Abstract
Background:
Optimal dosing of vancomycin in morbidly obese patients (>100 kg and at least 140% of their ideal body weight) has not been determined. Conventional dosing strategies have led to the observation of supratherapeutic trough concentrations (>20 mcg/mL).
Objective:
To evaluate the effectiveness of a new vancomycin dosing protocol in morbidly obese patients in achieving therapeutic trough concentrations between 10 and 20 mcg/mL and to determine patient-specific factors influencing the trough concentration attained.
Methodology:
A single-center, retrospective chart review included morbidly obese adult patients with a pharmacy-to-dose vancomycin consult and at least 1 trough concentration obtained at steady state. Patients were excluded if they had a creatinine clearance (CrCl) less than 35 mL/min or unstable renal function, were not dosed according to the revised protocol, or received vancomycin prior to initiation of the protocol.
Results:
Of the 48 patients included, 17 (35.4%) achieved a therapeutic vancomycin trough concentration. Subtherapeutic concentrations (<10 mcg/mL) were observed in 27 patients (56.3%) and supratherapeutic concentrations were observed in 4 (8.3%) patients. Age less than 45 years and CrCl greater than 100 mL/min were associated with subtherapeutic trough concentrations.
Conclusion:
This study demonstrates that the revised vancomycin dosing protocol led to the attainment of therapeutic trough concentrations in 35.4% of patients. The majority had subtherapeutic concentrations, which increases the risk of treatment failures and resistance. Further study is needed to determine the optimal dosing strategy in this patient population.
Keywords: dosing, obesity, therapeutic drug monitoring, vancomycin
Vancomycin hydrochloride is a glycopeptide antibiotic used in the treatment of gram-positive infections, most notably for infections caused by methicillin-resistant Staphylococcus aureus (MRSA).1 Currently, MRSA accounts for greater than 50% of all S. aureus infections in many institutions and exceeds 70% in many intensive care unit (ICU) settings.2 Vancomycin therapy is assessed by serum trough concentrations obtained when the drug reaches steady state. Vancomycin trough concentrations vary based on the severity of illness and by indication.3 Trough concentrations less than 10 mcg/mL have been associated with treatment failures, whereas elevated concentrations have been associated with toxicities.4 Although the toxicity profile has improved greatly with purification, vancomycin still has the potential to cause nephrotoxicity and ototoxicity. Prolonged duration of vancomycin therapy beyond 7 days, doses greater than or equal to 4 g/day, trough concentrations greater than or equal to 15 mcg/mL, and concomitant nephrotoxic therapy have been associated with the development of nephrotoxicity.5–9
Although the rate of obesity is increasing in the United States, little is known about the optimal dosing of vancomycin in this patient population. The 2009 consensus statement published by the Infectious Disease Society of America (IDSA), the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists provided recommendations for the dosing and monitoring of vancomycin in adult patients. These guidelines recommend a vancomycin loading dose of 25 to 30 mg/kg in critically ill patients followed by a maintenance dose of 15 mg/kg every 8 to 12 hours. Dosing is to be based on total body weight (TBW) and adjusted accordingly based on serum vancomycin concentrations.10
