Abstract
Background:
Neuromuscular electrical stimulation (NMES), often referred to as functional electrical stimulation (FES), has been used to activate paralyzed skeletal muscle in people with spinal cord injury (SCI). The goal of NMES has been to reverse some of the dramatic losses in skeletal muscle mass, to stimulate functional improvements in people with incomplete paralysis, and to produce some of the health benefits associated with exercise.
Objective:
The purpose of this brief review is to describe a quantifiable resistance training form of NMES developed by Gary A. Dudley.
Methods:
People with motor complete SCI were first tested to confirm that an NMES-induced muscle contraction of the quadriceps muscle could be achieved. The contraction stimulus consisted of biphasic pulses at 35 Hz performed with increasing current up to what was needed to produce full knee extension. Four sets of 10 knee extensions were elicited, if possible. Training occurred biweekly for 3 to 6 months, with ankle weights being increased up to an added weight of 9.1 kg if the 40 repetitions could be performed successfully for 2 sessions.
Results:
Many participants have performed this protocol without adverse events, and all participants showed progression in the number of repetitions and/or the amount of weight lifted. Large increases in muscle mass occur, averaging 30% to 40%. Additional physiological adaptations to stimulated muscle have also been reported.
Conclusions:
These results demonstrate that the affected skeletal muscle after SCI responds robustly to progressive resistance training many years after injury. Future work with NMES should determine whether gains in lean mass translate to improved health, function, and quality of life.
Key words: electrical stimulation, muscle hypertrophy, spinal cord injury
In the past few years, there have been significant advances in the management of people with spinal cord injury (SCI). New treatments such as stem cell therapies,1 epidural stimulation,2 and exoskeletons3 are showing great potential. Unfortunately, many people living with an SCI will not be able to take advantage of new treatments or the treatments may not be as effective at restoring their function because they have such deteriorated musculoskeletal systems. Currently, most rehabilitation treatments focus on compensating for lost function to increase independence in activities of daily living and neglect the musculoskeletal system. Therefore, rehabilitation interventions to preserve and/or restore the musculoskeletal system are critical for individuals with SCI to lead healthier lives and possibly take advantage of future, innovative treatments.
The consequences of SCI on the musculoskeletal system are quite devastating. Skeletal muscle is perhaps one of the most important tissues in human physiology, comprising approximately 40% of body mass and accounting for much of total energy expenditure. There are multiple adaptations that occur at the skeletal muscle level that have been documented. People with SCI suffer substantial muscle atrophy4 and altered body composition,5 which likely contribute to their increased susceptibility to secondary complications.6,7 Although considerable evidence has been compiled in animal models, we focus on relevant human studies. The early work in the late 1970s reported significant myofiber atrophy and a predominantly fast composition as compared to able-bodied counterparts.8–10 Longitudinal studies on the topic have been less abundant, but Burnham et al11 reported on serial biopsy samples in a few participants and Castro et al12 provided a detailed analysis after acute SCI, showing rapid losses in myofiber size with fibers approximately 60% the size of able-bodied controls and continuing atrophy for 18 more weeks. Studies of whole muscle with current imaging techniques show rapid skeletal muscle loss as evidenced by MRI early after injury4 or a significant difference in muscle mass in people several years after SCI.13
Of the countermeasures for muscle atrophy tested to date, including pharmacologic therapies and various modes of exercise training, only intense resistance exercise training has consistently been found to effectively increase muscle mass. Previous studies have shown that the muscles of people after SCI respond to neuromuscular electrical stimulation (NMES) contractions with some degree of adaptation. These studies used what has been termed functional electrical stimulation (FES), which often consists of pedaling a cycle ergometer or performing a walking exercise. When participants perform FES cycling, it is difficult to quantify the mixture of resistance and endurance exercise stimuli. Tetanic concentric contractions are performed by the various muscles in the leg, but once the participant can no longer reach the target work level, the device may move the limbs passively or the session is complete. Most studies using FES training report modest hypertrophy of about 10% to 12%.14–16 However, one study that lasted 12 months (training up to 5 days per week for 60 minutes) reported increases in thigh muscle cross-sectional area of 35.5% ± 18%.17 In 1999, Gary A. Dudley’s (Figure 1) laboratory published a short paper on a simple, innovative intervention that utilized NMES in combination with progressive resistance training principles to yield remarkable skeletal muscle hypertrophy in affected muscle after SCI.18 Dudley’s group effectively reversed 48 weeks of atrophy in the quadriceps femoris with a short intervention that was conducted 2 days per week for 8 weeks.
