Abstract
During July 2014, 14 brown anoles, Anolis sagrei Duméril and Bibron were collected from Orange County, Florida, U.S.A., and their faeces examined for coccidian parasites. One (7%) harboured an eimerian that we describe here as new. Oöcysts of Eimeria garmani sp. n. were ellipsoidal with a uni-layered wall and measured (length × width, L × W) 19.3 × 12.5 μm, with a length/width (L/W) ratio of 1.5. A micropyle, oöcyst residuum and polar granule were absent. Sporocysts were subspheroidal, 6.8 × 6.3 μm, L/W 1.1. Stieda, substieda and parastieda bodies were absent. A sporocyst residuum was present as dispersed granules. Endogenous stages were observed within the small intestine. This is the first coccidian reported from the brown anole and the third eimerian reported from anoles in the United States.
Introduction
The brown anole, Anolis sagrei Duméril and Bibron, is a brown to gray lizard that is native to Cuba, the Isla de Juventud, the Bahamas, Swan Island, and the Islas de la Bahia, Honduras (Schwartz & Henderson, 1991); it has been introduced into 13 US states and is now established in Alabama, Arkansas, Florida, Georgia, Louisiana, North Carolina, South Carolina and Texas (Conant & Collins, 1998; Parmley 2002; McAllister et al., 2003; Crother et al., 2012; Dixon 2013). Around 1887, brown anoles came to southern Florida (Garman, 1887) and, more recently, have colonized Hawaii (Goldberg et al., 2000), Jamaica (Bundy et al., 1987), and Taiwan (Norval et al., 2011). Brown anoles primarily feed on insects but they also consume lizards, including smaller conspecifics, native green anoles, Anolis carolinensis Voigt and nonindigenous red-sided curly-tailed lizards, Leiocephalus schreibersii (Gravenhorst) in Florida (Krysko & Wasilewski, 2013).
Although a great deal has been published on their helminth parasites (Bundy et al., 1987; Goldberg et al., 1994, 2002; Goldberg & Bursey, 2000; Norval et al., 2011; Langford et al., 2013), nothing is known of coccidia in these lizards (McAllister et al., 2014). Herein, we provide a description of an eimerian from A. sagrei from Florida.
Materials and methods
During July 2014, 14 juvenile and adult A. sagrei were collected from Orange County, Florida, USA, and their faeces examined for coccidian parasites. Faecal samples from the rectum were placed in individual vials containing 2.5% (w/v) aqueous potassium dichromate (K2Cr2O7). Samples were examined for coccidia by light microscopy after flotation in Sheather’s sugar solution (specific gravity = 1.30). Once they had completely sporulated, measurements were taken on 24 oöcysts from a single anole using a calibrated ocular micrometer or Olympus© cellSens 1.7 digital software and reported in micrometres (μm) with means followed by the ranges in parentheses; photographs were taken using Nomarski interference-contrast optics. Oöcysts were c.110 days old when measured and photographed. Tissue samples from the small intestine and gall bladder of a single infected anole were fixed in 10% neutral–buffered formalin and processed as histological sections (at 6 μm) following standard methods of staining with hematoxylin and eosin.
In an attempt to rupture oocyst walls and subsequently release sporocysts and their sporozoites from sporocyst valve sutures, 150 oocysts in ATL lysis buffer were subjected to 600 mAU/ml proteinase K enzyme (DNA Easy Blood and Tissue Kit, Qiagen, Hilden, Germany) following the manufacturer’s instructions. Oocysts were incubated at 56 C for 2, 6, 12 and 24 hr and, immediately after each time period, samples were placed on a microscopic slide and inspected by light microscopy under oil immersion at 1,000×. We also did another technique by freezing the oocysts for 6 hours at −80 C followed by boiling for 5 minutes. Again, samples were inspected at 1,000×. Because this resulted in about 80% of oocysts being shattered, we repeated this experiment hoping that the remaining intact oocysts would gradually open, releasing sporocysts.