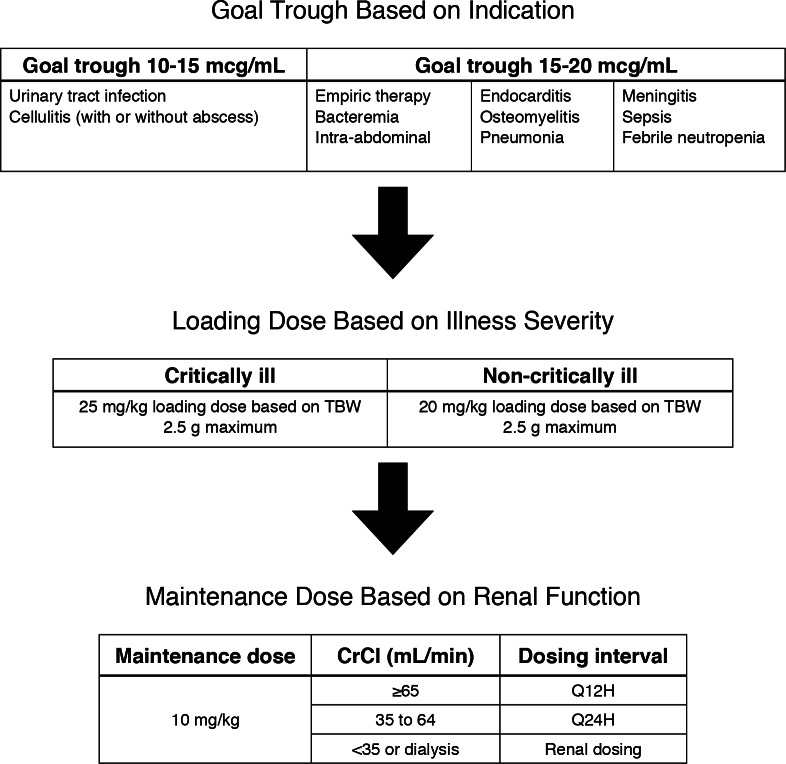
The previous pharmacy-to-dose vancomycin protocol at Carolinas Medical Center for patients with adequate renal function was consistent with the IDSA guideline recommendations; no specifications were made regarding morbidly obese patients. A medication use evaluation was conducted to assess this protocol across the Carolinas HealthCare System. Of the trough concentrations observed that exceeded 20 mcg/mL, 63.6% occurred in patients weighing more than 100 kg. As a result of this observation, a new pharmacyto- dose protocol was implemented in October 2013 with updated recommendations for dosing vancomycin in morbidly obese patients. The new dosing strategy recommended a 20 to 25 mg/kg loading dose for all patients (with the 25 mg/kg loading dose reserved for those with critical illness) followed by a 10 mg/ kg dose administered every 12 to 24 hours depending on renal function (Figure 1). All doses were based on TBW. The addition of a loading dose for all morbidly obese patients and a lower mg/kg maintenance dose differ from the IDSA recommendations. The new protocol was based upon a method identified by DeRyke et al12 and implemented in clinical practice at a large, academic medical center by Reynolds et al.11
Figure 1.
Vancomycin dosing protocol for morbidly obese patients. CrCl = creatinine clearance; TBW = total body weight; Q12H = every 12 hours; Q24H = every 24 hours.
The primary objective of this study was to evaluate the effectiveness of a new vancomycin dosing strategy in achieving therapeutic trough concentrations of 10 to 20 mcg/mL in morbidly obese patients.
Methods
This was a single-center, retrospective study conducted at Carolinas Medical Center, an 894-bed academic medical center. The study was approved by the institutional review board. Patients were identified through pharmacy consultations-to-dose vancomycin requested from June 1, 2013 to February 15, 2014 and screened for inclusion. Patients were included if they were 18 years old or older, morbidly obese (defined as ≥100 kg combined with a TBW ≥140% of ideal body weight [IBW]),11 and had at least one vancomycin trough concentration drawn at steady state, defined as 5 half-lives or prior to the fourth or fifth dose, within 25% of the dosing interval (ie, up to 3 hours before a dose in a patient being dosed every 12 hours). Patients were excluded if they had a creatinine clearance (CrCl) less than 35 mL/min at initiation of therapy or unstable renal function, defined as an increase in serum creatinine (SCr) of greater than or equal to 0.3 mg/dL from historical baseline, if available within the previous year, or from the first recorded SCr in the absence of physician documentation of acute renal failure in the first 48 hours of therapy. Additional exclusion criteria were pregnancy, dosing strategies outside of the revised vancomycin protocol, and initiation of vancomycin therapy prior to admission to Carolinas Medical Center. If a dose had been rescheduled by more than 50% of the interval between doses (ie, more than 6 hours after the original scheduled administration time with a 12-hour dosing interval), the patient was also excluded.