Figure 1.
Gary A. Dudley, PhD, evaluates MRIs of the thigh. The picture was taken at the University of Georgia around 1998.
Since the initial paper from Dudley, we19,20 and others21 have utilized this simple, home-based NMES-induced resistance training program to evoke substantial skeletal muscle hypertrophy. In these studies, quadriceps femoris cross-sectional area (CSA), measured by MRI, was increased by about 35% in just 12 weeks. For comparison, studies in able-bodied individuals, using similar imaging techniques, have reported increases in CSA of about 10% to 15% when using NMES22,23 or voluntary actions.24 In this short review, we detail the methodology and highlight data from published and previously unpublished work to support the use of this type of training for inducing robust increases in skeletal muscle mass in people with SCI.
We report data from 3 studies that utilized the same methodology to evoke skeletal muscle hypertrophy in participants with SCI. Portions of study 1 have previously been published (n = 14)20; study 2 was conducted over the course of 6 months (n = 6), with a 3-month subset (n = 5) previously published19; and study 3 was recently completed over 8 weeks (n = 6). Participants in all studies provided written, informed consent, and all studies were approved by the appropriate institutional review boards (University of Alabama at Birmingham or University of Georgia).
Inclusion Criteria
The Dudley protocol was initially designed to train people with clinically complete SCI, in whom the quadriceps muscles were affected. Initial inclusion criteria were based on self-reported injury level and self-reported observation of muscle spasms in the upper leg. Eligible participants were brought into the laboratory for baseline testing and an initial testing/training session. Because transportation to a training location is often a major barrier for participation, the training protocol was developed for use in the home rather than in the laboratory; but the protocol has been completed in both environments. Participants with higher levels of injury (reduced hand or arm function) were eligible for the studies, but they were asked to bring someone who could assist with their training to the initial orientation/training session. We found that electrical stimulation of the knee extensors needed to be confirmed in the laboratory prior to starting the training procedures, as some potential participants had evidence of lower motor neuron injury. The initial studies were limited to participants who could produce 2.77 kg•m of knee extension torque with tetanic contractions, but later studies were extended to all participants who could produce a visually detectable muscle contraction, even if the contraction was not able to result in complete knee extension. The initial study recruited male participants with injury durations as short as 1 year, but later studies included women and participants with injury durations of many years.
NMES-Induced Resistance Training Protocol (Dudley Protocol)
Dudley’s protocol for NMES-induced resistance training was first published in 1999.18 The goal of the training program is to have the participants perform 4 sets of 10 dynamic contractions of the quadriceps femoris muscle in the seated position, 2 days per week. Stimulation is performed with 2 electrodes (typically 7.6 × 12.7 cm) that are placed on the proximal vastus lateralis and the distal vastus medialis. Any commercially available electrical stimulator can be used, but we have utilized the Theratouch (Rich-Mar, Chattanooga, TN) or the BTL-4000 (BTL Industries LTD, United Kingdom). These units were chosen because many of the participants with complete motor injury require stimulation amplitudes above 100 mA and often up to 200 mA. The stimulation parameters for each contraction are 30 to 35 Hz and 400 to 600 μs biphasic pulses. Contractions are initiated by increasing the current from zero to the target level (50–200 mA) in 3 to 5 seconds, resulting in a tetanic muscle contraction that evokes full knee extension. Once the knee extension is produced, the current is rapidly turned down (another 3–5 seconds) to produce a gradual relaxation to the flexed position. Each set of knee extension exercises takes from 2 to 5 minutes to complete. Because both legs are trained in an alternating order, there is typically 2 to 5 minutes of rest between sets.
