Abstract
Natural products serve as an important source of novel compounds for drug development. Recently, peptides have emerged as a new class of therapeutic agents due to their versatility and specificity for biological targets. Yet, their effective application often requires use of a nanoparticle delivery system. In this chapter, we review the role of natural peptides in the design and creation of nanomedicines, with a particular focus on cell-penetrating peptides, antimicrobial peptides, and peptide toxins. The use of natural peptides in conjunction with nanoparticle delivery systems holds great promise for the development of new therapeutic formulations as well as novel platforms for the delivery of various cargoes.
Keywords: Natural peptide, Nanoparticle, Drug delivery, Cell-penetrating peptide, Antimicrobial peptide, Peptide toxin
1. Introduction
Since the dawn of medicine, natural products have served as a crucial source of therapeutic compounds for drug development. From the identification of salicylic acid as the active component of willow bark extract to the discovery of penicillin produced by the mold Penicillium rubens, natural compounds have formed the basis of many commonly used drugs throughout history. The classical paradigm of drug development from natural products includes screening of biological extracts and identification of active components, followed by structure determination and modification. In the past 20 years, this method of developing small-molecule drugs has largely been supplanted by high-throughput screening of synthetic chemical libraries, lead compound identification, and structural optimization (Koehn & Carter, 2005). During this same time period, however, peptides have emerged as an important new class of drugs due to their versatility and specificity for individual targets. An enormous number of potentially therapeutic natural peptides have been identified in the course of biological research. Their clinical translation, however, has been limited by the cost of large-scale synthesis and their inefficient delivery to therapeutic sites in vivo (Vlieghe, Lisowski, Martinez, & Khrestchatisky, 2010). Along with continual improvements in the efficiency of peptide synthesis, nanoparticle platforms have been proposed as a strategy to facilitate delivery of peptide drugs. Conversely, natural peptides with unique biological properties have been studied as tools to enhance delivery of nanoparticles bearing other therapeutic compounds. In this chapter, we will review the use of natural peptides in the design and creation of nanomedicines, with a particular focus on short cationic or amphipathic peptides. The sequence and natural origin of several of these peptides are listed in Table 1. These compounds, which may be classified as cell-penetrating peptides, antimicrobial peptides, and peptide toxins, exhibit interesting interactions with lipid membranes which influence their use as components of nanoparticle drug delivery systems. It is important to note that there is significant overlap between members of these classes and that a particular peptide may behave as a member of more than one class. As a result, the following sections have been constructed based primarily on the applications being discussed rather than individual peptide identities. For each section, we provide a brief review of the structure and function of peptides used for these purposes followed by a detailed description of their application in nanoparticle delivery systems.
Table 1.
Natural peptides used for nanoparticle and drug design
| Peptide | Sequence | Origin |
|---|---|---|
| Tat Peptide | YGRKKRRQRRR | HIV-1 trans-activator of transcription (Tat) protein |
| Penetratin | RQIKIWFQNRRMKWKK | Drosophila Antennapedia protein |
| Transportan | GWTLNSAGYLLGKINLKALAALAKKIL | Neuropeptide galanin / Wasp venom mastoparan |
| MPG | GALFLGFLGAAGSTMGAWSQPKKKRKV | HIV-1 glycoprotein 41 / Simian virus 40 large T antigen |
| Pep-1 | KETWWETWWTEWSQPKKKRKV | HIV-1 reverse transcriptase / Simian virus 40 large T antigen |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | European honeybee venom |
| Magainin II | GIGKWLHSAKKFGKAFVGEIMNS | African clawed frog secretion |
| Cecropin A | KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK | Cecropia moth hemolymph |
| Buforin II | TRSSRAGLQFPVGRVHRLLRK | Asian toad stomach tissue |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTESC | Human cathelicidin |
| Chlorotoxin | MCMPCFTTDHQMARKCDDCCGGKGRGKCYGPQCLCR | Deathstalker scorpion venom |
2. Role of Nanoparticles in Peptide Drug Delivery
Despite a great deal of enthusiasm, there remain several key pharmacological limitations to the development of peptide-based pharmaceutical agents (Craik, Fairlie, Liras, & Price, 2013). Short peptides (< 50 amino acids) have poor pharmacokinetic profiles due to their degradation by serum proteases and rapid clearance by renal filtration. Additionally, most peptide drugs are limited to extracellular targets due to their inability to cross the plasma membrane and, like all drugs, may have off-target effects when administered systemically. Nanoparticle delivery platforms hold great promise as tools to overcome these limitations and enhance the efficacy and utility of peptide drugs. The term “nanoparticle” refers to an extremely broad range of constructs between 1 and 500 nm in diameter which possess unique physical, chemical, and biological properties as a result of their size (Caruthers, Wickline, & Lanza, 2007). Although most types of nanoparticles have been applied in some way for drug delivery, lipid and polymer nanoparticles are by far the most extensively studied and commonly used for this purpose. The rest of this chapter will focus on the use of these nanoparticles as a platform for the delivery of natural peptides and their derivatives.
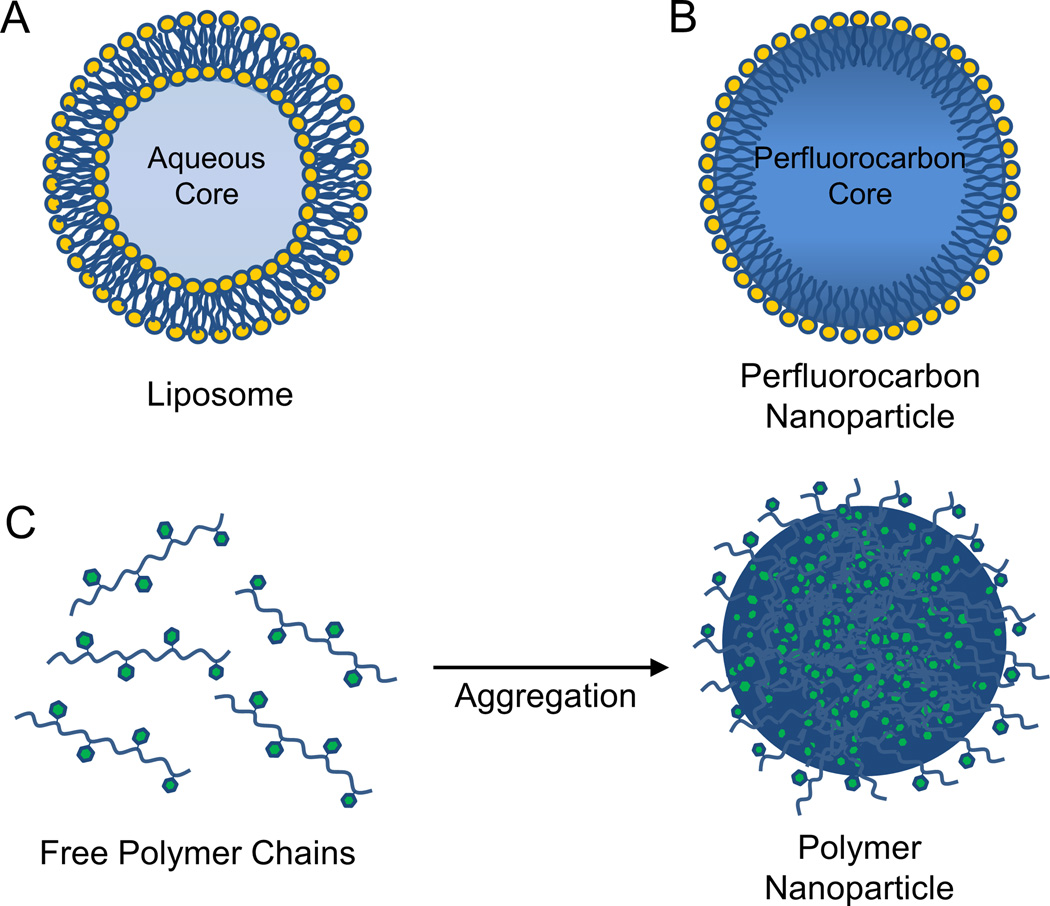
Lipid nanoparticles as a class include liposomes and perfluorocarbon nanoparticles, as well as a number of other lipid-based constructs. Liposomes are among the most well-studied nanostructures for drug delivery and include several formulations currently approved for clinical use (Allen & Cullis, 2013). As shown in Figure 1, liposomes are vesicles consisting of a phospholipid bilayer surrounding an aqueous core (Figure 1A). Based on their hydrophobicity, small-molecule compounds may either be encapsulated within the aqueous region (e.g., liposomal doxorubicin, Doxil®) or incorporated into the lipid bilayer (e.g., liposomal amphotericin B, AmBisome®). Perfluorocarbon nanoparticles, in contrast, consist of a hydrophobic perfluorocarbon core stabilized by a phospholipid monolayer shell (Figure 1B). These nanoparticles have been extensively used for targeted drug delivery and imaging via incorporation of targeting ligands into the phospholipid monolayer (Tran et al., 2007). For both liposomes and perfluorocarbon nanoparticles, peptides may be covalently attached to the phospholipid head groups or non-covalently associated with the lipid membrane. Due to their relatively large size, liposomes and perfluorocarbon nanoparticles alter the pharmacokinetic profile of attached peptides, preventing renal filtration and leading to an overall increase in the circulating peptide half-life. For instance, covalent attachment of the thrombin inhibitor D-phenylalanyl-L-prolyl-L-arginyl-chloromethylketone (PPACK) to the surface of liposomes (Palekar et al., 2013) or perfluorocarbon nanoparticles (Myerson, He, Lanza, Tollefsen, & Wickline, 2011) substantially increased its duration of antithrombotic activity relative to free PPACK. Additionally, insertion into the lipid monolayer of perfluorocarbon nanoparticles enhanced circulation time of melittin, a cytolytic peptide toxin derived from bee venom (Soman et al., 2009). Loading of melittin onto perfluorocarbon nanoparticles also protected melittin from cleavage by serum proteases and significantly reduced its hemolytic activity and toxicity when injected systemically. Interestingly, attempts to use liposomes as a melittin delivery vehicle resulted in membrane pore formation and complete disruption of liposome structure (Soman, Lanza, Heuser, Schlesinger, & Wickline, 2008). These findings illustrate the importance of selecting an appropriate nanoparticle carrier to enhance the therapeutic potential of peptide agents.
Figure 1.
Structure of lipid and polymer nanoparticles. (A) Liposomes consist of a phospholipid bilayer surrounding an aqueous core. (B) Perfluorocarbon nanoparticles consist of a phospholipid monolayer surrounding a hydrophobic perfluorocarbon core. (C) Polymer nanoparticles are often formed by aggregation of hydrophobic polymer chains in an aqueous environment.
Polymer nanoparticles are extremely versatile drug carriers which may be generated using a wide variety of natural or synthetic polymers. Biodegradable synthetic polymers, such as poly(lactic-co-glycolic acid) (PLGA) and poly(ε-caprolactone), often aggregate to form nanoparticles via hydrophobic interactions (Figure 1C). Additionally, several methods have been developed to synthesize nanoparticles composed of natural hydrophilic polymers, such as chitosan and gelatin (Soppimath, Aminabhavi, Kulkarni, & Rudzinski, 2001). One important characteristic of polymer nanoparticles is the ability to directly modify the polymer chain as needed to achieve desired functionality. For instance, PLGA nanoparticles containing polymer chains grafted with poly(ethylene glycol) and a cyclic RGD targeting peptide demonstrated enhanced delivery of paclitaxel and doxorubicin to a variety of in vitro and in vivo tumor models (Danhier et al., 2012). Peptide cargoes may be either covalently linked to the polymer backbone in this way or non-covalently associated with the polymer matrix via electrostatic and hydrophobic interactions. Interestingly, amphipathic peptides may self-assemble into nanoparticles, thereby facilitating their own delivery and that of any complexed cargo. Cell-penetrating peptides in particular have been used in this fashion to enhance translocation of nucleotides and other peptides across the cell membrane (Heitz, Morris, & Divita, 2009).