A host voucher was accessioned into the Henderson State University Collection (HSU), Arkadelphia, Arkansas, U.S.A. Photosyntypes of sporulated oöcysts were accessioned into the Harold W. Manter Laboratory of Parasitology (HWML), Lincoln, Nebraska, U.S.A. Scientific names of reptiles follow the TIGR Reptile Database (Uetz & Hošek, 2014).
Results
One (7%) of the A. sagrei was found to be passing oöcysts of a new species of coccidian, which is described below. Unfortunately, attempts at releasing sporozoites from sporocyst sutures was not successful using our two experimental approaches.
Eimeria garmani n. sp
Type-host: Brown anole, Anolis sagrei Duméril and Bibron, 1837 (Reptilia: Sauria: Dactyloidae) (adult male, symbiotype HSU 1801 collected 22.vii.2014).
Type-locality: 24 km SW Orlando, Orange County, Florida, USA (28.352785°N, 81.540483°W).
Type-material: Photosyntype deposited in the HWML 75132.
Prevalence: In 1 of 14 (7%) of the type host.
Sporulation time: Exogenous. All oöcysts were passed unsporulated or partially sporulated and fully sporulated within 5 days at c.23°C.
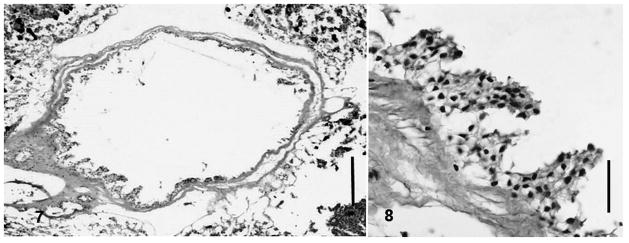
Site of infection: Small intestine (Figs. 5–6). Sections of gall bladder tissue (Figs. 7–8) did not reveal endogenous stages suggesting a Choleoeimeria sp.
Figs. 5–6.
Endogenous stages of Eimeria garmani n. sp. in columnar epithelium of small intestine. 5, Mature meront (ME) containing 6–7 merozoites. 6, Developing meront (ME) enclosed within parasitophorus vacuole. Scale bars: 10 μm.
Figs. 7–8.
Gallbladder sections. 7, Whole gallbladder. Scale bar: 100 μm. 8, Higher magnification of gallbladder showing only sloughed epithelial cells. Scale bar: 10 μm.
Etymology: The specific epithet is given in honor of Samuel Walton Garman (1843–1927), American zoologist and naturalist, who first reported A. sagrei in the U.S.A. in 1887. He was also the first official curator of fishes, amphibians and reptiles at the Museum of Comparative Zoology, Harvard University, Boston, Massachusetts, U.S.A.
Description (Figs. 1–6)
Figs. 1–3.
Nomarski interference-contrast photomicrographs of oocysts of Eimeria garmani n. sp. 1, Ellipsoidal oöcyst and sporocyst (SP) and sporocyst residuum (SR). 2, Oöcyst showing polar granule (PG). 3, Individual sporocyst showing suture (SU). Scale bars: Figs. 1–2, 10 μm; Fig. 3, 2 μm.
Sporulated oöcyst
Oöcyst (n = 24) colourless, smooth, ellipsoidal; 19.3 × 12.5 (17–22 × 12–14), length/width (L/W) ratio 1.5 (1.3–1.7). Wall single-layered, c.0.6 thick. Micropyle absent, oöcyst residuum absent, 1 (bi-lobed), rarely 2 polar granule(s) present.
Sporocyst
Sporocysts (n = 24) four, colourless, smooth, subspheroidal, 6.8 × 6.3 (6–8 × 5–7); L/W ratio 1.1 (1.0–1.2); wall single-layered c.0.5 thick, with valves joined by longitudinal sutures. Stieda body, sub-Stieda, and para-Stieda bodies absent; sporocyst residuum composed of large-sized granules in compact mass between sporozoites.
Sporozoite
Sporozoites 2, sausage-shaped, not measured; single subspheroidal anterior refractile body and subspheroidal posterior refractile body, with nucleus slightly posterior to midpoint.