Outcomes
The primary outcome measure was the proportion of patients attaining target trough concentrations of 10 to 20 mcg/mL extrapolated from the first measured trough at steady state. Secondary objectives included the frequencies of therapeutic trough concentrations ranging from 10 to 14.99 mcg/mL and 15 to 20 mcg/mL, frequencies of trough concentrations above and below target range, and frequency of trough concentrations greater than or equal to 30 mcg/mL, a quality indicator at our institution. An analysis of patient-specific factors including renal function, TBW, percentage above IBW, concomitant nephrotoxins, comorbid conditions, CrCl, and age was conducted to determine potential factors contributing to the attainment of a therapeutic (10–20 mcg/ mL) trough concentration. The incidence of nephrotoxicity during vancomycin therapy, as defined by the Acute Kidney Injury Network criteria as an increase in SCr greater than or equal to 0.3 mg/dL from baseline or an increase in SCr greater than or equal to 150% to 200% from baseline in less than 48 hours or if urine output decreased to less than 0.5 mL/kg/h for more than 6 hours, was evaluated.13 The frequency of dose and interval adjustments, length of therapy, ICU length of stay (LOS), and overall LOS were also assessed.
Data Collection
Study data were collected and managed using REDCap electronic data capture tools hosted at Carolinas Medical Center.14 Patient-specific demographic data collected included age, height, and weight as recorded by the pharmacist; data were used to calculate vancomycin doses, gender, race, and ICU status. IBW and percentage above IBW were calculated from these data using the Devine equations.15 Clinical data collected included vancomycin dose administered in both total mg and mg/kg, frequency of administration, goal trough concentration, and indication for vancomycin therapy. Loading doses administered as more than one dose were counted collectively as the first administration. Pertinent comorbid health conditions and concomitant anti-infectives and nephrotoxic agents, including contrast medication for radiologic procedures, were also collected. CrCl was calculated using adjusted body weight and the Cockcroft- Gault equation.16 In patients 65 years old and older, the SCr was rounded to 1 mg/dL to limit overestimation of renal function, which was the protocol for the institution at that time.
Statistical Analysis
Descriptive statistics including means, medians, standard deviations, and interquartile ranges (IQR) were calculated. Shapiro-Wilk tests were used to test for normality. Several variables were not normally distributed; therefore, nonparametric tests were used. The patients were divided into 2 groups: those who attained a therapeutic trough concentration, and those who did not. For data measured on an interval scale, Student t tests or Wilcoxon rank-sum tests were used. For nominal data, the chi-square or Fisher’s exact test was performed. To test for linear relationships among variables measured on an interval scale, Spearman’s correlation coefficients were calculated. Breakpoints were determined after reviewing continuous data such as age and CrCl in order to create categorical variables. In a separate analysis of only those subjects who obtained target trough concentrations of 10 to 20 mcg/mL, the subjects were divided into those who were in the 10 to 14.99 mcg/mL range and those who were in the 15 to 20 mcg/mL range. The proportion of patients in each of the subranges was reported. SAS Enterprise Guide, version 5.1, was used for all analyses. A two-tailed P value of less than .05 was considered statistically significant.
Population pharmacokinetics were used to extrapolate concentrations obtained to determine an estimate of true vancomycin trough concentrations. Equations utilized for the clearance of vancomycin (Clvancomycin), as described by Leonard and Boro17 and authenticated by Leong et al18; volume of distribution (Vd); the elimination constant (Ke); and the extrapolated trough concentration are displayed in Figure 2.19
Figure 2.