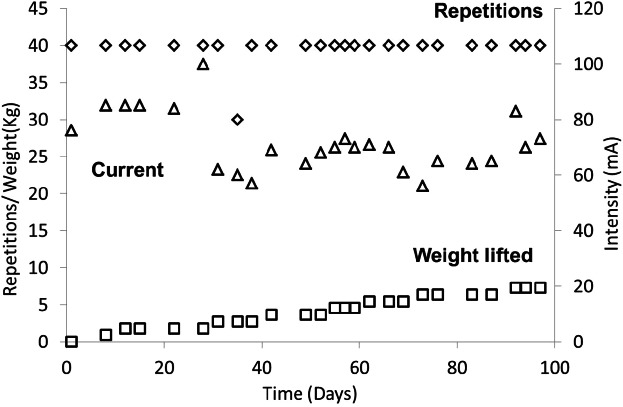
Individuals begin with the weight of their limb only. When the participant is able to complete 4 sets of 10 knee extension contractions with only their limb as resistance for at least 2 consecutive training sessions, external loads (~1–2 kg) are added. Both legs are trained; the sets of dynamic contractions are performed alternatively, one leg at a time. Loads are progressively increased when the participant can complete the full 40 repetitions (4 sets of 10 contractions) on 2 consecutive training days (Table 1). An example of a training program with a representative training progression (from study 1)20 is shown in Figure 2. The participant and assistant, if necessary, are instructed on the proper placement of the electrodes and use of the stimulator. Participants often train on a bed or in a chair and sometimes in their wheelchair. Care is taken to ensure that the training position includes back and trunk support and proper clearance for knee extensions. Care is also taken to make sure the leg is properly cushioned so that the heel and the back of the leg are not injured when the leg returns to the flexed position.
Table 1. Training program .
| Session | Repetitionsa | Sets | Load | Current (mA) | Range of movement |
| 1st | 10 per set | 4 | 0 | 70–200 | 90° to at least 10° |
| 2nd | 10 per set | 4 | 0 | 70–200 | 90° to at least 10° |
| Weekly | 10 per set | 4 | Add ~1 kg if successfully complete 40 repetitions in previous 2 sessions | 70–200 | 90° to at least 10° |
| Final | 10 per set | 4 | Maximum 9.1 kg | 70–200 | 90° to at least 10° |
Actual number based on observation of successful lifts.
Figure 2.
An example of a typical training program for a person with motor complete SCI.20 This participant was able to complete 4 × 10 repetitions with no added load on the first training session. The weight lifted in each session reached 7.3 kg at the end of the 4 months of training. Note that the current level needed to perform the lifts appeared to decrease slightly (90 to 75 mA) over the course of the training program.
We preloaded the stimulation parameters into the stimulator. A training time and location were negotiated, and the initial training session was performed by each participant with the investigators present in the laboratory. The initial training session typically lasted about 2 hours. The participants who trained at home were given the stimulator and enough electrodes to last 3 to 5 weeks. Additional electrodes were sent in the mail as needed. Each training session was supervised either in person or performed with the participant on the phone with a trained research staff member who recorded the number of repetitions, the weight lifted, the current required, and the degree of knee extension on each action. A key part of the training supervision was the recording of the amount of current needed. If more current was needed than was typically used, this was a sign that new electrodes were necessary. If higher than normal currents were needed even with new electrodes, this was used as evidence of contraction-induced muscle injury or a failure to positively respond to the training stimulus. We found that requiring participants to start with no added weight and only increasing the loads after 2 successful training sessions meant that participants did not need additional current to perform their lifts once new electrodes were used. No training-related adverse events were recorded in any of the studies.