3. Cell-penetrating peptides
In order for macromolecules and nanoparticles to exert therapeutic effects intracellularly, they must first cross the plasma membrane. Over the past 25 years, a large amount of work has focused on the development of peptide agents capable of facilitating this process, leading to enhanced cellular uptake of cargoes ranging from individual protein molecules (Schwarze, Ho, Vocero-Akbani, & Dowdy, 1999) to liposomes with diameters of 200 nm (Torchilin, Rammohan, Weissig, & Levchenko, 2001). Many of these cell-penetrating peptides (CPPs) are originally derived from natural proteins which translocate across biological membranes. The first reports of this phenomenon came from studies of the HIV-1 trans-activator of transcription (Tat) protein, which penetrates membranes and accumulates in the nucleus by an energy-independent mechanism (Frankel & Pabo, 1988; Green & Loewenstein, 1988). Further studies identified the minimal peptide sequence required for this uptake (Vivès, Brodin, & Lebleu, 1997) and led to the discovery of other CPPs such as penetratin, derived from the homeodomain of the Drosophila Antennapedia protein (Derossi, Joliot, Chassaing, & Prochiantz, 1994). Since then, a wide variety of CPPs have been identified from sources such as viral proteins (Elliott & O’Hare, 1997), animal venoms (Hou, Pan, Lanza, & Wickline, 2013) and synthetic peptide libraries (Gao, Simon, Hue, Morrison, & Banta, 2011). These diverse peptides are typically 5–30 amino acids in length and possess a variety of structural and chemical properties which impact their use as macromolecule and nanoparticle delivery agents. In this section, we will review structural characteristics of the most commonly used CPPs and examine their use in nanoparticle-based drug delivery systems. For a more comprehensive listing of CPPs, the reader is referred to the review by Milletti (Milletti, 2012).
3.1. Structure and Properties
The number and variety of CPPs reported in the literature make precise classification difficult. CPPs may be broadly divided into groups based on multiple considerations, including origin, sequence, and proposed mechanism of internalization. In particular, classification by sequence provides a logical framework for understanding the structure and function of CPPs, as well as their role in the design of nanostructures. In this system, CPPs are classified as either cationic, amphipathic, or hydrophobic.
Cationic CPPs
Cationic CPPs contain positively charged residues which are essential for uptake and often adopt random coil configurations both in solution and when associated with lipid membranes (Eiríksdóttir, Konate, Langel, Divita, & Deshayes, 2010). While the Tat peptide is the most commonly used member of this class, many other cationic CPPs have been identified from sources such as heparan binding proteins (de Coupade et al., 2005), bacteriophages (Futaki et al., 2001), and transcription factors (Balayssac et al., 2006). Internalization of cationic CPPs relies heavily on the presence of arginine residues, which are thought to interact strongly with anionic cell surface proteoglycans. In fact, polyarginine sequences themselves can serve as CPPs, with several studies identifying octaarginine as the minimal sequence for cellular uptake (El-Sayed, Futaki, & Harashima, 2009; Tünnemann et al., 2008). Following interaction with the cell surface, the mechanisms of CPP uptake become controversial (Heitz et al., 2009; Richard et al., 2003). Although one or more endocytotic mechanisms are likely involved, the contribution of each mechanism may depend on the particular CPP being studied and the cargo to which it is bound (Duchardt, Fotin-Mleczek, Schwarz, Fischer, & Brock, 2007).
Amphipathic CPPs
Amphipathic CPPs contain both hydrophilic and hydrophobic domains which are necessary for cellular internalization. In primary amphipathic CPPs, these segments are separated based on their position along the peptide chain. This class includes several natural peptides such as pVEC, which is derived from VE-cadherin (Elmquist, Lindgren, Bartfai, & Langel, 2001). Primary amphipathic CPPs may be designed as fusion constructs containing the targeting domain of one protein and the membrane-interacting domain of another. For instance, both MPG and Pep-1 contain the cationic Simian Virus 40 (SV40) nuclear localization sequence. However, MPG contains a hydrophobic domain derived from the fusion sequence of HIV-1 glycoprotein 41, while that of Pep-1 is derived from the dimerization motif of HIV-1 reverse transcriptase (May C. Morris, Depollier, Mery, Heitz, & Divita, 2001; M. C. Morris, Vidal, Chaloin, Heitz, & Divita, 1997). These different hydrophobic domains allow MPG to carry nucleic acid cargoes while Pep-1 preferentially complexes with other peptides and proteins (Deshayes, Morris, Divita, & Heitz, 2005).
Secondary amphipathic CPPs are another large class of peptides in which the separation between hydrophilic and hydrophobic domains occurs due to the formation of secondary structures, such as α-helices or β-sheets. Many of the most commonly used CPPs are members of this class. These include penetratin (Derossi et al., 1994), which is derived from the Antennapedia homeodomain, and transportan (Pooga, Hällbrink, Zorko, Uuml, & Langel, 1998), which is derived from fusion of the neuropeptide galanin with the wasp venom peptide mastoparan. Many antimicrobial peptides and animal toxins also resemble amphipathic CPPs in structure. In order for these peptides to be successfully used for drug delivery, however, they must first be modified to minimize their cytotoxicity. Although most amphipathic CPPs are cationic, there are a number of neutral and anionic peptides which retain their cell-penetrating ability (W. Li, Nicol, & Szoka Jr., 2004; Taylor et al., 2009). These findings indicate that cellular internalization is a consequence of these peptides’ amphipathic nature, not solely their charge.
Hydrophobic CPPs
Hydrophobic CPPs possess a low net charge and are composed of predominantly nonpolar amino acids. Several hydrophobic CPPs are derived from natural proteins, including α1-antitrypsin (Rhee & Davis, 2006) and fibroblast growth factor 12 (Nakayama et al., 2011). Nevertheless, this class includes the fewest CPPs discovered to date and most new hydrophobic CPPs are generated using synthetic peptide libraries (Marks, Placone, Hristova, & Wimley, 2011). This class also includes a number of chemically modified peptides, such as stapled peptides (Chu et al., 2014), prenylated peptides (Ochocki, Mullen, Wattenberg, & Distefano, 2011), and pepducins (Covic, Gresser, Talavera, Swift, & Kuliopulos, 2002). The use of these synthetic modifications to enhance peptide uptake raises exciting prospects for the development of cell-penetrating agents based on a wide variety of natural products.
3.2. Applications in Nanoparticle Delivery
Although nanoparticles possess several distinct advantages as drug delivery vehicles, their size generally prevents them from crossing the plasma membrane, thereby precluding their use for intracellular drug delivery. One promising approach to overcome this limitation involves incorporating CPPs directly into the nanoparticle structure. Depending on the type of nanoparticle, this conjugation may be either covalent or non-covalent.
Multiple studies have demonstrated that covalent addition of cationic or amphipathic CPPs to the surface of nanostructures is sufficient to enhance their cellular uptake. This technique has been successfully applied to a wide range of nanoparticles, including liposomes (Tseng, Liu, & Hong, 2002), iron oxide nanoparticles (M. Zhao, Kircher, Josephson, & Weissleder, 2002), gold nanoparticles (de la Fuente & Berry, 2005), and quantum dots (Ruan, Agrawal, Marcus, & Nie, 2007). Advantages of covalent conjugation include its simplicity, stability and applicability to a variety of well-defined nanoparticles. Nevertheless, continued interaction between the CPP and cargo following translocation into the cell may diminish the cargo’s biological activity (Juliano, Alam, Dixit, & Kang, 2008). In these cases, the use of cleavable linkers or non-covalent association of the CPP may be more appropriate.
The non-covalent approach for CPP incorporation relies on electrostatic and hydrophobic interactions between the CPP and cargo to maintain nanoparticle integrity. This technique was originally developed using the fusion peptide of influenza hemagglutinin, which condenses with DNA to form nanoscale complexes capable of escaping the endosome (Wagner, Plank, Zatloukal, Cotten, & Birnstiel, 1992). Recently, this approach has been extended to a number of other CPPs and has been successfully used for the delivery of nucleotide (Hou, Pan, Lanza, et al., 2013; Nakase et al., 2012), peptide (Grdisa, 2011) and small-molecule therapeutics (L. Liu et al., 2008). The wide variety of CPPs, nanoparticles, and conjugation methods available provides great freedom in the design of drug delivery systems. For the purposes of this chapter, we have classified studies based on the specific CPP used in each delivery system.
Tat Peptide
One of the most commonly used CPPs in the field of drug delivery is the Tat peptide, an 11-mer identified as the minimal sequence necessary for membrane translocation of the HIV-1 trans-activator of transcription (Tat) protein (Vivès et al., 1997). The first application of this peptide to enhance nanoparticle uptake involved its covalent attachment to the surface of superparamagnetic iron oxide nanoparticles (SPIO NPs) (Josephson, Tung, Moore, & Weissleder, 1999). SPIO NPs bearing the Tat peptide were internalized over 100-fold more efficiently than control nanoparticles, allowing labeled cells to be detected by magnetic resonance imaging and retained on magnetic separation columns. A similar technique was used by Torchilin et al. to enhance uptake of 200-nm liposomes by a variety of cell lines (Torchilin et al., 2001). This and other studies (Marty, Meylan, Schott, Ballmer-Hofer, & Schwendener, 2004) demonstrated that liposome uptake was dependent on the number of peptides attached to the liposome as well as their ability to interact with the plasma membrane and cell surface proteoglycans. Covalent functionalization with Tat peptide has subsequently been applied to a variety of lipid-based drug delivery systems. Liposomes are well-established as carriers to increase the efficacy and reduce off-target toxicity of chemotherapeutic agents (A. Z. Wang, Langer, & Farokhzad, 2012). Tseng et al. demonstrated that addition of Tat peptide to the liposomal surface increased uptake of doxorubicin by 12-fold in a number of cancer cell lines (Tseng et al., 2002). Interestingly, however, they also found that this enhanced uptake was not accompanied by increased cytotoxicity or inhibition of tumor growth in a subcutaneous xenograft model. Other studies have reported somewhat more positive results, with Qin et al. claiming that Tat peptide-conjugated liposomes enhanced cytotoxicity and moderately improved survival in a mouse model of brain glioma (Qin et al., 2011). In either case, limited penetration into the tumor and slow release of encapsulated drug have been identified as key obstacles to liposomal drug delivery (Manzoor et al., 2012) and may persist even with the use of CPPs. Nanostructured lipid carriers functionalized with Tat peptide have also been used in transdermal applications to deliver the non-steroidal anti-inflammatory drug celecoxib (Desai, Patlolla, & Singh, 2010). These agents significantly increased celecoxib accumulation in all skin layers compared to control carriers lacking the Tat peptide.
The first application of CPPs for nanoparticle-based gene delivery involved the use of lipoplexes formed by electrostatic interaction of Tat peptide-modified cationic liposomes with DNA (Torchilin et al., 2003). These nanoparticles were used to transfect fibroblasts and cardiomyocytes in vitro, as well as Lewis lung carcinoma cells growing subcutaneously in vivo. Similar lipoplexes demonstrated successful gene delivery to brain tumors (Gupta, Levchenko, & Torchilin, 2007) and ischemic myocardium (Ko, Hartner, Kale, & Torchilin, 2008) in animal models. Recently, polymer nanostructures have gained popularity as nucleotide delivery vehicles. These carriers often contain Tat peptide covalently linked to a cationic polymer which forms nanoscale complexes with nucleotides. Kleeman et al. demonstrated this concept using a complex of DNA with a Tat-peptide conjugated form of polyethyleneimine (Kleemann et al., 2005). The resulting polyplexes successfully transfected lung carcinoma cells in vitro, as well as bronchial and alveolar tissue following intratracheal instillation. A similar nanoparticle was later used to transfect neurotypic cells with possible implications for treatment of neurodegenerative diseases (Suk et al., 2006). Tat peptide-functionalized chitosan nanoparticles have also been used for this application and have successfully delivered siRNA to reduce in vitro production of ataxin, a protein overexpressed in spinocerebellar ataxia (Malhotra, Tomaro-Duchesneau, & Prakash, 2013). While Tat peptide-conjugated polymer carriers are often used for gene delivery, they have also been applied as alternatives to liposomes for the delivery of paclitaxel (Sawant & Torchilin, 2009; P. Zhao et al., 2010) and doxorubicin (Lee et al., 2011) to tumors, as well as the delivery of antibiotics such as ciprofloxacin (L. Liu et al., 2008) across the blood-brain barrier.