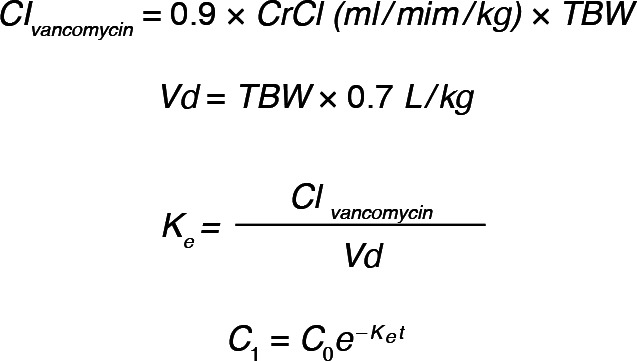
Equations for extrapolation of vancomycin concentrations.
Results
Patient Inclusion and Baseline Characteristics
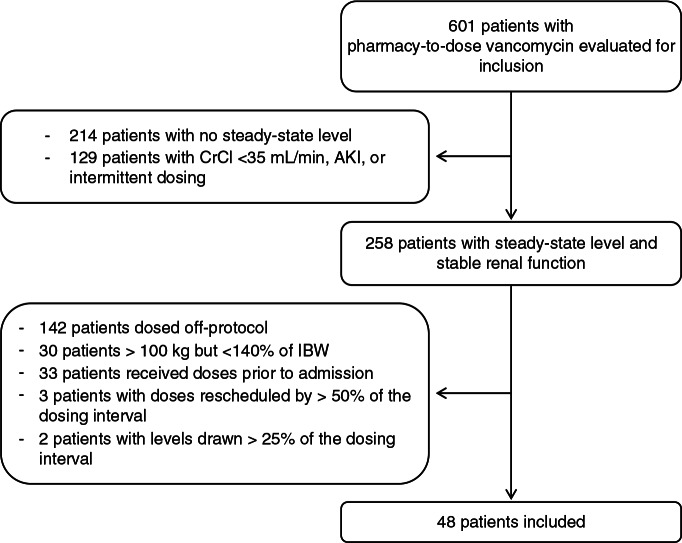
A total of 601 patients were screened for inclusion in the study via review of pharmacy-to-dose vancomycin data collection forms. A total of 48 patients were enrolled, representing an inclusion rate of 8% (Figure 3). Baseline characteristics are presented in Table 1. The median age was 52.5 years (IQR, 42.5–58.5), one-third of patients were in the ICU (33.3%; n = 16), and median CrCl was 122 mL/min (IQR, 93–153). Only 2 patients had a CrCl less than 65 mL/min. The most common indication for therapy was pneumonia (37.5%; n = 18) and the majority of patients had a goal trough target of 15 to 20 mcg/mL (77.1%; n = 37). The median duration of therapy was 4 days (IQR, 3–6).
Figure 3.
Patient screening and inclusion. AKI = acute kidney injury; CrCl = creatinine clearance; IBW = ideal body weight.
Table 1. Baseline characteristics (N = 48).