Results from the Training Program
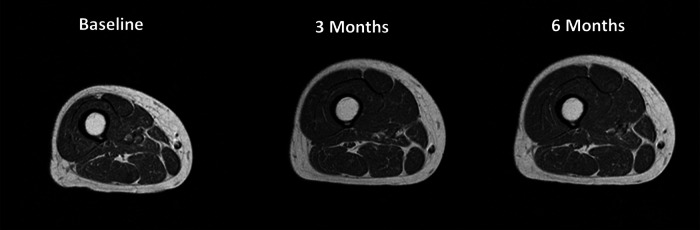
The primary method of assessing changes in stimulated muscle size was done with MRI. T1-weighted transaxial images were collected from both thigh areas with a 1.5 T or 3 T wholebody magnet. Images were segmented into fat, skeletal muscle, and background/bone regions. Regions of interest were manually drawn around the quadriceps (trained) and hamstrings (untrained) muscle groups. Percent fat was calculated using values of skeletal muscle and fat pixels from the histogram. The cortical bone and marrow area of the femur were used as an internal reference to ensure accurate pre-post comparisons of images. In our studies, testretest reliability scores were r = 0.99 for skeletal muscle pixels from segmentation, r = 0.98 for fat pixels from the histogram, and r = 0.99 for the whole region. Increases in muscle size of the quadriceps without significant increases in the hamstring muscle groups were typically observed (Figure 3).
Figure 3.
MR images from a representative participant in the training study. Note the larger change in muscle size of the quadriceps muscle groups where the electrodes were placed, compared to the hamstring muscle groups that were not stimulated.
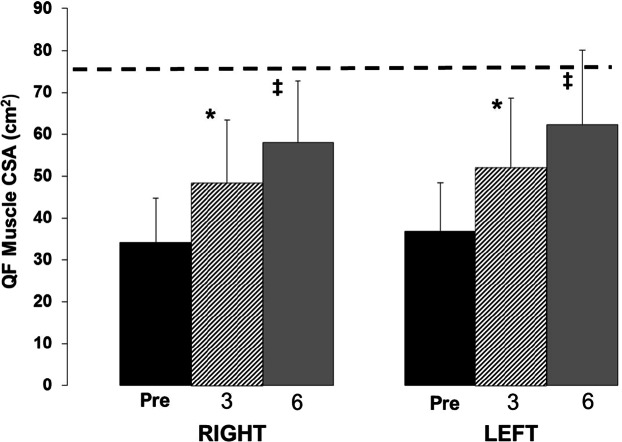
Portions of study 1 have been reported in a previous communication.20 After 3 months of training with NMES-induced resistance training, quadriceps muscle volume (average of both legs) was increased by 39% ± 27% (P < .05), and there was a slight increase in hamstring muscle volume by 7% ± 15% (P > .05); both legs responded in a similar fashion to training.20 In a second study that was carried out over 6 months, quadriceps muscle cross-sectional area was increased at 3 months on average by 45% ± 25% and 43% ± 23% of the right and left, respectively. After 6 months, the muscle continued to grow, averaging 80% ± 59% and 75% ± 41% of the right and left, respectively (Figure 4).
Figure 4.
The progression of muscle hypertrophy as measured with MRI over 6 months using the Dudley protocol. Hatched line indicates the average able-bodied muscle size as reported by Castro et al.4 The asterisk indicates a significant increase from baseline and the double dagger indicates a significant increase from 3 months (P < .05). CSA = cross-sectional area; QF = quadriceps femoris.
Female participants were able to successfully complete the training program and had increases in training progression and quadriceps muscle mass that were at least as great, if not greater, than those found in the male participants (Figure 5).20 Another method to quantify changes in lean mass that is often less expensive than MRI is dual energy x-ray absorptiometry (DXA). DXA can be used to evaluate changes in thigh muscle mass (assuming that changes in lean tissue in the segmented area represent changes in muscle mass). Because DXA cannot distinguish between the quadriceps and the hamstring muscles, the increases in muscle mass are much less than what are typically observed when using MRI analyses of specific muscle groups. In a third study, which was conducted over a shorter timeframe, bilateral thigh lean mass as measured by DXA (Prodigy; Lunar Radiation, Madison, WI) was higher following 8 weeks of NMES-induced resistance training in individuals with long-standing SCI (~22 years post injury). On average, after 8 weeks of training, bilateral thigh lean mass was increased by 5.5% ± 4.6% (0.6 ± 0.5 kg; n = 6; P = .05) compared to baseline.