Remarks
Because we observed endogenous stages in the small intestine (Figs. 5–6) and did not observe endogenous stages developing in hypertrophied, displaced cells of the gall bladder or biliary epithelium (Figs. 6–7) (see Jirků et al., 2002), we placed the new species in the genus Eimeria rather than Choleoeimeria (sensu Paperna & Landsberg, 1989). Even in the absence of distinct DNA sequences that separate these two genera (Jirků et al., 2002), we feel comfortable with this placement.
When the new species is compared to other ellipsoidal eimerians described from other Anolis spp., it is most similar to Eimeria intermedia Ruiz, 1959 from the intermediate anole, Anolis intermedius Peters from Costa Rica (Ruiz, 1959; McAllister et al., 2014). However, oocyst width of the new species is considerably smaller than E. intermedia (12.5 vs. 14.9 μm). It further differs by having subspheroidal to ovoidal sporocysts compared to the spheroidal sporocysts of E. intermedia as well as having polar granules that E. intermedia does not possess.
Discussion
McAllister et al. (2014) recently provided a summation of the coccidia of the lizard family Dactyloidae that included 17 species of coccidians from 10 Anolis spp. in the Western Hemisphere. The number of coccidians known to date is obviously a large underestimate of the total worldwide diversity as only 2.5% of the 395 species of Anolis (Uetz & Hošek 2014) have been reported to harbor coccidia and no one really knows how many have ever been examined in the past but not found to be passing oocysts. Within the United States alone, only native A. carolinensis has been reported to host eimerians, with Eimeria anolidis Daszak & Ball, 1991 from Florida (which actually may be a Choleoeimeria), and Eimeria robisoni McAllister, Seville, & Connior, 2014 from Arkansas. Prior to our study, none of the 10 exotic species of Anolis within the United States had ever been examined, to our knowledge, for coccidia, and here we describe the first coccidian from one of these introduced species. The establishment of A. sagrei within Florida is from multiple introductions, leading to a genetic structure of Florida populations that is unique from native populations within Cuba (Kolbe et al., 2004). It would be interesting to see if this new coccidian occurs within the native range of A. sagrei or of native A. carolinensis in Florida. We suggest additional surveys on other exotic Anolis in order to determine the geographic distribution, diversity, and abundance of coccidia within the continental United States.
Fig. 4.

Composite line drawing of oöcyst of Eimeria garmani n. sp. Scale bar: 5 μm.
Acknowledgments
We thank Drs. S.L. Gardner (HWML) and R. Tumlison (HSU) for expert curatorial assistance and Dr. Dagmara Motriuk-Smith (Univ. Wyoming) for technical assistance. A scientific collecting permit was provided to MBC by the Florida Fish and Wildlife Conservation Commission. This study was supported, in part, by a grant from the National Institute of General Medical Sciences (8P20GM103432-12), National Institutes of Health to R.S. Seville.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Chris T. McAllister, Email: cmcallister@se.edu, Science and Mathematics Division, Eastern Oklahoma State College, Idabel, OK 74745, USA