| Baseline characteristics | Median (IQR) or n (%) |
| Age, years | 52.5 (42.5–58.5) |
| % Female | 27 (56.3) |
| Race | |
| Caucasian | 27 (56.3) |
| African American | 19 (39.6) |
| Other | 2 (4.2) |
| ICU status/critically ill | |
| ICU | 16 (33.3) |
| Non-ICU | 32 (66.7) |
| Total body weight, kg | 122.5 (108.7–140.2) |
| Percent above ideal body weight | 206.5 (162–228.5) |
| SCr, mg/dL | 0.86 (0.63–0.99) |
| CrCl, mL/min | 122 (93–152.5) |
| Indication for therapy | |
| Pneumonia | 18 (37.5) |
| Cellulitis | 9 (18.8) |
| Empiric | 5 (10.4) |
| Febrile neutropenia | 3 (6.3) |
| Intra-abdominal infection | 3 (6.3) |
| Sepsis | 3 (6.3) |
| Meningitis | 2 (4.2) |
| Osteomyelitis | 1 (2.1) |
| UTI | 1 (2.1) |
| Other | 3 (6.3) |
| Goal trough | |
| 10–15 mcg/mL | 11 (22.9) |
| 15–20 mcg/mL | 37 (77.1) |
| Comorbidities | |
| Diabetes | 21 (43.8) |
| Malignancy | 12 (25) |
| Congestive heart failure | 8 (16.7) |
| Other | 7 (14.6) |
| End-stage liver disease | 1 (2.1) |
| Chronic kidney disease | 1 (2.1) |
| Hypermetabolic state | 1 (2.1) |
| No comorbidities | 14 (29.2) |
| Concomitant nephrotoxins | |
| Antimicrobials | 46 (95.8) |
| Piperacillin-tazobactam | 27 (56.3) |
| Other beta-lactams | 20 (41.7) |
| Fluoroquinolones | 11 (22.9) |
| Aminoglycosides | 3 (6.3) |
| Other antimicrobials | 12 (25.0) |
| Other nephrotoxins | 32 (66.7) |
| Diuretics | 23 (47.9) |
| Contrast medication | 17 (35.4) |
| NSAIDs | 4 (8.3) |
| Length of stay, days | 10.5 (7–19.5) |
| ICU length of stay, days | 7.5 (3.5–15) |
| Duration of therapy, days | 4 (3–6) |
Note: CrCl = creatinine clearance; ICU = intensive care unit; NSAIDs = nonsteroidal anti-inflammatory drugs; SCr = serum creatinine; UTI = urinary tract infection.
Vancomycin Dosing
The median loading dose was 19.4 mg/kg (IQR, 17.8–20.5), with the 2,500 mg maximum dose administered to 79.2% of patients (n = 38), 2,250 mg administered to 2% of patients (n = 1), and 2,000 mg administered to 18.8% of patients (n = 9). The median maintenance dose was 9.9 mg/kg (IQR, 9.6–10.2) or 1,250 mg (IQR, 1,000–1,500). All doses were administered every 12 hours, consistent with protocol recommendations and baseline renal function.
Vancomycin Trough Concentrations
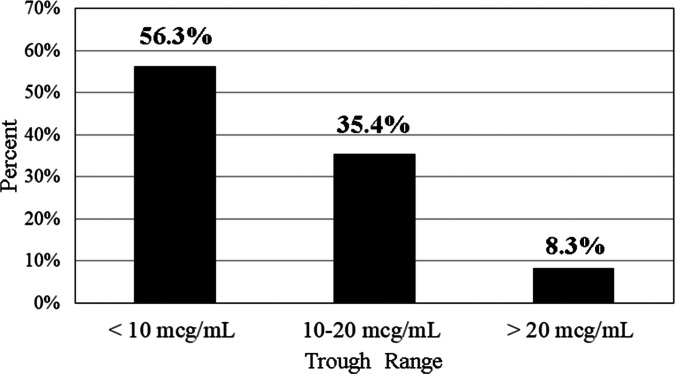
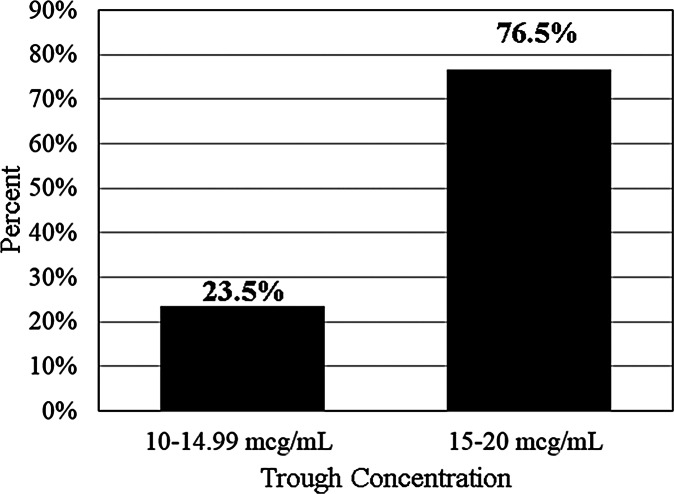
Initial vancomycin trough concentrations are presented in Figure 4. Subtherapeutic trough concentrations of less than 10 mcg/mL were observed in 56.3% of patients (n = 27), therapeutic trough concentrations of 10 to 20 mcg/mL were observed in 35.4% of patients (n = 17), and supratherapeutic concentrations of less than 20 mcg/mL were observed in 8.3% of patients (n = 4). Of the therapeutic trough concentrations, 23.5% (n = 4) were in the 10 to 14.99 mcg/mL range, while 76.5% (n = 13/17) were in the 15 to 20 mcg/mL range (Figure 5). The median trough value after extrapolation was 9.5 mcg/mL (IQR, 6.7–13.6). No patients had trough concentrations greater than 30 mcg/mL. The trough concentrations attained for patients with goal troughs of 10 to 15 mcg/mL and 15 to 20 mcg/mL are further detailed in Table 2.