Figure 5.
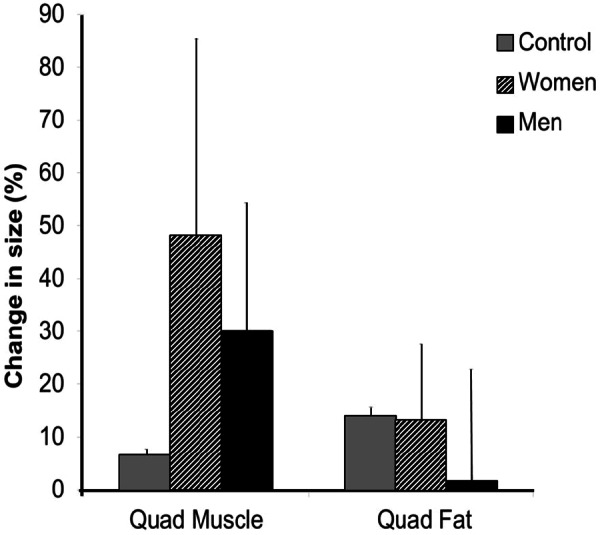
Percent change in quadriceps femoris muscle and fat cross-sectional area of men (n = 9) and women (n = 3) participating in neuromuscular electrical stimulation–induced resistance training for 16 weeks. Data adapted from Ryan et al,20 and untrained SCI controls added for comparison.
Discussion
These results demonstrate the potent effect of the NMES-induced resistance training protocol, first described by Gary A. Dudley in 1999,18 on skeletal muscle hypertrophy in people with chronic SCI. The protocol requires only 40 contractions per day, 2 days per week, to evoke substantial hypertrophy of the quadriceps femoris. Since the initial article, results have been replicated using training periods of 12 to 16 weeks.19–21,25 We also present previously unpublished data to suggest that with longer training periods, the skeletal muscle after SCI can continue to respond by growing about 3% per week. We are not aware of any other interventions in people with SCI that can claim an 80% increase in muscle cross-sectional area in 6 months. Increased levels of lean tissue are generally associated with better overall health in the general population, so finding appropriate interventions to do the same in people with SCI seems appropriate. It should be noted that the health-related benefits of the gains reported in this article require further study.
The robust response of the skeletal muscle after SCI reported by studies utilizing the Dudley protocol are superior to other studies that used alternative modes of exercise. For example, Mohr et al15 utilized an FES cycle ergometer and trained people with SCI about 3 times per week for 1 year and reported an increase in muscle cross-sectional area of 12%. A similar yet shorter duration study conducted by Sloan et al26 utilized FES cycling and reported an increase of quadriceps femoris muscle CSA of about 10%. Frotzler et al17 found that FES cycling produced increases in muscle cross-sectional area similar to that reported for the Dudley protocol. That study, which was focused on bone health, trained participants about 5 times per week for 60-minute sessions for 1 year to produce increases in muscle size. The majority of FES cycling exercise studies do not produce the same gains in muscle mass as the Dudley protocol. In order to obtain substantial gains in muscle mass, the loads need to be high and both concentric and eccentric muscle actions are essential.27 The unique aspect of the Dudley protocol is that it has an eccentric phase to each repetition. Another advantage of the Dudley protocol is that it requires minimal equipment (leg weights and an NMES unit) as opposed to a more expensive FES cycle that may require expert assistance to set up and monitor.