One major drawback to the use of CPPs for drug delivery is their lack of specificity for a particular tissue or cell type. An early mouse study which involved intraperitoneal injection of a Tat peptide-β-galactosidase fusion protein found that this construct accumulated in all tissues analyzed, including the brain (Schwarze et al., 1999). While this property may be desirable for some applications, such as systemic gene delivery, it could result in off-target effects and increased dose requirement for many drug delivery systems. Thus, recent efforts using Tat peptide have focused on developing nanocarriers in which the internalizing effects of the CPP are activated only under specific conditions. Kale et al. developed one such system in which liposomes were functionalized with both Tat peptide and pH-sensitive poly(ethylene glycol) (PEG) chains (Kale & Torchilin, 2007a, 2007b). The PEG coating sterically hindered membrane interaction of Tat peptide, thereby preventing liposome internalization until the PEG chains were hydrolyzed in the acidic tumor microenvironment. A similar concept has been applied to liposomes bearing a monoclonal antibody to enhance interaction with tumor cells (Koren, Apte, Jani, & Torchilin, 2012). These multifunctional liposomes enhanced delivery of doxorubicin to ovarian cancer both in vitro and in vivo (Apte, Koren, Koshkaryev, & Torchilin, 2014). Drug delivery systems activated by tumor-associated proteases have also been developed. Gullotti et al. reported a Tat peptide-functionalized polymer nanoparticle bearing PEG chains anchored by a matrix metalloproteinase-2 (MMP-2) cleavable peptide sequence (Gullotti, Park, & Yeo, 2013). Treatment with MMP-2 led to increased nanoparticle uptake and delivery of paclitaxel in an ovarian cancer cell line. Similarly, Liu et al. generated a Tat peptide-modified liposome activated by legumain, a protease overexpressed in a variety of tumor tissues (Z. Liu et al., 2014). In this study, however, the cleavable group was covalently linked to Tat peptide, reducing its cell-penetrating activity prior to cleavage by legumain. These strategies for CPP activation may be used in conjunction with other techniques to locally enhance nanoparticle uptake. The application of ultrasound energy, for instance, improved the ability of Tat peptide-modified bubble liposomes to escape the endosome, leading to a 30-fold increase in gene delivery (Omata et al., 2011).
Penetratin
Penetratin, one of the first CPPs to be discovered, is a 16-mer peptide derived from the third helix of the Drosophila Antennapedia homeodomain (Derossi et al., 1994). It is commonly used as an alternative or complement to Tat peptide for enhancing cellular uptake of nanoparticles. Like Tat peptide, penetratin is often covalently linked to the nanoparticle surface. One of the most common applications for penetratin-modified nanoparticles is to facilitate drug delivery across the blood-brain barrier. Xia et al. found that polymer nanoparticles functionalized with penetratin demonstrated increased cellular uptake in vitro and improved pharmacokinetics in vivo (Xia et al., 2012). These nanoparticles accumulated to a greater extent in the brain and to a lesser extent in other tissues than unmodified nanoparticles. Similarly, addition of penetratin to the surface of liposomes improved liposomal accumulation and delivery of encapsulated doxorubicin to brain tumors (Sharma, Modgil, Zhong, Sun, & Singh, 2014). Penetratin-functionalized polymer nanoparticles have also been used to deliver peptide nucleic acids, a new class of therapeutics, to malignant lymphocytes in a mouse model of lymphoma (Babar et al., 2012). Interestingly, this study found that pre-B cells preferentially internalized nanoparticles coated with penetratin when compared to other CPPs, such as Tat peptide and polyarginine.
MPG/Pep-1
Covalent linkage of Tat peptide and penetratin has been shown to inhibit both cellular uptake and biological activity of certain types of cargo, particularly nucleotides (Presente & F. Dowdy, 2013). To avoid this limitation, several CPPs have been designed which form non-covalent nanoscale complexes with cargo via electrostatic and hydrophobic interactions. These complexes enhance cellular internalization while protecting cargo from degradation by serum nucleases and proteases. MPG is an amphipathic peptide generated by using a flexible peptide linker to join the hydrophobic fusion sequence of HIV-1 glycoprotein 41 with the cationic nuclear localization sequence of Simian Virus 40 (SV40) large T antigen (M. C. Morris et al., 1997). This peptide forms 200-nm complexes with nucleic acids and was initially used for the delivery of oligonucleotides and plasmid DNA into cultured cells (May C. Morris, Chaloin, Méry, Heitz, & Divita, 1999). More recently, the sequence of this construct was optimized for siRNA delivery by introducing a point mutation to the nuclear localization sequence, thereby facilitating nanoparticle accumulation and siRNA release in the cytoplasm (Simeoni, Morris, Heitz, & Divita, 2003). Although this and other MPG derivatives have been successfully used for gene delivery to a variety of cell lines in vitro, it appears that endosomal entrapment remains an important limitation to transfection efficiency (Veldhoen, Laufer, Trampe, & Restle, 2006). For in vivo applications, biodistribution presents yet another potential obstacle to effective gene delivery. To address this issue, Crombez et al. functionalized an MPG derivative with cholesterol and demonstrated that this modification improved tissue distribution following systemic administration in mice (Crombez et al., 2009). The use of this system to deliver cyclin B1 siRNA reduced tumor growth and increased survival following intravenous injection in a mouse xenograft cancer model.
Given the success of MPG for enhancing nucleotide delivery, Morris et al. developed a similar peptide which facilitates uptake of proteins via formation of non-covalent complexes (May C. Morris et al., 2001). This peptide, known as Pep-1, contains the same hydrophilic domain and peptide linker as MPG, but a different hydrophobic domain derived from a tryptophan-rich cluster found in HIV-1 reverse transcriptase. A study of Pep-1 complexation with the cell cycle inhibitor protein p27Kip1 found an optimal Pep-1:p27 molar mixing ratio of 20:1, which resulted in discrete nanoparticles with diameters of approximately 100 nm (Muñoz-Morris, Heitz, Divita, & Morris, 2007). Addition of these complexes to cultured fibroblasts led to cell cycle arrest in 70% of cells analyzed. Pep-1 has been used to deliver proteins to a wide variety of cell lines (Gros et al., 2006), including primary neurons (Gallo, 2003), hepatocytes (Bardag-Gorce et al., 2003), and macrophages (Garnon et al., 2005). This peptide has also been commercialized for research purposes under the trade name Chariot™ (Active Motif, Carlsbad, CA). In vivo applications of Pep-1 have been promising, with successful protein delivery demonstrated following intratracheal instillation in a mouse model of emphysema (Aoshiba, Yokohori, & Nagai, 2003). Recently, however, Pep-1 has primarily been used as a covalent attachment to facilitate penetration of nanoparticles into tumors (Kang, Park, Kang, Park, & Choi, 2013; B. Wang et al., 2014) and generate fusion proteins with enhanced tissue accumulation (Ahn et al., 2014; He et al., 2013). These applications resemble those of other CPPs and demonstrate the versatility of Pep-1 as a tool for improving drug delivery.
Antimicrobial Peptides
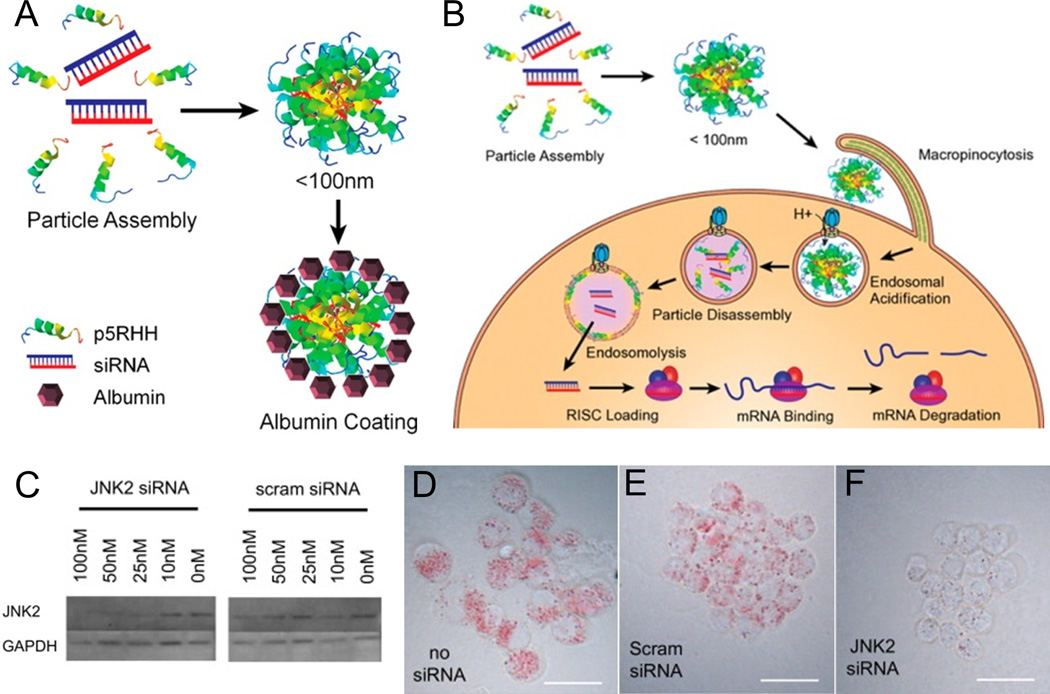
Like CPPs, antimicrobial peptides (AMPs) often possess cationic or amphipathic segments which enhance their interaction with lipid membranes. Indeed, some authors have proposed that both CPPs and AMPs belong to a class of “membrane-active peptides”, with their primary distinguishing feature being the extent of membrane translocation vs. permeabilization induced at a particular peptide concentration (Henriques, Melo, & Castanho, 2006). Several AMPs have been reported to exhibit cell-penetrating properties (Splith & Neundorf, 2011). However, their practical application as drug delivery vehicles remains limited by their toxicity to mammalian cells. Judicious peptide sequence modifications may substantially reduce cytotoxicity while maintaining cell-penetrating activity. For instance, Pan et al. reported that N-terminal truncation of melittin, an AMP derived from bee venom, reduced cytotoxicity by two orders of magnitude without significantly affecting the ability of melittin to insert into lipid membranes (Pan et al., 2010). Addition of arginine and histidine residues to the C-terminus of this peptide gave rise to p5RHH, a transfection agent capable of forming nanoscale complexes with siRNA (Hou, Pan, Lanza, et al., 2013). As shown in Figure 2, these complexes are stable in serum and have been successfully used to transfect macrophages, B16 melanoma cells, and human umbilical vein endothelial cells in vitro. Mechanistic studies indicated that endosomal acidification led to disassembly of these complexes and lysis of the endosomal membrane, thereby facilitating siRNA release into the cytoplasm (Hou, Pan, Ratner, Schlesinger, & Wickline, 2013). These complexes accumulated at inflammatory sites following intravenous injection in a mouse model of rheumatoid arthritis. Incorporation of siRNA targeting the p65 subunit of NF-κB led to dramatic improvements in joint inflammation without eliciting a humoral immune response or affecting p65 expression in other tissues (Zhou et al., 2014).
Figure 2.
Self-assembly and efficacy of p5RHH-siRNA nanocomplexes. (A) Schematic diagram of p5RHH and siRNA complexation. (B) Schematic diagram of p5RHH-siRNA complex internalization and disassembly following endosomal acidification. (C) Application of p5RHH-siRNA complexes containing JNK2 siRNA lowered JNK2 expression in a macrophage cell line. (D, E, and F) Application of p5RHH-siRNA complexes containing JNK2 siRNA reduced foam cell formation following exposure to acetylated LDL. Panels A and B adapted with permission from Hou, K. K., Pan, H., Ratner, L., Schlesinger, P. H., & Wickline, S. A. (2013). Mechanisms of Nanoparticle-Mediated siRNA Transfection by Melittin-Derived Peptides. ACS Nano, 7(10), 8605–8615. Copyright 2013 American Chemical Society. Panels C, D, E, and F adapted with permission from Hou, K. K., Pan, H., Lanza, G. M., & Wickline, S. A. (2013). Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials 34(12), 3110–3119.Copyright 2013 Elsevier.