Figure 4.
Initial trough concentration (N = 48).
Figure 5.
Trough concentrations of patients in therapeutic range (n / N = 17/48).
Table 2. Vancomycin trough concentrations at steady state (N = 48).
| Variable | Median (IQR) or n (%) |
| Therapeutic trough achieved | |
| Yes | 17 (35.4) |
| No | 31 (64.6) |
| Goal trough 10–15 mcg/mL | |
| (n = 11) | |
| <10 mcg/mL | 6 (54.6) |
| 10–14.99 mcg/mL | 2 (18.2) |
| >14.99 mcg/mL | 3 (27.3) |
| Goal trough 15–20 mcg/mL | |
| (n = 37) | |
| <15 mcg/mL | 30 (81.1) |
| 15–20 mcg/mL | 4 (10.8) |
| >20 mcg/mL | 3 (8.1) |
| Extrapolated trough, mcg/mL | 9.5 (6.7–13.6) |
Secondary Trough Extrapolations
Eight patients with trough concentrations less than 10 mcg/mL continued therapy with changes in dose and follow-up steady-state concentrations. Six of these patients ultimately reached a therapeutic trough goal of 10 to 20 mcg/mL. It took 6 to 11 doses to achieve the target trough, with concentrations ranging from 11.2 to 17 mcg/mL. Of these trough concentrations, 5 were in the 10 to 14.99 mcg/mL range and 1 was in the 15 to 20 mcg/mL range. Doses ranged from 9.8 to 14.3 mg/kg, with 3 patients requiring an 8-hour dosing interval to reach therapeutic trough concentrations and 3 patients requiring a 12-hour interval.
Nephrotoxicity
One patient experienced nephrotoxicity while receiving vancomycin therapy. The patient’s SCr peaked at 1.55 mg/dL from a baseline of 1.05 mg/dL, with a vancomycin level of 29.1 mcg/mL on the day of the peak SCr. Of note, the patient was also receiving diuretics and piperacillin/tazobactam.
Analysis of Patient-Specific Variables on Trough Concentrations
Patient-specific variables were analyzed to determine whether any of these factors influenced trough concentrations. As we found the majority of patients had subtherapeutic troughs, we compared the group that achieved therapeutics troughs from 10 to 20 mcg/mL to the group that achieved troughs less than 10 mcg/mL (n = 44). Variables included were age, SCr, CrCl, ICU status, TBW, percentage above IBW, 1 or more comorbidities, and other nephrotoxic therapy. Table 3 shows the P values for these factors of which age younger than 45 years (P = .0073) and CrCl greater than or equal to 100 mL/ min (P = .0170) were associated with subtherapeutic trough concentrations. Several of these variables were related, including age with extrapolated trough (r = 0.52856, P = .0002), CrCl with extrapolated trough (r = −0.45405, P = .002), and age with CrCl (r = −0.68121, P < .0001).
