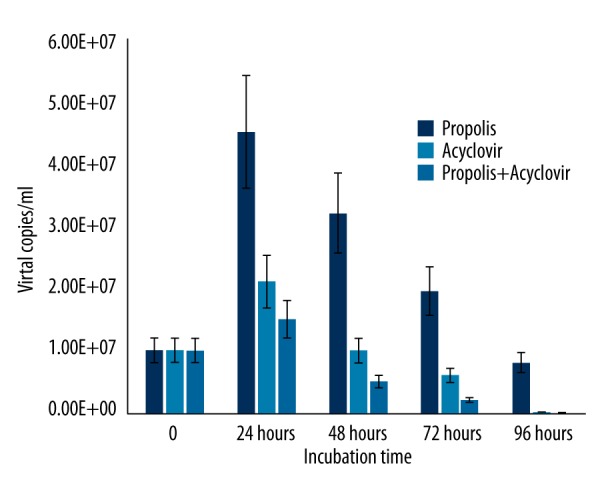
The synergistic effects of Hatay propolis and acyclovir on HSV-2 replication as compared to acyclovir.
Results of scanning electron microscopy (SEM)
Many atypical structures on the surfaces of HEp-2 cells infected with HSV-1 and 2 were found in the evaluations carried out by electron microscopy examinations. We found no disruption of membrane integrity of HEp-2 cells, and they were in the typical form in infected cells treated with propolis (Figure 9). Compared to the control group, there was no significant difference between the cells treated with propolis and the control group cells (Figure 10).
Figure 9.
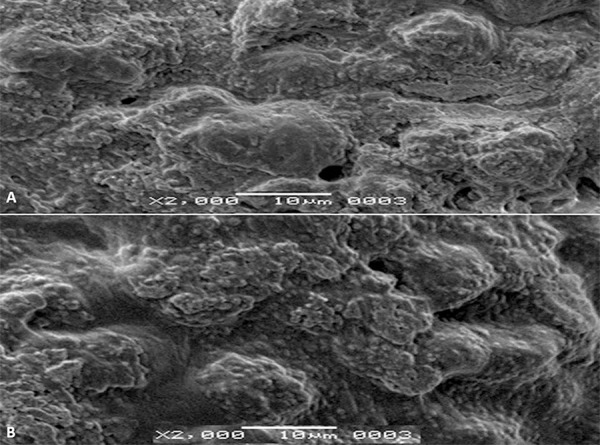
HSV-infected cells were treated with Hatay propolis. Propolis was shown to inhibit HSV replication. (A) HSV-1-infected cells, (B) HSV-2-infected cells. There are no atypical forms on the HEp-2 cell surface.
Figure 10.
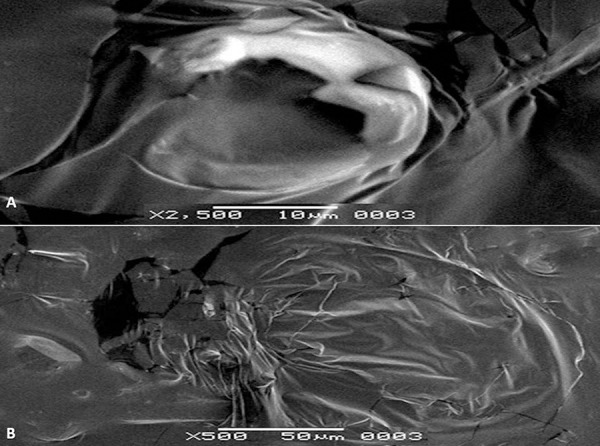
(A) HSV-infected HEp-2 cells. HEp-2 cells were infected by HSV-1. (B) The virus disrupted membrane integrity on the surface of HEp-2 cells.
Discussion
Herpes simplex viruses cause a very extensive spectrum of infection in humans, ranging from simple skin lesions to meningitis [35,36] and can cause significant morbidity and mortality. Although HSV-1 and HSV-2 usually cause orofacial infections and genital infections, respectively, both HSV-1 and HSV-2 can be isolated to be orofacial or genital infections. Orofacial infections can be closely connected with oral health, especially at young ages [37]. Although there are antiviral drugs available for the treatment of infections, herpes viruses can lead to very serious infections, especially in immunocompromised patients. It is also reported that there is serious viral resistance to existing antiviral drugs [38,39]. Increasing drug resistance in these infections stimulates the search for drugs that are more potent and easily accessible. Lately, antiviral drug research has concentrated on natural products because of their low toxicity [40].