4. Antimicrobial peptides
With the rise of antibiotic resistance and the emergence of multidrug-resistant organisms (Andersson & Hughes, 2011; Bush et al., 2011), there is a pressing need to develop new antimicrobial agents which avoid conventional mechanisms of resistance. One promising set of compounds which could form the basis for a new class of therapeutics are antimicrobial peptides (AMPs). These peptides are produced by a wide variety of organisms and generally exert their antimicrobial effect by disrupting biological membranes, leading to depolarization, lysis, and cellular death. Like cell-penetrating peptides, AMPs are often short (10–50 amino acids), cationic (overall charge +2 to +9), and possess a number of hydrophobic residues to facilitate their interaction with lipid membranes (Hancock & Sahl, 2006). This class of compounds is also extremely diverse, with over 1,200 peptides divided into five structural classes (G. Wang, Li, & Wang, 2009) and a variety of proposed mechanisms of action (Nguyen, Haney, & Vogel, 2011). In contrast to conventional antibiotics, AMPs do not target a specific molecule or biochemical process, giving them broad-spectrum activity and making development of resistance difficult (Zasloff, 2002). Nevertheless, there are some important limitations preventing clinical translation of these compounds. While many natural AMPs demonstrate some degree of preference for bacterial membranes, toxicity to eukaryotic cells remains a key concern (Marr et al., 2006). Additionally, like many short peptides, free AMPs are rapidly cleared by the kidney and possess a poor pharmacokinetic profile (Bradshaw, 2003). Recent efforts to address these concerns include sequence modifications to enhance antimicrobial activity while minimizing toxicity to eukaryotic cells, as well as development of nanoparticle-based strategies to improve AMP delivery while further reducing off-target treatment effects.
4.1. Sequence Modifications
As with cell-penetrating peptides, the ability of an AMP to disrupt biological membranes depends primarily on its general structure and physicochemical properties rather than its specific amino acid composition. Thus, sequence modifications intended to change peptide activity often alter key peptide characteristics such as charge and hydrophobicity. Addition of cationic residues to either the N- or C-terminus of magainin II, an AMP derived from the African clawed frog, substantially improved its antibacterial activity without significantly altering its hemolytic activity (Bessalle, Haas, Goria, Shalit, & Fridkin, 1992). This effect was attributed to enhanced electrostatic interaction between the magainin derivatives and bacterial membranes, which contain high concentrations of anionic phospholipids. A similar trend was observed using cationic derivatives of the AMP V681, which is derived from a fusion construct of cecropin A, an AMP isolated from the Hyalophora cecropia moth, and melittin, an AMP found in bee venom (Jiang et al., 2008). However, this study also identified a charge threshold above which hemolytic activity of the V681 derivatives sharply increased.
The effects of hydrophobicity on antibacterial and hemolytic activity are similarly difficult to predict. Replacing two leucine residues with more hydrophobic hexafluoroleucine residues enhanced antibacterial activity without altering hemolytic activity in buforin II, an AMP derived from stomach tissue of the Asian toad (Meng & Kumar, 2007). However, similar alterations of the magainin sequence increased hemolytic activity without substantially affecting antibacterial activity. Many other sequence modifications have been applied to a wide variety of AMPs in order to enhance their selectivity for bacterial membranes or gain information regarding their mechanism of action. For a more comprehensive assessment of these efforts, the reader is referred to the review by Findlay et al. (Findlay, Zhanel, & Schweizer, 2010).
Modification of an AMP sequence may also be performed to introduce properties which are beneficial for a particular application. For instance, Pan et al. recently developed a peptide linker to non-covalently attach cargo to the surface of lipid nanoparticles (Pan et al., 2010). This linker is generated by deleting seven amino acids from the N-terminus of the AMP melittin. The resulting peptide is substantially less cytotoxic and hemolytic than free melittin but retains the ability of melittin to stably bind and insert into lipid membranes. A fusion construct of this linker with the Nemo Binding Domain peptide was used to generate perfluorocarbon nanoparticles which potently inhibited NF-κB signaling in leukemia/lymphoma cells (Pan et al., 2011). Additionally, this linker was used to create perfluorocarbon nanoparticles targeted to the inflammatory marker VCAM-1 (Pan et al., 2013). These nanoparticles accumulated in atherosclerotic plaques and breast tumors to a much greater extent than untargeted nanoparticles, making them promising vehicles for drug delivery to these sites.
While truncation of an AMP sequence permanently reduces its cytotoxicity, AMP activity may be reversibly inhibited by addition of a removable anionic blocking sequence. Desgranges et al. demonstrated this concept using a cecropin-magainin hybrid peptide containing an N-terminal polyglutamate sequence joined via a trialanine linker (Desgranges et al., 2011). This linker sequence was designed to be cleaved by neutrophil elastase, leading to activation of cytolytic activity only in the presence of inflammation. While this compound exhibited potent activity against Pseudomonas aeruginosa following incubation with recombinant neutrophil elastase, activation by bronchoalveolar lavage specimens was much less pronounced despite the presence of similar elastase concentrations (Forde, Humphreys, Greene, Fitzgerald-Hughes, & Devocelle, 2014). This effect resulted from inhibition of cytolytic activity by anionic components of the bronchoalveolar lavage sample, demonstrating the complexity of AMP development for clinical applications.
4.2. Nanoparticle Delivery Strategies
In addition to sequence modifications, nanoparticle delivery vehicles may be used to minimize off-target effects and improve AMP release kinetics. For instance, incorporation of melittin into the lipid monolayer of perfluorocarbon nanoparticles has been shown to reduce toxicity to sperm and vaginal epithelium (Jallouk et al., 2014) while retaining antiviral activity against HIV-1 (Hood, Jallouk, Campbell, Ratner, & Wickline, 2013). This preferential inactivation of HIV-1 virions was attributed to their relatively small surface area, high internal pressure, and inability to repair membrane defects. These findings demonstrated the potential utility of melittin-loaded nanoparticles as a topical vaginal virucide. Similarly, LL-37, an AMP derived from human cathelicidin, retained activity against Escherichia coli following incorporation into polymer nanoparticles (Chereddy et al., 2014). Intradermal administration of LL-37-loaded nanoparticles led to sustained LL-37 release and improved wound healing in a mouse injury model.
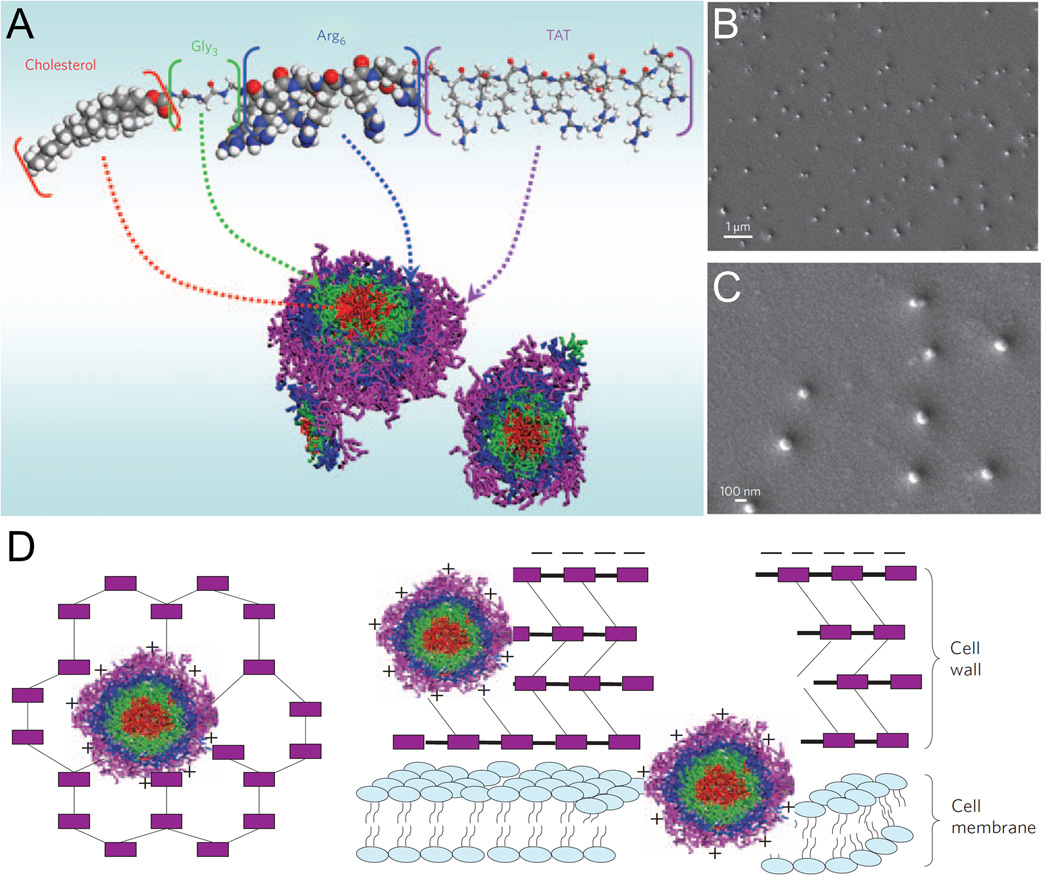
Like cell-penetrating peptides, amphipathic AMPs may self-assemble to form nanoparticles via electrostatic and hydrophobic interactions (Hamley, 2011; X. Zhao et al., 2010). This self-assembly process, in turn, may be influenced through covalent addition of cationic or hydrophobic groups to the peptide sequence. Liu et al. generated one such peptide, known as CG3R6TAT, which consists of cholesterol joined via a triglycine linker to a hexaarginine-modified version of Tat peptide (L. Liu et al., 2009). As shown in Figure 3, this construct self-assembles into 150-nm nanoparticles with core-shell morphology. The presence of a cell-penetrating peptide modified with additional cationic and hydrophobic groups enhances the ability of these nanoparticles to disrupt and translocate across biological membranes. These nanoparticles exhibited antimicrobial activity against a wide range of bacteria, yeast, and other fungi in vitro, and demonstrated an antibacterial effect in a rabbit model of Staphylococcus aureus-induced meningitis. In a more detailed study using a rabbit model of Cryptococcus neoformans-induced meningitis, these nanoparticles were shown to penetrate the blood-brain barrier and appeared to have an antifungal effect similar to amphotericin B with fewer off-target effects on liver and kidney function (Huaying Wang et al., 2010).
Figure 3.
Self-assembly and mechanism of action of CG3R6TAT nanoparticles. (A) Schematic diagram of CG3R6TAT self-assembly into nanoparticles with core-shell morphology. (B and C) Scanning electron micrographs of CG3R6TAT nanoparticles. (D) Schematic diagram of CG3R6TAT nanoparticle antimicrobial activity. Electrostatic interaction with anionic cellular components leads to cell wall and cell membrane disruption. All panels adapted with permission from Liu, L., Xu, K., Wang, H., Tan, P. K. J., Fan, W., Venkatraman, S. S., Li, L., & Yang, Y.-Y. (2009). Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nature Nanotechnology, 4(7), 457–463. Copyright 2009 Nature Publishing Group.
5. Peptide toxins
In addition to their primary role in defense from predators and capture of prey, animal venoms represent a potent reservoir of potentially bioactive molecules with broad therapeutic applications (Bailey & Wilce, 2001). Venom is composed of many different bioactive peptides which contribute to the pharmacologic response after delivery to the recipient (Lewis & Garcia, 2003). Due to their high potency and specificity, venom peptides remain an attractive tool for probing the structure and function of their various targets, including voltage-gated ion channels, nicotinic acetylcholine receptors, N-methyl-D-aspartate (NMDA) receptors, and acid-sensing ion channels (ASICs) (Dutertre & Lewis, 2010). Additionally, venom peptides have been used as structural templates for the development of drugs targeting these receptors, many of which have been successfully translated to the clinic. In this section, we will discuss venom peptides derived from several different organisms, as well as their potential therapeutic applications.
5.1. Mechanisms of Action
The mechanism of action of individual peptide toxins varies greatly based on the organism from which they are derived and their intended biological effect. Nevertheless, many peptides derived from animal venoms are toxic to bacteria, fungi and animal cells by virtue of their ability to disrupt biological membranes. The exact mechanism of membrane disruption is heavily debated and can vary based on the peptide being studied. However, most proposed mechanisms are based on several commonly accepted models (Shai, 1999). In the “carpet” model, peptides in an α-helical conformation coat the membrane and alter its curvature in a detergent-like fashion. Increased peptide deposition eventually leads to collapse of the membrane and leakage of its contents. In the “barrel stave” model, peptides bind electrostatically to membrane phospholipids and oligomerize, resulting in the formation of transmembrane pores and osmotic lysis. In the “toroidal pore” model, insertion of peptides into the membrane results in conformational strain leading to increased distance between phospholipid head groups, cavitation, pore formation, and osmotic lysis. This disruption of biological membranes forms the basis for therapeutic application of a number of peptide toxins.