R. Scott Seville, Department of Zoology and Physiology, University of Wyoming, Casper, WY 82601, USA.
Matthew B. Connior, Health and Natural Sciences, South Arkansas Community College, El Dorado, AR 71730, USA
Stanley E. Trauth, Department of Biological Sciences, Arkansas State University, State University, AR 72467, USA
References
- Bundy DAP, Vogel P, Harris EA. Helminth parasites of Jamaican anoles (Reptilia: Iguanidae): A comparison of the helminth fauna of 6 Anolis species. Journal of Helminthology. 1987;61:77–83. [Google Scholar]
- Conant R, Collins JT. A field guide to reptiles and amphibians of eastern and central North America. 3. Boston: Houghton Mifflin; 1998. p. 616. expanded. [Google Scholar]
- Crother BI., Committee Chair Scientific and standard English names of amphibians and reptiles of North America north of Mexico, with comments regarding confidence in our understanding. Society for the Study of Amphibians and Reptiles Herpetological Circular. 2012;39:1–101. [Google Scholar]
- Dixon JR. Amphibians and reptiles of Texas: With keys, taxonomic synopses, bibliography, and distribution maps. College Station: Texas A&M University Press; 2013. p. 447. [Google Scholar]
- Garman S. On West Indian Iguanidae and on West Indian Scincidae. Bulletin of the Essex Institute. 1887;19:1–29. [Google Scholar]
- Goldberg SR, Bursey CR. Transport of helminths to Hawaii via the brown anole, Anolis sagrei (Polychrotidae) Journal of Parasitology. 2000;86:750–755. doi: 10.1645/0022-3395(2000)086[0750:TOHTHV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Bursey CR, Kraus F. Seasonal variation in the helminth community of the brown anole, Anolis sagrei (Sauria: Polychrotidae), from Oahu, Hawaii. American Midland Naturalist. 2002;148:409–415. [Google Scholar]
- Goldberg SR, Bursey CR, Tawil R. Helminth parasites of the bark anole, Anolis distichus and the brown anole, Anolis sagrei (Polychridae) from Florida and the Bahamas. Caribbean Journal of Science. 1994;30:275–277. [Google Scholar]
- Jirků M, Modrý D, Šlapeta JR, Koudela B, Lukes J. The phylogeny of Goussia and Choleoeimeria (Apicomplexa: Eimeriorina) and the evolution of excystation structures in coccidia. Protist. 2002;153:379–390. doi: 10.1078/14344610260450118. [DOI] [PubMed] [Google Scholar]
- Kolbe JJ, Glor RE, Schettino LR, Lara AC, Larson A, Losos JB. Genetic variation increases during biological invasion by a Cuban lizard. Nature. 2004;431:177–181. doi: 10.1038/nature02807. [DOI] [PubMed] [Google Scholar]
- Krysko KL, Wasilewski JA. Natural history notes: Anolis sagrei. Herpetological Review. 2013;43:477–478. [Google Scholar]
- Paperna I, Landsberg JH. Description and taxonomic discussion of eimerian coccidia from African and Levantine geckoes. South African Journal of Zoology. 1989;24:245–355. [Google Scholar]
- Langford GJ, Willobee BA, Isidoro LF. Transmission, host specificity, and seasonal occurrence of Cyrtosomum penneri (Nematoda: Atractidae) in lizards from Florida. Journal of Parasitology. 2013;99:241–246. doi: 10.1645/12-30.1. [DOI] [PubMed] [Google Scholar]
- McAllister CT, Seville RS, Connior MB. A new caryosporan and eimerian (Apicomplexa: Eimeriidae) from green anoles, Anolis carolinensis (Sauria: Dactyloidae), from Arkansas and Louisiana, with a summary of the coccidia of the Dactyloidae. Journal of Parasitology. 2014;100:480–484. doi: 10.1645/13-459.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CT, Trauth SE, Harris CS. Geographic distribution: Anolis sagrei. Herpetological Review. 2003;34:261–262. [Google Scholar]
- Norval G, Bursey CR, Goldberg SR, Mao JJ, Slater K. Origin of the helminth community of an exotic invasive lizard, the brown anole, Anolis sagrei (Squamata: Polychrotidae), in southwestern Taiwan. Pacific Science. 2011;65:383–390. [Google Scholar]
- Parmley D. Northernmost record of the brown anole (Anolis sagrei) in Georgia. Georgia Journal of Science. 2002;60:191–193. [Google Scholar]
- Ruiz A. Eimeria intermedia n. sp., parásitia de la lagarija Anolis intermedius Peters. Revista de Biologia Tropica. 1959;7:109–112. [Google Scholar]
- Schwartz A, Henderson RW. Amphibians and reptiles of the West Indies: Descriptions, distributions, and natural history. Gainesville: University of Florida Press; 1991. p. 720. [Google Scholar]
- Uetz P, Hošek J. The TIGR Reptile Database. [Accessed 6 December 2014];World Wide Web electronic publication. 2014 http://www.reptile-database.org/