Plants or herbal products have long been used in treatment of many diseases in folk medicine. New drug research, especially a limited number of antiviral drug studies, are ongoing around the world. Compared with antibacterial or antimicrobial drugs, the use of antivirals is very limited. In recent years, drug resistance against available antiviral agents is seen as a significant problem [38,39].
Propolis is a plant-derived bee product that is used in folk medicine to treat various diseases. Propolis has many pharmacological properties, such as antimicrobial, anti-inflammatory, and antioxidant effects [41]. Propolis is a non-toxic natural product and in previous studies was reported to produce very few adverse effects in people and animals, with the exception of allergy and contact dermatitis. These allergic reactions are mild and not life-threatening. It has been reported that these allergic reactions may arise from the allergy to honey or allergens in flowers from which the bees made the honey [42–44].
Propolis is a bee product. Because component ingredients of propolis are associated with plant flora, richness in propolis components is directly related to plant flora and the season during which it was collected [10]. Therefore, component structures of propolis can vary from country to country, from region to region, and from city to city in the same region. In this study, we aimed to determine the antiviral efficacy against HSV-1 and HSV-2 of propolis collected from Hatay province in southern Turkey. We determined that propolis has extremely high efficacy against these 2 virus types. Hatay propolis samples had important levels of antiviral efficacy compared with acyclovir. Acyclovir is a guanosine nucleoside analog that strongly prevents DNA replication of Herpes Simplex Virus [45]. The antiviral efficacy created by propolis in synergy with acyclovir produced higher efficacy than acyclovir alone. In particular, the synergy produced by antiviral activity of propolis and acyclovir together had a stronger effect against HSV-1 and HSV-2 than acyclovir alone. We believe that some components of propolis that affect cell division may increase the effect of acyclovir. Hence, a strong synergy between propolis and acyclovir against herpes virus can be created.
In these experiments, synergistic effects of propolis with acyclovir against HSV-1 were found to be higher than effects against HSV-2. We believe that some minor structural differences between HSV-1 and HSV-2 may lead to different antiviral effects against these 2 virus types. It has been reported that there are 4 different transcript patterns (UL4, UL29, UL30, and UL31) between HSV-1 and HSV-2. The UL29 protein has been reported to play an important role in virus replication in culture. The possible reasons for the difference in kinetics of inhibition of HSV-1 versus HSV-2 may be the transcript differences between these viruses [46,47].
The mechanism by which the antiviral activity of propolis occurs is very complicated and has been reported to be associated with some of the major components of propolis. The possible mechanism of synergism between acyclovir and propolis may be attributed to some of the components of propolis. In the literature, ethanol extract of propolis has been shown to interfere in the growth of microorganisms by inhibiting protein synthesis [48]. In addition, some components of propolis have been reported to inhibit the enzymatic activity of pathogenic microorganisms [49]. In our study, a significant synergy between acyclovir and Hatay propolis was determined against both HSV-1 and HSV-2.
Conclusions
In this study, Hatay propolis has been shown to be quite effective against the replication of HSV-1 and HSV-2. We believe that Hatay propolis may be a potential drug for the treatment of Herpes Simplex Virus infections, but this needs further study. We found that Hatay propolis increased the activity/action of acyclovir. Although further studies are needed in this regards, the findings obtained from this study are quite striking. It is necessary to further explore the efficacy of Hatay propolis by conducting detailed and broad studies of propolis components.