5.2. Therapeutic Applications
Amphibian Toxins
Toxins from amphibians, particularly frogs and toads, are typically delivered as a secretion from the skin, where they play a major role in defending the host from invasion by potentially lethal bacteria and fungi. Studies of skin secretions from amphibians have isolated several potential therapeutic agents with antimicrobial properties. Like other antimicrobial peptides, these toxins have been studied for their ability to disrupt bacterial membranes and induce cell death. The first of these peptides to be isolated was magainin, derived from the African clawed frog Xenopus laevis. Additional work has resulted in a library of more than 300 cytolytic peptides found in skin secretions, ranging from 10–48 amino acids in length and generally cationic due to the presence of several lysine residues (Conlon, Kolodziejek, & Nowotny, 2004). Toxins commonly studied for their antimicrobial activity include dermaseptin, dermatoxin, distinctin, phylloseptin, phylloxin, and plasticins, among others (Calderon, Silva, Ciancaglini, & Stábeli, 2011). Despite their efficacy as antimicrobial agents, clinical translation of many amphibian toxins has been slow due to their nonspecific toxicity.
In addition to these antimicrobial peptides, other components of amphibian secretions have been isolated for their anticancer properties, such as the bufadienolide family of compounds derived from the skin glands of Chinese toads (Bufo melanostictus Schneider and Bufo bufo gargarzinas Gantor). Bufadienolides are a class of C-24 steroids consisting of a characteristic α-pyrone ring at C-17 and include bufalin, cinobufagin, and resibufogenin (F. Li, Wang, He, & Tang, 2008). These compounds have been shown to induce reactive oxygen species-dependent autophagy pathways in a number of cancer cell lines. Yet, their clinical translation has been limited by their cardiotoxicity and low solubility in water, which presents significant formulation challenges (Yin et al., 2012).
Several nanoparticle-based strategies have been employed to enhance the therapeutic potential of amphibian toxins. As with antimicrobial peptides, nanoparticles may sequester peptide toxin activity until release at a desired therapeutic site (Calderon et al., 2011). For poorly soluble compounds such as bufadienolides, delivery and therapeutic efficacy have been improved following encapsulation in nanoparticles with hydrophobic components, including liposomes (F. Li et al., 2008; F. Li, Yang, Weng, & Tang, 2009) and polymer nanoparticles composed of pluronic polyetherimide (Hu et al., 2014) or mPEG-PLGA-PLL-cRGD block copolymers (Yin et al., 2012).
Bee Venom
The bee venom component most commonly studied for its cytolytic properties is melittin, a peptide derived from the venom of the European honeybee, Apis mellifera. Melittin is a cationic amphipathic peptide composed of 26 amino acids and represents approximately 50% of the dry weight of bee venom. Characterized as a host defense peptide, melittin has demonstrated potent antitumor efficacy as a result of its cytolytic activity (Gajski & Garaj-Vrhovac, 2013). The ability of melittin to disrupt lipid membranes arises from oligomerization of melittin peptides to form transmembrane channels which lead to osmotic cell lysis (Tosteson & Tosteson, 1981). Each peptide chain of melittin consists of two α-helical segments which are joined in a “bent rod” configuration due to disruption of the overall helical structure at amino acid positions 10–12 (Bechinger, 1997). Despite its great promise as an antimicrobial and antitumor agent, therapeutic applications of melittin have been severely limited by its nonspecific cytotoxicity and hemolytic activity (Hoskin & Ramamoorthy, 2008).
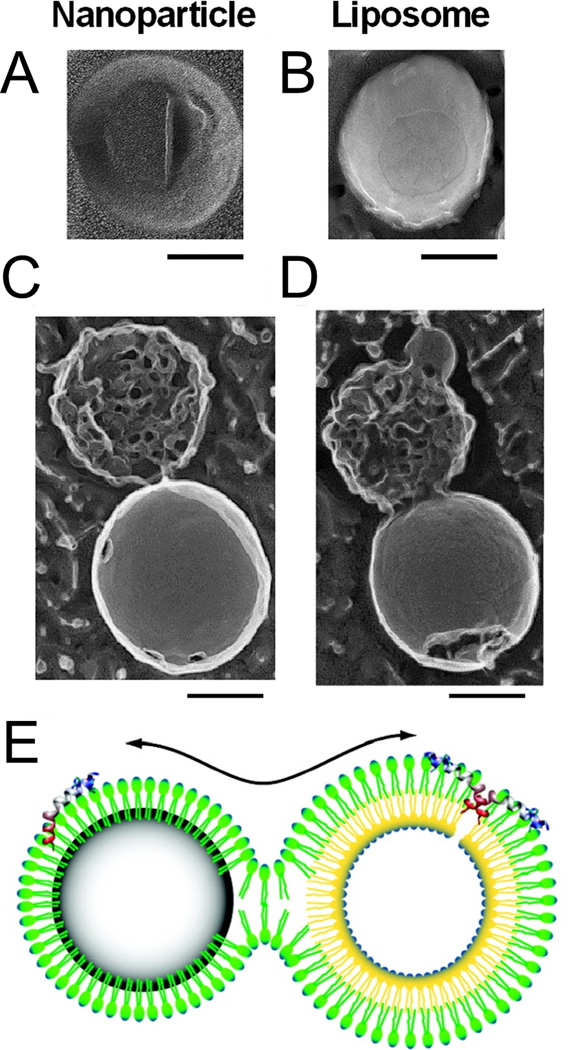
Nanoparticle delivery systems have been shown to reduce off-target toxicity of melittin and enhance its delivery to therapeutic sites in vivo. Soman et al. first demonstrated the ability of melittin to stably bind and insert into the lipid monolayer of perfluorocarbon nanoparticles (Soman et al., 2008). Due to the biophysical properties of the lipid monolayer and the hydrophobicity of the perfluorocarbon core, nanoparticle-bound melittin peptides did not oligomerize or disrupt nanoparticle structure in this construct. In the presence of cell membranes or other lipid nanoparticles, however, formation of a hemifusion stalk between perfluorocarbon nanoparticles and target membranes allowed for melittin transfer and subsequent membrane disruption as shown in Figure 4. Incorporation of melittin into perfluorocarbon nanoparticles significantly reduced systemic toxicity following intravenous administration while retaining antitumor activity against established melanoma tumors and precancerous skin lesions (Soman et al., 2009). N-terminal fusion of melittin with an amphipathic α-helical peptide and subsequent incorporation into lipid nanoparticles also resulted in substantial antitumor activity with reduced nonspecific cytotoxicity (C. Huang et al., 2013)
Figure 4.
Interaction of melittin-loaded perfluorocarbon nanoparticles with liposomes. (A) Deep etch platinum replica of a perfluorocarbon nanoparticle. (B) Deep etch platinum replica of a liposome. (C and D) Deep etch platinum replicas of perfluorocarbon nanoparticle-liposome complexes. Scale bars correspond to 100 nm. (E) Schematic diagram of hemifusion stalk formation and melittin transfer between perfluorocarbon nanoparticles and liposomes. All panels adapted with permission from Soman, N. R., Lanza, G. M., Heuser, J. M., Schlesinger, P. H., & Wickline, S. A. (2008). Synthesis and Characterization of Stable Fluorocarbon Nanostructures as Drug Delivery Vehicles for Cytolytic Peptides. Nano Letters, 8(4), 1131–1136. Copyright 2008 American Chemical Society.
Scorpion Venom
Scorpion venom contains numerous compounds which may be adapted for cancer therapy. Of these, peptides which block ion channels have been the most heavily studied. The extent of interaction with different types of ion channels depends on the properties of each individual peptide. Longer peptides (60–70 amino acids) with multiple disulfide bridges have been shown to interact with sodium channels while shorter peptides (30–40 amino acids) primarily interact with potassium and chloride channels. The most commonly used scorpion venom toxin is the 36-amino acid peptide chlorotoxin, which is derived from the deathstalker scorpion Leiurus quinquestriatus and has historically been used for the study of voltage-gated chloride channels. In addition to its use as a biological research tool, chlorotoxin has been identified as a potential agent for identification and treatment of gliomas. Chlorotoxin binds reliably and specifically to gliomas and other tumors of neuroectodermal origin (Lyons, O’Neal, & Sontheimer, 2002). Fluorescently labeled chlorotoxin has been shown to stain glioma cells in vitro, in situ, and in patient tissue samples (Ding, Chua, Bay, & Gopalakrishnakone, 2014). Additionally, synthetic chlorotoxin labeled with radioactive 131I is currently in clinical trials for the treatment of solid tumors and recurrent gliomas. Many different types of nanoparticles have been conjugated to chlorotoxin in order to facilitate delivery of contrast agents (R. Huang et al., 2011; Veiseh et al., 2010) and anticancer therapies (Veiseh et al., 2009, 2010; Hao Wang, Gu, Xiao, Ye, & Xu, 2014) to gliomas. Furthermore, several studies have demonstrated the efficacy of chlorotoxin itself in preventing invasion and migration of glioma cells (Deshane, Garner, & Sontheimer, 2003; Soroceanu, Manning, & Sontheimer, 1999). In addition to chlorotoxin, several other ion channel blockers have been isolated from scorpion venom, including iberiotoxin, margatoxin, and charybdotoxin, all of which could serve as potential therapeutic agents due to their antiproliferative effects on cancer cells.
Snake Venom
Constituents of snake venom have been extensively studied for over 30 years due to their effects on a wide variety of physiological targets. Snake venom peptides and their derivatives have found particular success in the field of cardiovascular medicine. Bradykinin potentiating factors were first isolated from the venom of the lancehead viper Bothrops jararaca in the 1960s and demonstrated potent inhibition of angiotensin-converting enzyme (ACE) activity. Structure-function studies of these peptides identified the chemical groups most responsible for this inhibition, eventually leading to the development of the first orally active ACE inhibitor, captopril (Koh & Kini, 2012). ACE inhibitors continue to be extremely popular drugs, with one study reporting that ACE inhibitors are prescribed to 27% of all patients with hypertension (Smith & Ashiya, 2007).
Peptides derived from snake venom have had a substantial impact on the fields of thrombosis and hemostasis due to their pro- and anti-coagulant activity. Factor X activators derived from elapid and viperid venoms, such as Russell’s Viper Venom-X, have been primarily used as diagnostic tools in the hematology laboratory for detection of lupus anticoagulants (Takeya et al., 1992; Thiagarajan, Pengo, & Shapiro, 1986). Similarly, prothrombin and factor V activators are beneficial for diagnostic use due to the high specificity of venom components for their substrates. For instance, factor V remains the only known substrate for Russell’s Viper Venom-V, allowing this compound to be developed into a specific tool for detection of factor V (Esmon & Jackson, 1973; McCleary & Kini, 2013). Ecarin, an enzyme derived from the venom of the saw-scaled viper Echis carinatus promotes conversion of prothrombin to meizothrombin but is inhibited by hirudin, thereby serving as the basis for a clotting time assay for patients treated with hirudin (Nowak, 2003). Additionally, the class of enzymes known as snake venom thrombin-like enzymes (SVTLEs) has found use in diagnostic labs to defibrinogenate blood samples, as addition of SVTLEs results in formation of loose fibrin clots due to cleavage at only one of the two fibrinogen chains (Koh & Kini, 2012). Other venom components have been utilized for their procoagulant effects on platelets, where snaclec (snake C-type lectin-like) toxins have demonstrated platelet activation through binding of von Willebrand factor (e.g., botrocetin, bitiscetin) or direct binding to platelet glycoprotein receptors (e.g., convulxin) (Sajevic, Leonardi, & Križaj, 2011). Platelet aggregation has also been shown to occur through activation of protease activated receptors on platelets by snake venom serine proteases such as thrombocytin (McCleary & Kini, 2013).
The major classes of anticoagulants present in snake venom include enzymes such as phospholipase A2 (Carredano et al., 1998) and peptides such as snaclecs and “three finger toxins” (Kini, 2006). Snaclecs, including anticoagulation factor I, bind to coagulation factors IX and X, resulting in inhibition of clotting as measured by activated partial thromboplastin time (aPTT), prothrombin time (PTT), and thrombin time (TT) (Zhang et al., 2012). Similarly, three-finger toxins, which consist of 4–5 disulfide bridges and three β-stranded loops (Kini, 2006) , have been studied for their anticoagulant effects on the extrinsic pathway of the clotting cascade (Kini, Haar, & Evans, 1988) and factor VIIa inhibition (Banerjee, Mizuguchi, Iwanaga, & Kini, 2005).