In addition to increases in muscle mass of the quadriceps muscles, changes in other physiological variables have been reported. The protocol has resulted in reduced muscle fatigue to a 4-minute duration tetanic stimulation fatigue protocol (35 Hz trains, 1 sec on/4 sec off ).28 These results could have been due to increased muscle metabolism20 or reduced muscle injury.13,29 The Dudley protocol has also resulted in about a 25% increase in muscle mitochondrial capacity as measured by 31P magnetic resonance spectroscopy20 and increases in the operating range of the posterior tibial artery.30
One day, there may be a cure for SCI. Should this occur, most individuals with SCI may not be able to take advantage of such treatments due to deteriorated musculoskeletal systems and generally poor health. Therefore, NMES-induced exercise treatments may provide the necessary stimulus to benefit the musculoskeletal system. The most up-to-date, evidence-based exercise guidelines for people with SCI recommend that aerobic and strength-training activities be performed 2 times per week.31 We support these guidelines and suggest that the Dudley protocol could be incorporated into exercise programs with the appropriate instructions and available equipment. Currently, individuals with SCI need specific exercise guidelines to maintain their musculoskeletal integrity; NMES-induced resistance training is a simple and effective tool that should be considered.
We are not aware of any studies that have utilized the Dudley protocol and investigated the specific impact of training on the affected bone. Whereas significant muscle atrophy occurs after SCI, the bone also undergoes major remodeling in response to the resultant unloading. Both cortical32 and trabecular bone33,34 are lost, likely due to the lack of repetitive loading that occurs with ambulation and exercise. The work conducted by Shields’ group suggests that long-term NMES training, when started soon after injury, can slow the loss of bone35; however, it is unclear what impact longterm training with the Dudley protocol described in this report has on the skeletal system. We suggest that future studies should carefully consider the impact of this type of NMES training on the bone of people with chronic SCI.
The NMES-induced resistance training protocol first described by Dudley in 1999 can yield significant muscle hypertrophy, as evidenced by studies conducted over 12 to 24 weeks. It is superior to any other intervention that we are aware of at inducing large gains in muscle mass for people with SCI. However, the impact of increased skeletal muscle mass on health and function in people with SCI needs further study.
Acknowledgments
The authors have no known conflicts of interest. Portions of this work were supported by grants from the National Institutes of Health (R01HD039676 to K.K.M.) and the National Institute on Disability, Independent Living, and Rehabilitation Research (H133F130051 to C.Y.F.).
References
- 1. Zhang D, He X. A meta-analysis of the motion function through the therapy of spinal cord injury with intravenous transplantation of bone marrow mesenchymal stem cells in rats. PloS One. 2014;9(4):e93487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137(pt 5):1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cruciger O, Tegenthoff M, Schwenkreis P, Schildhauer TA, Aach M. Locomotion training using voluntary driven exoskeleton (HAL) in acute incomplete SCI. Neurology. 2014;83(5):474. [DOI] [PubMed] [Google Scholar]
- 4. Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(4):373–378. [DOI] [PubMed] [Google Scholar]
- 5. Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: A cross-sectional study. J Appl Physiol. 2003;95(6):2398–2407. [DOI] [PubMed] [Google Scholar]
- 6. Cragg JJ, Noonan VK, Noreau L, Borisoff JF, Kramer JK. Neuropathic pain, depression, and cardiovascular disease: A national multicenter study. Neuroepidemiology. 2015;44(3):130–137. [DOI] [PubMed] [Google Scholar]
- 7. Gater DR., Jr Obesity after spinal cord injury. Phys Med Rehabil Clinics N Am. 2007;18(2):333–351, vii. [DOI] [PubMed] [Google Scholar]
- 8. Grimby G, Broberg C, Krotkiewska I, Krotkiewski M. Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med. 1976;8(1):37–42. [PubMed] [Google Scholar]
- 9. Stilwill EW, Sahgal V. Histochemical and morphologic changes in skeletal muscle following cervical cord injury: A study of upper and lower motor neuron lesions. Arch Phys Med Rehabil. 1977;58(5):201–206. [PubMed] [Google Scholar]