Footnotes
Source of support: Departmental sources
References
- 1.Cragg GM, Newman DJ. Natural products: A continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830(6):3670–95. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra BB, Tiwari VK. Natural products: An evolving role in future drug discovery. Eur J Med Chem. 2011;46:4769–807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 3.Duran N, Muz M, Culha G, et al. GC-MS analysis and antileishmanial activities of two Turkish propolis types. Parasitol Res. 2011;108(1):95–105. doi: 10.1007/s00436-010-2039-z. [DOI] [PubMed] [Google Scholar]
- 4.Onlen Y, Duran N, Atik E, et al. Antibacterial activity of propolis against MRSA and synergism with topical mupirocin. J Altern Complement Med. 2007;13(7):713–18. doi: 10.1089/acm.2007.7021. [DOI] [PubMed] [Google Scholar]
- 5.Onlen Y, Tamer C, Oksuz H, et al. Comparative trial of different anti-bacterial combinations with propolis and ciprofloxacin on Pseudomonas keratitis in rabbits. Microbiol Res. 2007;162(1):62–68. doi: 10.1016/j.micres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Duran N, Koc A, Oksuz H, et al. The protective role of topical propolis on experimental keratitis via nitric oxide levels in rabbits. Mol Cell Biochem. 2006;281(1–2):153–61. doi: 10.1007/s11010-006-0720-4. [DOI] [PubMed] [Google Scholar]
- 7.Oksuz H, Duran N, Tamer C, et al. Effect of propolis in the treatment of experimental Staphylococcus aureus keratitis in rabbits. Ophthalmic Res. 2005;37(6):328–34. doi: 10.1159/000087943. [DOI] [PubMed] [Google Scholar]
- 8.Salim EI, Abd El-Magid AD, Farara KM, et al. Antitumoral and antioxidant potential of Egyptian propolis against the PC3 prostate cancer cell line. Asian Pac J Cancer Prev. 2015;16(17):7641–51. doi: 10.7314/apjcp.2015.16.17.7641. [DOI] [PubMed] [Google Scholar]
- 9.Mendonca IC, Porto IC, do Nascimento TG, et al. Brazilian red propolis: phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement Altern Med. 2015;15(1):357–69. doi: 10.1186/s12906-015-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nina N, Quispe C, Jimenez-Aspee F, et al. Antibacterial activity, antioxidant effect and chemical composition of propolis from the region del Maule, Central Chile. Molecules. 2015;20(10):18144–67. doi: 10.3390/molecules201018144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvan AK, Prabhu T. Extraction of propolis from beehives and characterization of its constituens and medicinal properties: A review. International Journal of Advanced Engineering Technology. 2010;1(3):50–3. [Google Scholar]
- 12.Schnitzler P, Neuner A, Nolkemper S. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res. 2010;24(1):20–28. doi: 10.1002/ptr.2868. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho RS, Baltazar F, Aguiar CA. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Alternat Med. 2015:206439. doi: 10.1155/2015/206439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waggoner-Fountain LA, Grossman LB. Herpes simplex virus. Pediatr Rev. 2004;25(3):86–93. doi: 10.1542/pir.25-3-86. [DOI] [PubMed] [Google Scholar]
- 15.Glinsek Biskup U, Ursic T, Petrovec M. Laboratory diagnosis and epidemiology of herpes simplex 1 and 2 genital infections. Acta Dermatovenerol Alp Pannonica Adriat. 2015;24(2):31–35. doi: 10.15570/actaapa.2015.9. [DOI] [PubMed] [Google Scholar]
- 16.Aranda AM, Epstein AL. Herpes simplex virus type 1 latency and reactivation: an update. Med Sci (Paris) 2015;31(5):506–14. doi: 10.1051/medsci/20153105012. [DOI] [PubMed] [Google Scholar]
- 17.Duran N. Genital herpes infections. Mikrobiyol Bul. 2003;37(2–3):205–13. [PubMed] [Google Scholar]
- 18.Duran N, Yarkin F, Evruke C, et al. Asymptomatic Herpes Simplex Virus type 2 (HSV-2) infection among pregnant women in Turkey. Indian J Med Res. 2004;120(2):106–10. [PubMed] [Google Scholar]
- 19.Whitley RJ. Herpes Simplex virus infections of the central nervous system. Continuum (Minneap Minn) 2015;21(6 Neuroinfectious Disease):1704–13. doi: 10.1212/CON.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 20.Looker KJ, Magaret AS, May MT, et al. Global and regional estimates of prevalent and incident Herpes Simplex Virus Type 1 infections in 2012. PLoS One. 2015;10(10):e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Looker KJ, Magaret AS, Turner KM, et al. Global estimates of prevalent and incident Herpes Simplex Virus type 2 infections in 2012. PLoS One. 2015;10(1):e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rostamzadeh Khameneh Z, Sepehrvand N, Taghizadeh-Afshari A, et al. Seroprevalence of Herpes Simplex Virus-2 in kidney transplant recipients: A single-center experience. Iran J Kidney Dis. 2010;4(2):158–61. [PubMed] [Google Scholar]
- 23.Holzinger D, Kuhn J, Ehlert K, et al. HSV-1 viremia as a potential cause of febrile neutropenia in an immunocompromised child. J Pediatr Hematol Oncol. 2010;32(1):e19–21. doi: 10.1097/MPH.0b013e3181b79669. [DOI] [PubMed] [Google Scholar]