Outside of the cardiovascular field, peptides derived from snake venom have been studied as anticancer therapies due to their proapoptotic properties. To this end, silica nanoparticles bearing venom from the black snake Walterinnesia aegyptia have been shown to enhance function of normal lymphocytes (Badr, Al-Sadoon, El-Toni, & Daghestani, 2012), as well as induce apoptosis in human prostate cancer cells (Badr, Al-Sadoon, Rabah, & Sayed, 2013) and mouse models of multiple myeloma (Al-Sadoon, Rabah, & Badr, 2013).
Conclusion
Natural peptides possess unique biological, chemical, and physical properties which underlie their application as therapeutic agents. The use of natural peptides in conjunction with nanoparticle delivery systems holds great promise for the development of new therapeutic formulations as well as novel platforms for the delivery of various cargoes.
Contributor Information
Andrew P. Jallouk, Email: jallouka@wusm.wustl.edu.
Rohun U. Palekar, Email: rpalekar@cmrl.wustl.edu.
Hua Pan, Email: hpan@dom.wustl.edu.
Paul H. Schlesinger, Email: phschlesinger@gmail.com.
Samuel A. Wickline, Email: wicklines@aol.com.
References
- Ahn EH, Kim DW, Shin MJ, Kim HR, Kim SM, Woo SJ, Choi SY. PEP-1-PEA-15 protects against toxin-induced neuronal damage in a mouse model of Parkinson’s disease. Biochimica et Biophysica Acta (BBA) - General Subjects. 2014;1840(6):1686–1700. doi: 10.1016/j.bbagen.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Al-Sadoon MK, Rabah DM, Badr G. Enhanced anticancer efficacy of snake venom combined with silica nanoparticles in a murine model of human multiple myeloma: Molecular targets for cell cycle arrest and apoptosis induction. Cellular Immunology. 2013;284(1–2):129–138. doi: 10.1016/j.cellimm.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Andersson DI, Hughes D. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiology Reviews. 2011;35(5):901–911. doi: 10.1111/j.1574-6976.2011.00289.x. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Yokohori N, Nagai A. Alveolar Wall Apoptosis Causes Lung Destruction and Emphysematous Changes. American Journal of Respiratory Cell and Molecular Biology. 2003;28(5):555–562. doi: 10.1165/rcmb.2002-0090OC. [DOI] [PubMed] [Google Scholar]
- Apte A, Koren E, Koshkaryev A, Torchilin VP. Doxorubicin in TAT peptide-modified multifunctional immunoliposomes demonstrates increased activity against both drug-sensitive and drug-resistant ovarian cancer models. Cancer Biology & Therapy. 2014;15(1):69–80. doi: 10.4161/cbt.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB, Saltzman WM, Slack FJ. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proceedings of the National Academy of Sciences. 2012;109(26):E1695–E1704. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G, Al-Sadoon MK, El-Toni AM, Daghestani M. Walterinnesia aegyptia venom combined with silica nanoparticles enhances the functioning of normal lymphocytes through PI3K/AKT, NFkappaB and ERK signaling. Lipids in Health and Disease. 2012;11(1):27. doi: 10.1186/1476-511X-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G, Al-Sadoon MK, Rabah DM, Sayed D. Snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles induce apoptosis and growth arrest in human prostate cancer cells. Apoptosis. 2013;18(3):300–314. doi: 10.1007/s10495-012-0787-1. [DOI] [PubMed] [Google Scholar]
- Bailey P, Wilce J. Venom as a source of useful biologically active molecules. Emergency Medicine. 2001;13(1):28–36. doi: 10.1046/j.1442-2026.2001.00174.x. [DOI] [PubMed] [Google Scholar]
- Balayssac S, Burlina F, Convert O, Bolbach G, Chassaing G, Lequin O. Comparison of Penetratin and Other Homeodomain-Derived Cell-Penetrating Peptides: Interaction in a Membrane-Mimicking Environment and Cellular Uptake Efficiency†. Biochemistry. 2006;45(5):1408–1420. doi: 10.1021/bi0518390. [DOI] [PubMed] [Google Scholar]
- Banerjee Y, Mizuguchi J, Iwanaga S, Kini RM. Hemextin AB Complex, a Unique Anticoagulant Protein Complex from Hemachatus haemachatus (African Ringhals Cobra) Venom That Inhibits Clot Initiation and Factor VIIa Activity. Journal of Biological Chemistry. 2005;280(52):42601–42611. doi: 10.1074/jbc.M508987200. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, Riley N, Nguyen V, Montgomery RO, French BA, Li J, French SW. The mechanism of cytokeratin aggresome formation: the role of mutant ubiquitin (UBB+1) Experimental and Molecular Pathology. 2003;74(2):160–167. doi: 10.1016/s0014-4800(02)00024-2. [DOI] [PubMed] [Google Scholar]
- Bechinger B. Structure and Functions of Channel-Forming Peptides: Magainins, Cecropins, Melittin and Alamethicin. The Journal of Membrane Biology. 1997;156(3):197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- Bessalle R, Haas H, Goria A, Shalit I, Fridkin M. Augmentation of the antibacterial activity of magainin by positive-charge chain extension. Antimicrobial Agents and Chemotherapy. 1992;36(2):313–317. doi: 10.1128/aac.36.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J. Cationic antimicrobial peptides: issues for potential clinical use. BioDrugs: Clinical Immunotherapeutics, Biopharmaceuticals and Gene Therapy. 2003;17(4):233–240. doi: 10.2165/00063030-200317040-00002. [DOI] [PubMed] [Google Scholar]
- Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Zgurskaya HI. Tackling antibiotic resistance. Nature Reviews Microbiology. 2011;9(12):894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon L, de A, Silva A, de AE, Ciancaglini P, Stábeli RG. Antimicrobial peptides from Phyllomedusa frogs: from biomolecular diversity to potential nanotechnologic medical applications. Amino Acids. 2011;40(1):29–49. doi: 10.1007/s00726-010-0622-3. [DOI] [PubMed] [Google Scholar]
- Carredano E, Westerlund B, Persson B, Saarinen M, Ramaswamy S, Eaker D, Eklund H. The three-dimensional structures of two toxins from snake venom throw light on the anticoagulant and neurotoxic sites of phospholipase A2. Toxicon. 1998;36(1):75–92. doi: 10.1016/s0041-0101(97)00051-2. [DOI] [PubMed] [Google Scholar]
- Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Current Opinion in Biotechnology. 2007;18(1):26–30. doi: 10.1016/j.copbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Chereddy KK, Her C-H, Comune M, Moia C, Lopes A, Porporato PE, Préat V. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. Journal of Controlled Release. 2014;194:138–147. doi: 10.1016/j.jconrel.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Chu Q, Moellering RE, Hilinski GJ, Kim Y-W, Grossmann TN, Yeh JT-H, Verdine GL. Towards understanding cell penetration by stapled peptides. Med. Chem. Commun. 2014 [Google Scholar]
- Conlon JM, Kolodziejek J, Nowotny N. Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2004;1696(1):1–14. doi: 10.1016/j.bbapap.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proceedings of the National Academy of Sciences. 2002;99(2):643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik DJ, Fairlie DP, Liras S, Price D. The Future of Peptide-based Drugs. Chemical Biology & Drug Design. 2013;81(1):136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- Crombez L, Morris MC, Dufort S, Aldrian-Herrada G, Nguyen Q, Master GM, Divita G. Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth. Nucleic Acids Research. 2009;37(14):4559–4569. doi: 10.1093/nar/gkp451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhier F, Pourcelle V, Marchand-Brynaert J, Jérôme C, Feron O, Préat V. Methods in Enzymology. Vol. 508. Elsevier; 2012. Targeting of Tumor Endothelium by RGD-Grafted PLGA-Nanoparticles; pp. 157–175. Retrieved from http://europepmc.org/abstract/MED/22449925. [DOI] [PubMed] [Google Scholar]
- De Coupade C, Fittipaldi A, Chagnas V, Michel M, Carlier S, Tasciotti E, Cailler F. Novel human-derived cell-penetrating peptides for specific subcellular delivery of therapeutic biomolecules. Biochemical Journal. 2005;390(2):407. doi: 10.1042/BJ20050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente JM, Berry CC. Tat Peptide as an Efficient Molecule To Translocate Gold Nanoparticles into the Cell Nucleus. Bioconjugate Chemistry. 2005;16(5):1176–1180. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. Journal of Biological Chemistry. 1994;269(14):10444–10450. [PubMed] [Google Scholar]
- Desai P, Patlolla RR, Singh M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Molecular Membrane Biology. 2010;27(7):247–259. doi: 10.3109/09687688.2010.522203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges S, Prieult FL, Daly A, Lydon J, Brennan M, Rai DK, Devocelle M. In Vitro Activities of Synthetic Host Defense Propeptides Processed by Neutrophil Elastase against Cystic Fibrosis Pathogens. Antimicrobial Agents and Chemotherapy. 2011;55(5):2487–2489. doi: 10.1128/AAC.01384-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshane J, Garner CC, Sontheimer H. Chlorotoxin Inhibits Glioma Cell Invasion via Matrix Metalloproteinase-2. Journal of Biological Chemistry. 2003;278(6):4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cellular and Molecular Life Sciences CMLS. 2005;62(16):1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Chua P-J, Bay B-H, Gopalakrishnakone P. Scorpion venoms as a potential source of novel cancer therapeutic compounds. Experimental Biology and Medicine. 2014;239(4):387–393. doi: 10.1177/1535370213513991. [DOI] [PubMed] [Google Scholar]
- Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A Comprehensive Model for the Cellular Uptake of Cationic Cell-penetrating Peptides. Traffic. 2007;8(7):848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Lewis RJ. Use of Venom Peptides to Probe Ion Channel Structure and Function. Journal of Biological Chemistry. 2010;285(18):13315–13320. doi: 10.1074/jbc.R109.076596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiríksdóttir E, Konate K, Langel Ü, Divita G, Deshayes S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2010;1798(6):1119–1128. doi: 10.1016/j.bbamem.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Elliott G, O’Hare P. Intercellular Trafficking and Protein Delivery by a Herpesvirus Structural Protein. Cell. 1997;88(2):223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Elmquist A, Lindgren M, Bartfai T, Langel Ü. VE-Cadherin-Derived Cell-Penetrating Peptide, pVEC, with Carrier Functions. Experimental Cell Research. 2001;269(2):237–244. doi: 10.1006/excr.2001.5316. [DOI] [PubMed] [Google Scholar]
- El-Sayed A, Futaki S, Harashima H. Delivery of Macromolecules Using Arginine-Rich Cell-Penetrating Peptides: Ways to Overcome Endosomal Entrapment. The AAPS Journal. 2009;11(1):13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon CT, Jackson CM. The factor V activating enzyme of Russell’s viper venom. Thrombosis Research. 1973;2(6):509–524. [Google Scholar]
- Findlay B, Zhanel GG, Schweizer F. Cationic Amphiphiles, a New Generation of Antimicrobials Inspired by the Natural Antimicrobial Peptide Scaffold. Antimicrobial Agents and Chemotherapy. 2010;54(10):4049–4058. doi: 10.1128/AAC.00530-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde É, Humphreys H, Greene CM, Fitzgerald-Hughes D, Devocelle M. Potential of Host Defense Peptide Prodrugs as Neutrophil Elastase-Dependent Anti-Infective Agents for Cystic Fibrosis. Antimicrobial Agents and Chemotherapy. 2014;58(2):978–985. doi: 10.1128/AAC.01167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y. Arginine-rich Peptides AN ABUNDANT SOURCE OF MEMBRANE-PERMEABLE PEPTIDES HAVING POTENTIAL AS CARRIERS FOR INTRACELLULAR PROTEIN DELIVERY. Journal of Biological Chemistry. 2001;276(8):5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Gajski G, Garaj-Vrhovac V. Melittin: A lytic peptide with anticancer properties. Environmental Toxicology and Pharmacology. 2013;36(2):697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Gallo G. Methods in Cell Biology. Vol. 71. Academic Press; 2003. Making Proteins into Drugs: Assisted Delivery of Proteins and Peptides into Living Neurons; pp. 325–338. Retrieved from http://www.sciencedirect.com/science/article/pii/S0091679×0301015X. [DOI] [PubMed] [Google Scholar]
- Gao S, Simon MJ, Hue CD, Morrison B, Banta S. An Unusual Cell Penetrating Peptide Identified Using a Plasmid Display-Based Functional Selection Platform. ACS Chemical Biology. 2011;6(5):484–491. doi: 10.1021/cb100423u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnon J, Lachance C, Marco SD, Hel Z, Marion D, Ruiz MC, Radzioch D. Fragile X-related Protein FXR1P Regulates Proinflammatory Cytokine Tumor Necrosis Factor Expression at the Post-transcriptional Level. Journal of Biological Chemistry. 2005;280(7):5750–5763. doi: 10.1074/jbc.M401988200. [DOI] [PubMed] [Google Scholar]
- Grdisa M. The Delivery of Biologically Active (Therapeutic) Peptides and Proteins into Cells. Current Medicinal Chemistry. 2011;18(9):1373–1379. doi: 10.2174/092986711795029591. [DOI] [PubMed] [Google Scholar]
- Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55(6):1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Gros E, Deshayes S, Morris MC, Aldrian-Herrada G, Depollier J, Heitz F, Divita G. A non-covalent peptide-based strategy for protein and peptide nucleic acid transduction. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2006;1758(3):384–393. doi: 10.1016/j.bbamem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Gullotti E, Park J, Yeo Y. Polydopamine-Based Surface Modification for the Development of Peritumorally Activatable Nanoparticles. Pharmaceutical Research. 2013;30(8):1956–1967. doi: 10.1007/s11095-013-1039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Levchenko TS, Torchilin VP. TAT peptide-modified liposomes provide enhanced gene delivery to intracranial human brain tumor xenografts in nude mice. Oncology Research. 2007;16(8):351–359. doi: 10.3727/000000006783980946. [DOI] [PubMed] [Google Scholar]