Table 3. Factors influencing achievement of therapeutic trough concentrations of 10–20 mcg/mL.
| Variable | P |
| Age | .0160 |
| Age ≥ 45 years | .0073 |
| SCr | .3979 |
| CrCl <100 mL/min | .0170 |
| ICU status | .0850 |
| Total body weight | .1350 |
| Percentage above ideal body weight | .0567 |
| Comorbidities ≥1 | .7395 |
| Diabetes | .9772 |
| Other nephrotoxic therapy | .6034 |
Note: CrCl = creatinine clearance; ICU = intensive care unit; SCr = serum creatinine.
Discussion
This study adds to the scarce literature available to guide vancomycin dosing in the morbidly obese patient population. The optimal dosing strategy in this patient population is yet to be determined, and there is considerable pharmacokinetic variability between patients. Historically, studies have concluded that vancomycin should be dosed by TBW rather than IBW. Several studies have shown an increase in vancomycin clearance in obese patients secondary to increased glomerular filtration rate due to increased blood volume, kidney size, and a greater number of functional nephrons. These studies have also shown increased but nonsignificant elevations in volume of distribution of vancomycin.20–23 Conflicting studies suggest that the increased volume of distribution in morbidly obese patients is responsible for drug accumulation and supratherapeutic vancomycin concentrations.8,23
Reynolds and colleagues observed supratherapeutic trough concentrations in greater than 50% of their population prior to the implementation of the revised protocol. This retrospective, observational study conducted at an 808-bed urban teaching facility included 64 patients in the original protocol group and 74 patients in the revised protocol group. The majority of the patients were treated for skin and soft tissue infections. This new dosing strategy, consistent with the strategy we utilized in our protocol, resulted in a significantly higher frequency of trough concentrations within the goal range of 10 to 20 mcg/mL (59% vs 36%; P = .006) and a lower frequency of trough concentrations greater than 20 mcg/ mL (18% vs 55%; P < .001). A higher frequency of trough concentrations less than or equal to 10 mcg/mL was also observed (23% vs 9%; P = .03). The authors identified estimated CrCl of greater than 150 mL/min and a SCr of less than 0.8 mg/dL as factors that were associated with subtherapeutic trough concentrations.11 With the exception of indication for therapy, the patients in our study had similar baseline characteristics but did not achieve a similar proportion of therapeutic troughs. This may be due to an underlying difference in patient population that was not captured by the retrospective, observational nature of our study. Similarly to Reynolds et al, we identified that patients with better baseline renal function may not be able to achieve trough concentrations between 10 and 20 mcg/mL by this dosing strategy, although our cutoff was observed at a lower CrCl value of greater than or equal to 100 mL/min.
As a single-center, retrospective study, several limitations were present. One of the largest limitations was the lack of a retrospective comparator group due to the absence of a consistent and standardized dosing protocol for attaining goal trough concentrations of 10 to 20 mcg/mL prior to the implementation of the protocol revision. The small sample size of the entire population as well as subgroups and the non-normally distributed data may have increased the risk of both type I and type II errors. The retrospective nature of this study also leads to the potential for bias. An additional limitation was a relatively young, healthy patient population with normal to above-average renal function observed, which limits the applicability of these data to other patient populations. The relatively short duration of therapy may have been responsible for the lack of vancomycin accumulation beyond the first steady-state concentration. Treatment outcomes and culture data were not assessed due to the largely short and empiric courses of therapy.