- 24.Marzano AV, Tourlaki A, Merlo V, et al. Herpes simplex virus infection and pemphigus. Int J Immunopathol Pharmacol. 2009;22(3):781–86. doi: 10.1177/039463200902200324. [DOI] [PubMed] [Google Scholar]
- 25.Piaserico S, Sandini E, Peserico A, et al. Cutaneous viral infections in organ transplant patients. G Ital Dermatol Venereol. 2014;149(4):409–15. [PubMed] [Google Scholar]
- 26.Saijo M. Diagnostic and therapeutic strategy for acyclovir-resistant herpes encephalitis. Rinsho Shinkeigaku. 2014;54(12):1024–27. doi: 10.5692/clinicalneurol.54.1024. [DOI] [PubMed] [Google Scholar]
- 27.Dokey AT, Haug SJ, McDonald HR, et al. Acute retinal necrosis secondary to multidrug-resistant Herpes Simplex Virus 2 in an immunocompetent adolescent. Retin Cases Brief Rep. 2014;8(4):260–64. doi: 10.1097/ICB.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 28.Frobert E, Burrel S, Ducastelle-Lepretre S. Resistance of Herpes Simplex Viruses to acyclovir: an update from a ten-year survey in France. Antiviral Res. 2014;111:36–41. doi: 10.1016/j.antiviral.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 29.McGahon AJ, Martin SJ, Bissonnette RP. The end of the (cell) line: methods for the study of apoptosis in-vitro. Methods Cell Biol. 1995;46:153–85. doi: 10.1016/s0091-679x(08)61929-9. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 31.Readand SJ, Kurtz JB. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J Clin Microbiol. 1999;37(5):1352–55. doi: 10.1128/jcm.37.5.1352-1355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayat MA. Principles and techniques of electron microscopy: biological applications. Cambridge University Press; London: 2000. [Google Scholar]
- 33.McFall RC, Nagy RM, Nagle BT. Scanning electron microscopic observation of two retinoblastoma cell lines. Cancer Res. 1978;38:2827–35. [PubMed] [Google Scholar]
- 34.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein DI, Bellamy AR, Hook EW, III, et al. Epidemiology, clinical presentation, and antibody response to primary infection with Herpes Simplex Virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56:344–51. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley H, Markowitz LE, Gibson T. Seroprevalence of Herpes Simplex Virus types 1 and 2-United States, 1999–2010. J Infect Dis. 2014;209:325–33. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 37.Sauerbrei A, Schmitt S, Scheper T, et al. Seroprevalence of Herpes Simplex Virus type 1 and type 2 in Thuringia, Germany, 1999 to 2006. Euro Surveill. 2011;16(44) pii: 20005. [PubMed] [Google Scholar]
- 38.Ziyaeyan M, Alborzi A, Japoni A, et al. Frequency of acyclovir-resistant Herpes Simplex Viruses isolated from the general immunocompetent population and patients with acquired immunodeficiency syndrome. Int J Dermatol. 2007;46(12):1263–66. doi: 10.1111/j.1365-4632.2007.03449.x. [DOI] [PubMed] [Google Scholar]
- 39.Morfin F, Thouvenot D. Herpes Simplex Virus resistance to antiviral drugs. J Clin Virol. 2003;26:29–37. doi: 10.1016/s1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 40.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang S, Zhang CP, Wang K, et al. Recent advances in the chemical composition of propolis. Molecules. 2014;19(12):19610–32. doi: 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callejo A, Armentia A, Lombardero M, et al. Propolis, a new bee-related allergen. Allergy. 2001;56:579. doi: 10.1034/j.1398-9995.2001.056006579.x. [DOI] [PubMed] [Google Scholar]
- 43.Mani F, Damasceno HCR, Novelli ELB, et al. Biochemical determinations of propolis-treated rats: effects of different concentrations, extracts and intake period. Biosaude. 2008;10(1):3–16. [Google Scholar]
- 44.Rudeschko O, Machnik A, Dorfelt H, et al. A novel inhalation allergen present in the working environment of beekeepers. Allergy. 2004;59(3):332–37. doi: 10.1111/j.1398-9995.2003.00401.x. [DOI] [PubMed] [Google Scholar]
- 45.Bacon TH, Levin MJ, Leary JJ, et al. Herpes Simplex Virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16(1):114–28. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liptak LM, Uprichard SL, Knipe DM. Functional order of assembly of Herpes Simplex Virus DNA replication proteins into prereplicative structures. J Virol. 1996;70:1759–67. doi: 10.1128/jvi.70.3.1759-1767.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguilar JS, Devi-Rao GV, Rice MK, et al. Quantitative comparison of the HSV-1 and HSV-2 transcriptomes using DNA microarray analysis. Virology. 2006;348(1):233–41. doi: 10.1016/j.virol.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 48.Takaisi-Kikuni NB, Schilcher H. Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med. 1994;60:222–17. doi: 10.1055/s-2006-959463. [DOI] [PubMed] [Google Scholar]
- 49.Koo H, Rosalen PL, Cury JA, et al. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosil transferase activity. Antimicrob Agents Chemother. 2002;46:1302–9. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