- Hamley IW. Self-assembly of amphiphilic peptides. Soft Matter. 2011;7(9):4122. [Google Scholar]
- Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnology. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. British Journal of Pharmacology. 2009;157(2):195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques ST, Melo MN, Castanho MARB. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochemical Journal. 2006;399(1):1. doi: 10.1042/BJ20061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X-H, Wang Y, Yan X-T, Wang Y-L, Wang C-Y, Zhang Z-Z, Jiang H-X. Transduction of PEP-1-Heme Oxygenase-1 Fusion Protein Reduces Myocardial Ischemia/Reperfusion Injury in Rats. Journal of Cardiovascular Pharmacology. 2013;62(5):436–442. doi: 10.1097/FJC.0b013e3182a0b638. [DOI] [PubMed] [Google Scholar]
- Hood JL, Jallouk AP, Campbell N, Ratner L, Wickline SA. Cytolytic nanoparticles attenuate HIV-1 infectivity. Antiviral Therapy. 2013;18(1):95–103. doi: 10.3851/IMP2346. [DOI] [PubMed] [Google Scholar]
- Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2008;1778(2):357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou KK, Pan H, Lanza GM, Wickline SA. Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials. 2013;34(12):3110–3119. doi: 10.1016/j.biomaterials.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou KK, Pan H, Ratner L, Schlesinger PH, Wickline SA. Mechanisms of Nanoparticle-Mediated siRNA Transfection by Melittin-Derived Peptides. ACS Nano. 2013;7(10):8605–8615. doi: 10.1021/nn403311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Jin H, Qian Y, Qi S, Luo H, Luo Q, Zhang Z. Hybrid Melittin Cytolytic Peptide-Driven Ultrasmall Lipid Nanoparticles Block Melanoma Growth in Vivo. ACS Nano. 2013;7(7):5791–5800. doi: 10.1021/nn400683s. [DOI] [PubMed] [Google Scholar]
- Huang R, Han L, Li J, Liu S, Shao K, Kuang Y, Jiang C. Chlorotoxin-modified macromolecular contrast agent for MRI tumor diagnosis. Biomaterials. 2011;32(22):5177–5186. doi: 10.1016/j.biomaterials.2011.03.075. [DOI] [PubMed] [Google Scholar]
- Hu Q, Liang B, Sun Y, Guo X-L, Bao Y-J, Xie D-H, Peng Z-H. Preparation of bufalin-loaded pluronic polyetherimide nanoparticles, cellular uptake, distribution, and effect on colorectal cancer. International Journal of Nanomedicine. 2014;9:4035–4041. doi: 10.2147/IJN.S64708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallouk AP, Moley KH, Omurtag K, Hu G, Lanza GM, Wickline SA, Hood JL. Nanoparticle Incorporation of Melittin Reduces Sperm and Vaginal Epithelium Cytotoxicity. PLoS ONE. 2014;9(4):e95411. doi: 10.1371/journal.pone.0095411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Vasil AI, Hale JD, Hancock REW, Vasil ML, Hodges RS. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Peptide Science. 2008;90(3):369–383. doi: 10.1002/bip.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson L, Tung C-H, Moore A, Weissleder R. High-Efficiency Intracellular Magnetic Labeling with Novel Superparamagnetic-Tat Peptide Conjugates. Bioconjugate Chemistry. 1999;10(2):186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Research. 2008;36(12):4158–4171. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegylated liposomes. Journal of Drug Targeting. 2007a;15(7-8):538–545. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale AA, Torchilin VP. “Smart” Drug Carriers: PEGylated TATp-Modified pH-Sensitive Liposomes. Journal of Liposome Research. 2007b;17(3-4):197–203. doi: 10.1080/08982100701525035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Park SH, Kang MH, Park MJ, Choi YW. Folic acid-tethered Pep-1 peptide-conjugated liposomal nanocarrier for enhanced intracellular drug delivery to cancer cells: conformational characterization and in vitro cellular uptake evaluation. International Journal of Nanomedicine. 2013;8:1155–1165. doi: 10.2147/IJN.S39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini RM. Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochemical Journal. 2006;397(3):377. doi: 10.1042/BJ20060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini RM, Haar NC, Evans HJ. Non-enzymatic inhibitors of coagulation and platelet aggregation from Naja nigricollis venom are cardiotoxins. Biochemical and Biophysical Research Communications. 1988;150(3):1012–1016. doi: 10.1016/0006-291x(88)90729-2. [DOI] [PubMed] [Google Scholar]
- Kleemann E, Neu M, Jekel N, Fink L, Schmehl T, Gessler T, Kissel T. Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG-PEI. Journal of Controlled Release. 2005;109(1-3):299–316. doi: 10.1016/j.jconrel.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nature Reviews Drug Discovery. 2005;4(3):206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- Koh CY, Kini RM. From snake venom toxins to therapeutics - Cardiovascular examples. Toxicon. 2012;59(4):497–506. doi: 10.1016/j.toxicon.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Koren E, Apte A, Jani A, Torchilin VP. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. Journal of Controlled Release. 2012;160(2):264–273. doi: 10.1016/j.jconrel.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YT, Hartner WC, Kale A, Torchilin VP. Gene delivery into ischemic myocardium by double-targeted lipoplexes with anti-myosin antibody and TAT peptide. Gene Therapy. 2008;16(1):52–59. doi: 10.1038/gt.2008.135. [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Choi Y-S, Suh J-S, Kwon Y-M, Yang VC, Lee S-J, Park Y-J. Cell-penetrating chitosan/doxorubicin/TAT conjugates for efficient cancer therapy. International Journal of Cancer. 2011;128(10):2470–2480. doi: 10.1002/ijc.25578. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Garcia ML. Therapeutic potential of venom peptides. Nature Reviews Drug Discovery. 2003;2(10):790–802. doi: 10.1038/nrd1197. [DOI] [PubMed] [Google Scholar]
- Li F, Wang T, He HB, Tang X. The properties of bufadienolides-loaded nano-emulsion and submicro-emulsion during lyophilization. International Journal of Pharmaceutics. 2008;349(1-2):291–299. doi: 10.1016/j.ijpharm.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Li F, Yang R, Weng Y, Tang X. Preparation and evaluation of lyophilized liposome-encapsulated bufadienolides. Drug Development and Industrial Pharmacy. 2009;35(9):1048–1058. doi: 10.1080/03639040902762987. [DOI] [PubMed] [Google Scholar]
- Liu L, Guo K, Lu J, Venkatraman SS, Luo D, Ng KC, Yang Y-Y. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood-brain barrier. Biomaterials. 2008;29(10):1509–1517. doi: 10.1016/j.biomaterials.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Liu L, Xu K, Wang H, Tan PKJ, Fan W, Venkatraman SS, Yang Y-Y. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nature Nanotechnology. 2009;4(7):457–463. doi: 10.1038/nnano.2009.153. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xiong M, Gong J, Zhang Y, Bai N, Luo Y, Xiang R. Legumain protease-activated TAT-liposome cargo for targeting tumours and their microenvironment. Nature Communications. 2014;5 doi: 10.1038/ncomms5280. [DOI] [PubMed] [Google Scholar]
- Li W, Nicol F, Szoka FC., Jr GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Advanced Drug Delivery Reviews. 2004;56(7):967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39(2):162–173. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- Malhotra M, Tomaro-Duchesneau C, Prakash S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials. 2013;34(4):1270–1280. doi: 10.1016/j.biomaterials.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Manzoor AA, Lindner LH, Landon CD, Park J-Y, Simnick AJ, Dreher MR, Dewhirst MW. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Research. 2012 doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JR, Placone J, Hristova K, Wimley WC. Spontaneous Membrane-Translocating Peptides by Orthogonal High-Throughput Screening. Journal of the American Chemical Society. 2011;133(23):8995–9004. doi: 10.1021/ja2017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty C, Meylan C, Schott H, Ballmer-Hofer K, Schwendener RA. Enhanced heparan sulfate proteoglycan-mediated uptake of cell-penetrating peptide-modified liposomes. Cellular and Molecular Life Sciences CMLS. 2004;61(14):1785–1794. doi: 10.1007/s00018-004-4166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary RJR, Kini RM. Snake bites and hemostasis/thrombosis. Thrombosis Research. 2013;132(6):642–646. doi: 10.1016/j.thromres.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Meng H, Kumar K. Antimicrobial Activity and Protease Stability of Peptides Containing Fluorinated Amino Acids. Journal of the American Chemical Society. 2007;129(50):15615–15622. doi: 10.1021/ja075373f. [DOI] [PubMed] [Google Scholar]
- Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discovery Today. 2012;17(15-16):850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Morris MC, Chaloin L, Méry J, Heitz F, Divita G. A novel potent strategy for gene delivery using a single peptide vector as a carrier. Nucleic Acids Research. 1999;27(17):3510–3517. doi: 10.1093/nar/27.17.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nature Biotechnology. 2001;19(12):1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- Morris MC, Vidal P, Chaloin L, Heitz F, Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Research. 1997;25(14):2730–2736. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Morris MA, Heitz F, Divita G, Morris MC. The peptide carrier Pep-1 forms biologically efficient nanoparticle complexes. Biochemical and Biophysical Research Communications. 2007;355(4):877–882. doi: 10.1016/j.bbrc.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Myerson J, He L, Lanza G, Tollefsen D, Wickline S. Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. Journal of Thrombosis and Haemostasis. 2011;9(7):1292–1300. doi: 10.1111/j.1538-7836.2011.04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase I, Akita H, Kogure K, Gräslund A, Langel Ü, Harashima H, Futaki S. Efficient Intracellular Delivery of Nucleic Acid Pharmaceuticals Using Cell-Penetrating Peptides. Accounts of Chemical Research. 2012;45(7):1132–1139. doi: 10.1021/ar200256e. [DOI] [PubMed] [Google Scholar]
- Nakayama F, Yasuda T, Umeda S, Asada M, Imamura T, Meineke V, Akashi M. Fibroblast Growth Factor-12 (FGF12) Translocation into Intestinal Epithelial Cells Is Dependent on a Novel Cell-penetrating Peptide Domain INVOLVEMENT OF INTERNALIZATION IN THE IN VIVO ROLE OF EXOGENOUS FGF12. Journal of Biological Chemistry. 2011;286(29):25823–25834. doi: 10.1074/jbc.M110.198267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends in Biotechnology. 2011;29(9):464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Nowak G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiology of Haemostasis and Thrombosis. 2003;33(4):173–183. doi: 10.1159/000081505. [DOI] [PubMed] [Google Scholar]
- Ochocki JD, Mullen DG, Wattenberg EV, Distefano MD. Evaluation of a cell penetrating prenylated peptide lacking an intrinsic fluorophore via in situ click reaction. Bioorganic & Medicinal Chemistry Letters. 2011;21(17):4998–5001. doi: 10.1016/j.bmcl.2011.04.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata D, Negishi Y, Hagiwara S, Yamamura S, Endo-Takahashi Y, Suzuki R, Aramaki Y. Bubble Liposomes and Ultrasound Promoted Endosomal Escape of TAT-PEG Liposomes as Gene Delivery Carriers. Molecular Pharmaceutics. 2011;8(6):2416–2423. doi: 10.1021/mp200353m. [DOI] [PubMed] [Google Scholar]
- Palekar RU, Myerson JW, Schlesinger PH, Sadler JE, Pan H, Wickline SA. Thrombin-Targeted Liposomes Establish a Sustained Localized Anticlotting Barrier against Acute Thrombosis. Molecular Pharmaceutics. 2013;10(11):4168–4175. doi: 10.1021/mp400210q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Ivashyna O, Sinha B, Lanza GM, Ratner L, Schlesinger PH, Wickline SA. Post-formulation peptide drug loading of nanostructures for metered control of NF-κB signaling. Biomaterials. 2011;32(1):231–238. doi: 10.1016/j.biomaterials.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Myerson JW, Hu L, Marsh JN, Hou K, Scott MJ, Wickline SA. Programmable nanoparticle functionalization for in vivo targeting. The FASEB Journal. 2013;27(1):255–264. doi: 10.1096/fj.12-218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Myerson JW, Ivashyna O, Soman NR, Marsh JN, Hood JL, Wickline SA. Lipid Membrane Editing with Peptide Cargo Linkers in Cells and Synthetic Nanostructures. The FASEB Journal. 2010;24(8):2928–2937. doi: 10.1096/fj.09-153130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooga M, Hällbrink M, Zorko M, Uuml Langel L. Cell penetration by transportan. The FASEB Journal. 1998;12(1):67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- Presente A, F. Dowdy S. [Retrieved November 8, 2014];PTD/CPP Peptide-Mediated Delivery of siRNAs [Text] 2013 May; from http://wustl.library.ingentaconnect.com.beckerproxy.wustl.edu/content/ben/cpd/2013/00000019/00000016/art00008.