- 10. Scelsi R, Marchetti C, Poggi P, Lotta S, Lommi G. Muscle fiber type morphology and distribution in paraplegic patients with traumatic cord lesion. Histochemical and ultrastructural aspects of rectus femoris muscle. Acta Neuropathol (Berl). 1982;57(4):243–248. [DOI] [PubMed] [Google Scholar]
- 11. Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord. 1997;35(2):86–91. [DOI] [PubMed] [Google Scholar]
- 12. Castro MJ, Apple DF, Jr., Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86(1):350–358. [DOI] [PubMed] [Google Scholar]
- 13. Bickel CS, Slade JM, Dudley GA. Long-term spinal cord injury increases susceptibility to isometric contraction-induced muscle injury. Eur J Appl Physiol. 2004;91(2–3):308–313. [DOI] [PubMed] [Google Scholar]
- 14. Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord. 1999;37(4):264–268. [DOI] [PubMed] [Google Scholar]
- 15. Mohr T, Andersen JL, Biering-Sorensen F, et al. Longterm adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35(1):1–16. [DOI] [PubMed] [Google Scholar]
- 16. Rochester L, Chandler CS, Johnson MA, Sutton RA, Miller S. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 1. Contractile properties. Paraplegia. 1995;33(8):437–449. [DOI] [PubMed] [Google Scholar]
- 17. Frotzler A, Coupaud S, Perret C, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43(1):169–176. [DOI] [PubMed] [Google Scholar]
- 18. Dudley GA, Castro MJ, Rogers S, Apple DF., Jr A simple means of increasing muscle size after spinal cord injury: A pilot study. Eur J Appl Physiol Occup Physiol. 1999;80(4):394–396. [DOI] [PubMed] [Google Scholar]
- 19. Mahoney ET, Bickel CS, Elder C, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86(7):1502–1504. [DOI] [PubMed] [Google Scholar]
- 20. Ryan TE, Brizendine JT, Backus D, McCully KK. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil. 2013;94(11):2166–2173. [DOI] [PubMed] [Google Scholar]
- 21. Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44(1):165–174. [DOI] [PubMed] [Google Scholar]
- 22. Ruther CL, Golden CL, Harris RT, Dudley GA. Hypertrophy, resistance training, and the nature of skeletal muscle activation. J Strength Cond Res. 1995;9(3):155–159. [Google Scholar]
- 23. Stevenson SW, Dudley GA. Dietary creatine supplementation and muscular adaptation to resistive overload. Med Sci Sports Exerc. 2001;33(8): 1304–1310. [DOI] [PubMed] [Google Scholar]
- 24. McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81(5):2004–2012. [DOI] [PubMed] [Google Scholar]
- 25. Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: Case report. J Spinal Cord Med. 2010;33(1):90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sloan KE, Bremner LA, Byrne J, Day RE, Scull ER. Musculoskeletal effects of an electrical stimulation induced cycling programme in the spinal injured. Paraplegia. 1994;32(6):407–415. [DOI] [PubMed] [Google Scholar]
- 27. Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand. 1991;143(2):177–185. [DOI] [PubMed] [Google Scholar]
- 28. Sabatier MJ, Stoner L, Mahoney ET, et al. Electrically stimulated resistance training in SCI individuals increases muscle fatigue resistance but not femoral artery size or blood flow. Spinal Cord. 2006;44(4):227–233. [DOI] [PubMed] [Google Scholar]
- 29. Mahoney E, Puetz TW, Dudley GA, McCully KK. Low-frequency fatigue in individuals with spinal cord injury. J Spinal Cord Med. 2007;30(5):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stoner L, Sabatier MJ, Mahoney ET, Dudley GA, McCully KK. Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury. Spinal Cord. 2007;45(1):49–56. [DOI] [PubMed] [Google Scholar]
- 31. Ginis KA, Hicks AL, Latimer AE, et al. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord. 2011;49(11):1088–1096. [DOI] [PubMed] [Google Scholar]
- 32. Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA. Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone. 2005;36(2):331–339. [DOI] [PubMed] [Google Scholar]
- 33. Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res. 2004;19(1):48–55. [DOI] [PubMed] [Google Scholar]
- 34. Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA. Trabecular bone is more deteriorated in spinal cord injured versus estrogenfree postmenopausal women. Osteoporos Int. 2005;16(3):263–272. [DOI] [PubMed] [Google Scholar]
- 35. Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: Effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95(4):2380–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]