This revised dosing strategy led to 35.4% of patients achieving a goal trough concentration, with the majority (56.3%) of the patients having concentrations that fell in the subtherapeutic range. This potentially places patients dosed by this protocol at risk for treatment failures as well as the development of vancomycin-resistant organisms, although these parameters were not assessed in this study. Very few supratherapeutic troughs (8.3%) were observed, and only one case of nephrotoxicity was observed. No trough concentrations greater than 30 mcg/mL were observed, suggesting that this dosing strategy poses little harm to patients, potentially as a result of the majority of troughs being subtherapeutic. Our study indicated that CrCl less than 100 mL/min and age greater than 45 years were associated with the attainment of therapeutic trough concentrations. These variables were found to have a statistically significant relationship, although the r values observed represented weak correlations. As a result, the value of one variable cannot be used to reliably predict the value of another. As such, the clinical impact of this relationship cannot be assessed. Our findings suggest that age and renal function may contribute to attainment of target trough concentrations in our patient population. A dosing strategy more consistent with the IDSA guideline recommendations of a 15 mg/kg maintenance dose may represent a potential revision to our dosing protocol for patients younger than 45 years and with CrCl greater than 100 mL/min. Weight and percentage of TBW above IBW did not significantly influence the trough concentration obtained, suggesting that the degree of morbid obesity does not play a role in attainment of therapeutic trough concentrations.
This new vancomycin dosing protocol achieved therapeutic troughs in 35.4% of patients at a large, urban, academic medical center. Younger patients (<45 years old) and patients with average or aboveaverage renal function, defined as CrCl greater than 100 mL/min, may have difficulty obtaining goal troughs when utilizing this conservative dosing strategy. Further studies including larger patient populations and differing dosing strategies are warranted.
Acknowledgments
The authors have no conflicts of interest or funding to disclose.
References
- 1.Vancomycin [package insert]. Lake Forest, IL: Hospira, Inc.; 2012.
- 2.National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:1–38. [DOI] [PubMed] [Google Scholar]
- 4.Charles PG, Ward PB, Johnson PD, et al. Clinical features associated with bacteremia due to heterogenous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2011;38:448–451. [DOI] [PubMed] [Google Scholar]
- 5.Wong-Beringer A, Joo J, Tse E, et al. Vancomycin-associated nephprotoxicity: Critical appraisal of risk with high-dose therapy. Int J Microb Agents. 2011;37:91–95. [DOI] [PubMed] [Google Scholar]
- 6.Jeffres MN, Isakov W, Doherty JA, et al. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health-care associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP, Lomaestro B, Graves J, et al. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52:1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farber B, Moellering RC. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother. 1983;23:138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced neprotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Disease Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds DC, Waite LH, Alexander DP, et al. Performance of a vancomycin dosage regimen developed for obese patients. Am J Health Syst Pharm. 2012;69:944–950. [DOI] [PubMed] [Google Scholar]
- 12.DeRyke CA, Alexander DP. Optimizing vancomycin dosing through pharmacodynamic assessment targeting area under the concentration-time curve/minimum inhibitory concentration. Hosp Pharm. 2009;44:751–765. [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Workgroup. KDIGO practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devine BJ. Gentamicin therapy. Drug Intell Clin Pharm. 1974;8:650–655. [Google Scholar]
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 17.Leonard AE, Boro MS. Vancomycin pharmacokinetics in middle aged and elderly men. Am J Hosp Pharm. 1994;51:798–800. [PubMed] [Google Scholar]
- 18.Leong J, Boro MS, et al. Determining vancomycin clearance in an overweight and obese population. Am J Health Syst Pharm. 2011;68:599–603. [DOI] [PubMed] [Google Scholar]
- 19.Burton ME, Shaw LM, Schentag JJ, Evans WE. Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 20.Ducharme MP, Slaughter RL, Edwards DJ. Vancomycin pharmacokinetics in a patient population: Effect of age, gender, and body weight. Ther Drug Monit. 1994;16: 513–518. [DOI] [PubMed] [Google Scholar]
- 21.Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol. 1998;54: 621–625. [DOI] [PubMed] [Google Scholar]
- 22.Blounin RA, Bauer LA, Miller DD. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vance-Bryan K, Guay DP, Gilliland SS, et al. Effect of obesity on vancomycin pharmacokinetic parameters as determined by using a Bayesian forecasting technique. Antimicrob Agents Chemother. 1993;37:436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]