- Qin Y, Chen H, Zhang Q, Wang X, Yuan W, Kuai R, He Q. Liposome formulated with TAT-modified cholesterol for improving brain delivery and therapeutic efficacy on brain glioma in animals. International Journal of Pharmaceutics. 2011;420(2):304–312. doi: 10.1016/j.ijpharm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Rhee M, Davis P. Mechanism of Uptake of C105Y, a Novel Cell-penetrating Peptide. Journal of Biological Chemistry. 2006;281(2):1233–1240. doi: 10.1074/jbc.M509813200. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Lebleu B. Cell-penetrating Peptides A REEVALUATION OF THE MECHANISM OF CELLULAR UPTAKE. Journal of Biological Chemistry. 2003;278(1):585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Ruan G, Agrawal A, Marcus AI, Nie S. Imaging and Tracking of Tat Peptide-Conjugated Quantum Dots in Living Cells: New Insights into Nanoparticle Uptake, Intracellular Transport, and Vesicle Shedding. Journal of the American Chemical Society. 2007;129(47):14759–14766. doi: 10.1021/ja074936k. [DOI] [PubMed] [Google Scholar]
- Sajevic T, Leonardi A, Križaj I. Haemostatically active proteins in snake venoms. Toxicon. 2011;57(5):627–645. doi: 10.1016/j.toxicon.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Sawant RR, Torchilin VP. Enhanced cytotoxicity of TATp-bearing paclitaxel-loaded micelles in vitro and in vivo. International Journal of Pharmaceutics. 2009;374(1-2):114–118. doi: 10.1016/j.ijpharm.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In Vivo Protein Transduction: Delivery of a Biologically Active Protein into the Mouse. Science. 1999;285(5433):1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1999;1462(1-2):55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- Sharma G, Modgil A, Zhong T, Sun C, Singh J. Influence of Short-Chain Cell-Penetrating Peptides on Transport of Doxorubicin Encapsulating Receptor-Targeted Liposomes Across Brain Endothelial Barrier. Pharmaceutical Research. 2014;31(5):1194–1209. doi: 10.1007/s11095-013-1242-x. [DOI] [PubMed] [Google Scholar]
- Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Research. 2003;31(11):2717–2724. doi: 10.1093/nar/gkg385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RET, Ashiya M. Antihypertensive therapies. Nature Reviews Drug Discovery. 2007;6(8):597–598. doi: 10.1038/nrd2354. [DOI] [PubMed] [Google Scholar]
- Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, Heuser JE, Schlesinger PH. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. Journal of Clinical Investigation. 2009;119(9):2830–2842. doi: 10.1172/JCI38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soman NR, Lanza GM, Heuser JM, Schlesinger PH, Wickline SA. Synthesis and Characterization of Stable Fluorocarbon Nanostructures as Drug Delivery Vehicles for Cytolytic Peptides. Nano Letters. 2008;8(4):1131–1136. doi: 10.1021/nl073290r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release. 2001;70(1-2):1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- Soroceanu L, Manning TJ, Sontheimer H. Modulation of Glioma Cell Migration and Invasion Using Cl− and K+ Ion Channel Blockers. The Journal of Neuroscience. 1999;19(14):5942–5954. doi: 10.1523/JNEUROSCI.19-14-05942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splith K, Neundorf I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. European Biophysics Journal. 2011;40(4):387–397. doi: 10.1007/s00249-011-0682-7. [DOI] [PubMed] [Google Scholar]
- Suk JS, Suh J, Choy K, Lai SK, Fu J, Hanes J. Gene delivery to differentiated neurotypic cells with RGD and HIV Tat peptide functionalized polymeric nanoparticles. Biomaterials. 2006;27(29):5143–5150. doi: 10.1016/j.biomaterials.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya H, Nishida S, Miyata T, Kawada S, Saisaka Y, Morita T, Iwanaga S. Coagulation factor X activating enzyme from Russell’s viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. Journal of Biological Chemistry. 1992;267(20):14109–14117. [PubMed] [Google Scholar]
- Taylor BN, Mehta RR, Yamada T, Lekmine F, Christov K, Chakrabarty AM, Gupta TKD. Noncationic Peptides Obtained From Azurin Preferentially Enter Cancer Cells. Cancer Research. 2009;69(2):537–546. doi: 10.1158/0008-5472.CAN-08-2932. [DOI] [PubMed] [Google Scholar]
- Thiagarajan P, Pengo V, Shapiro SS. The use of the dilute Russell viper venom time for the diagnosis of lupus anticoagulants. Blood. 1986;68(4):869–874. [PubMed] [Google Scholar]
- Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D’Souza GGM. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proceedings of the National Academy of Sciences. 2003;100(4):1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proceedings of the National Academy of Sciences. 2001;98(15):8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson MT, Tosteson DC. The sting. Melittin forms channels in lipid bilayers. Biophysical Journal. 1981;36(1):109–116. doi: 10.1016/S0006-3495(81)84719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Caruthers SD, Hughes M, Marsh JN, Cyrus T, Winter PM, Lanza GM. Clinical applications of perfluorocarbon nanoparticles for molecular imaging and targeted therapeutics. International Journal of Nanomedicine. 2007;2(4):515–526. [PMC free article] [PubMed] [Google Scholar]
- Tseng Y-L, Liu J-J, Hong R-L. Translocation of Liposomes into Cancer Cells by Cell-Penetrating Peptides Penetratin and Tat: A Kinetic and Efficacy Study. Molecular Pharmacology. 2002;62(4):864–872. doi: 10.1124/mol.62.4.864. [DOI] [PubMed] [Google Scholar]
- Tünnemann G, Ter-Avetisyan G, Martin RM, Stöckl M, Herrmann A, Cardoso MC. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. Journal of Peptide Science. 2008;14(4):469–476. doi: 10.1002/psc.968. [DOI] [PubMed] [Google Scholar]
- Veiseh O, Gunn JW, Kievit FM, Sun C, Fang C, Lee JSH, Zhang M. Inhibition of Tumor-Cell Invasion with Chlorotoxin-Bound Superparamagnetic Nanoparticles. Small. 2009;5(2):256–264. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiseh O, Kievit FM, Fang C, Mu N, Jana S, Leung MC, Zhang M. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials. 2010;31(31):8032–8042. doi: 10.1016/j.biomaterials.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen S, Laufer SD, Trampe A, Restle T. Cellular delivery of small interfering RNA by a non-covalently attached cell-penetrating peptide: quantitative analysis of uptake and biological effect. Nucleic Acids Research. 2006;34(22):6561–6573. doi: 10.1093/nar/gkl941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivès E, Brodin P, Lebleu B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. Journal of Biological Chemistry. 1997;272(25):16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discovery Today. 2010;15(1-2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proceedings of the National Academy of Sciences. 1992;89(17):7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle Delivery of Cancer Drugs. Annual Review of Medicine. 2012;63(1):185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- Wang B, Lv L, Wang Z, Zhao Y, Wu L, Fang X, Xin H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α2-mediated endocytosis. Biomaterials. 2014;35(22):5897–5907. doi: 10.1016/j.biomaterials.2014.03.068. [DOI] [PubMed] [Google Scholar]
- Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Research. 2009;37(suppl 1):D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gu W, Xiao N, Ye L, Xu Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. International Journal of Nanomedicine. 2014;9:1433–1442. doi: 10.2147/IJN.S58783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu K, Liu L, Tan JPK, Chen Y, Li Y, Li L. The efficacy of self-assembled cationic antimicrobial peptide nanoparticles against Cryptococcus neoformans for the treatment of meningitis. Biomaterials. 2010;31(10):2874–2881. doi: 10.1016/j.biomaterials.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Xia H, Gao X, Gu G, Liu Z, Hu Q, Tu Y, Chen H. Penetratin-functionalized PEG-PLA nanoparticles for brain drug delivery. International Journal of Pharmaceutics. 2012;436(1-2):840–850. doi: 10.1016/j.ijpharm.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Yin P, Wang Y, Qiu Y, Hou L, Liu X, Qin J, Li Q. Bufalin-loaded mPEG-PLGA-PLL-cRGD nanoparticles: preparation, cellular uptake, tissue distribution, and anticancer activity. International Journal of Nanomedicine. 2012;7:3961–3969. doi: 10.2147/IJN.S32063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial Peptides in Health and Disease. New England Journal of Medicine. 2002;347(15):1199–1200. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu X, Shen D, Song J, Guo M, Yan X. Anticoagulation factor I, a snaclec (snake C-type lectin) from Agkistrodon acutus venom binds to FIX as well as FX: Ca2+ induced binding data. Toxicon. 2012;59(7-8):718–723. doi: 10.1016/j.toxicon.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Zhao M, Kircher MF, Josephson L, Weissleder R. Differential Conjugation of Tat Peptide to Superparamagnetic Nanoparticles and Its Effect on Cellular Uptake. Bioconjugate Chemistry. 2002;13(4):840–844. doi: 10.1021/bc0255236. [DOI] [PubMed] [Google Scholar]
- Zhao P, Wang H, Yu M, Cao S, Zhang F, Chang J, Niu R. Paclitaxel-Loaded, Folic-Acid-Targeted and TAT-Peptide-Conjugated Polymeric Liposomes: In Vitro and In Vivo Evaluation. Pharmaceutical Research. 2010;27(9):1914–1926. doi: 10.1007/s11095-010-0196-5. [DOI] [PubMed] [Google Scholar]
- Zhao X, Pan F, Xu H, Yaseen M, Shan H, Hauser CAE, Lu JR. Molecular self-assembly and applications of designer peptide amphiphiles. Chemical Society Reviews. 2010;39(9):3480–3498. doi: 10.1039/b915923c. [DOI] [PubMed] [Google Scholar]
- Zhou H, Yan H, Pan H, Hou KK, Akk A, Springer LE, Pham CTN. Peptide-siRNA nanocomplexes targeting NF-κB subunit p65 suppress nascent experimental arthritis. Journal of Clinical Investigation. 2014;124(10):4363–4374. doi: 10.1172/JCI75673. [DOI] [PMC free article] [PubMed] [Google Scholar]