Abstract
Cholestatic liver disorders encompass hepatobiliary diseases of diverse etiologies characterized by the accumulation of bile acids, bilirubin and cholesterol as the result of impaired secretion of bile. Members of the nuclear receptor (NR) family of ligand-modulated transcription factors are implicated in the adaptive response to cholestasis. NRs coordinately regulate bile acid and phospholipid transporter genes required for hepatobiliary transport, as well as the phases I and II metabolizing enzymes involved in processing of their substrates. In this review we will focus on FXR and PXR, two members of the NR family whose activities are regulated by bile acids. In addition, we also discuss the potential of pharmacological modulators of these receptors as novel therapies for cholestatic disorders.
Keywords: FXR, PXR, Cholestasis, Nuclear receptor, Liver, Review
1. Introduction
Cholestatic liver disorders include a spectrum of hepatobiliary diseases of diverse etiologies that are characterized by impaired hepatocellular secretion of bile, resulting in accumulation of bile acids, bilirubin and cholesterol. Causes of cholestasis include extrahepatic biliary obstruction (e.g. stones, tumors, biliary atresia), intrahepatic biliary obstruction (e.g. primary biliary cirrhosis, primary sclerosing cholangitis) and intrahepatic cholestasis (e.g. drugs, genetic transporter defects, or infections) [1,2]. In patients with cholestasis, the major abnormalities observed are an elevation of circulating levels of primary bile acids and an increase in the formation of sulfated bile acids. The major mechanism for bile acid elimination in severely cholestatic patients is renal excretion, with the relatively hydrophilic tetrahydroxy bile acids found in their urine [3]. In advanced cholestasis, the ratio of cholic acid (CA) to chenodeoxycholic acid (CDCA) increases in the serum, the proportion of unconjugated bile acids is reduced, and concentrations of the secondary bile acid deoxycholic acid (DCA) decreases [4]. The physiological consequences of reduced intestinal bile acids include maldigestion of fat and malabsorption of fat-soluble vitamins. In addition, increased circulating bile acids may contribute to pruritis [4], as well as apoptosis or necrosis of hepatocytes [5]. Progressive hepatic fibrosis and cirrhosis can ensue leading to death due to hepatic failure or the complications of portal hypertension.
Hepatobiliary transport of bile acids and phospholipids is mediated by specific transporters expressed at the canalicular membrane of the hepatocyte. Impaired function of these transporters leads to reduced bile formation or cholestasis and mutations in these genes are associated with a variety of hereditary cholestatic syndromes. At the transcriptional level, these transporters and the phases I and II metabolizing enzymes involved in processing of their substrates are coordinately regulated by members of the nuclear receptor (NR) family of ligand-modulated transcription factors. In this review, we will focus on FXR and PXR, two members of the NR family whose activities are regulated by bile acids and which are implicated in the adaptive response to cholestasis.
2. FXR (farnesoid X receptor)
FXR (farnesoid X receptor, NR1H4, also known as bile acid receptor, BAR) was the first nuclear receptor identified to have bile acids as endogenous and physiologically relevant ligands [6,9]. FXR serves as a sensor for bile acids and promotes enterohepatic clearance of bile acids by controlling the expression of genes involved in their transport and metabolism. In addition, FXR counteracts liver X receptor (LXR) in both cholesterol and triglyceride metabolism [7]. FXR is abundantly expressed in the liver and intestine as well as the kidney and adrenal gland [8]. Endogenous bile acids have differing abilities to activate FXR. In vitro studies indicate that CDCA is a potent FXR ligand at physiological concentrations (Fig. 1), whereas other bile acids such as lithocholic acid (LCA), DCA, and CA are less effective, and hydroxylated CDCA metabolites (muricholic acids) do not activate FXR [6,9]. Interestingly, although LCA functions as a weak agonist for FXR, it strongly antagonizes CDCA-stimulated activation of FXR [10]. Synthetic FXR agonists have been identified, including GW4064, an isoxazole derivative [11], and fexaramine [12] as well as the semi-synthetic CDCA derivative, 6-ethyl CDCA [13] (Fig. 1). In addition, the natural product guggulsterone is a promiscuous nuclear receptor ligand that antagonizes FXR [14], but activates PXR [15] and the progesterone receptor [16]. FXR binds DNA in most instances as a heterodimer with retinoid X receptor α (RXRα), to response elements consisting of tandem repeats of the AGGTCA hexamer. FXR preferentially binds as a heterodimer to an IR-1 element (inverted repeat with a single nucleotide spacer), but can also activate transcription through other DNA elements, such as DR-3 or DR-4 motifs. Less commonly it can activate transcription by binding to DNA as a monomer, independent of RXR [17,18].
Fig. 1.
Structures of ligands that interact with FXR and/or PXR. Compounds that are agonists for FXR or PXR are shown in panels (A) and (B), respectively. The effective agonist concentration that elicits 50% of maximum activation of the respective human receptor (EC50) is provided, with the exception of PCN, a selective mouse PXR ligand, where the EC50 for mouse PXR is shown. Guggulsterone, a promiscuous nuclear receptor ligand that acts as both an FXR antagonist and a PXR agonist, is shown in (C). Ursodeoxycholic acid, a naturally occurring epimer of chenodeoxycholic acid that is used in the therapy of some cholestatic liver disorders, but is not thought to significantly interact with nuclear receptors, is shown for comparative purposes in (D). CDCA, chenodeoxycholic acid; PCN, pregnenolone 16α-carbonitrile.
Characterization of mice with targeted deletions of FXR has provided insight into the physiological importance of this nuclear receptor. Sinal et al. [19] showed that Fxr knockout (Fxr KO) mice display high serum bile acids, cholesterol and triglycerides and decreased fecal excretion of bile acids, a phenotype similar to Byler’s disease [19]. Surprisingly, increased serum bile acid levels were associated with a reduction of total bile acid pool size in the Fxr KO animals, suggesting that bile acid synthesis was suppressed in the absence of functional FXR [19]. In another study, however, elevated serum bile acid concentrations in Fxr KO mice were associated with an increased biliary bile acid output as well as increased cholate pool size and total bile acid pool size, corresponding to an increase in bile acid synthesis [20]. Fxr KO mice fed with high concentrations of dietary CA exhibited increased hepatocyte necrosis and increased mortality as compared to wild-type mice, most likely due to liver failure secondary to toxic bile acid accumulation [21]. A shorter duration of 1% CA feeding resulted in a marked increase in serum, liver and urine bile acid concentrations, and severe hepatotoxicity in Fxr KO mice, associated with the loss of expression of the apical hepatocyte bile acid transporter, Abcb11, and reduced biliary excretion of bile acids [19–22]. Compensatory increases in expression of the basolateral bile acid efflux transporters, Mrp3 and Mrp4 have been demonstrated in these mice [23]. Fxr KO mice also fail to repress Cyp7a1 appropriately in response to bile acid (BA) feeding, resulting in increased bile acid synthesis, consistent with loss of induction of short heterodimer partner (Shp) [19,24].
3. Regulation of bile acid transport and metabolism by FXR
Activation of FXR in vivo is associated with increased hepatobiliary circulation of bile acids, inhibition of hepatic bile acid biosynthesis, and a reduction in plasma triglycerides [25]. Below we will discuss the roles of FXR in bile acid transport and metabolism and the implications for cholestatic disorders (Fig. 2).
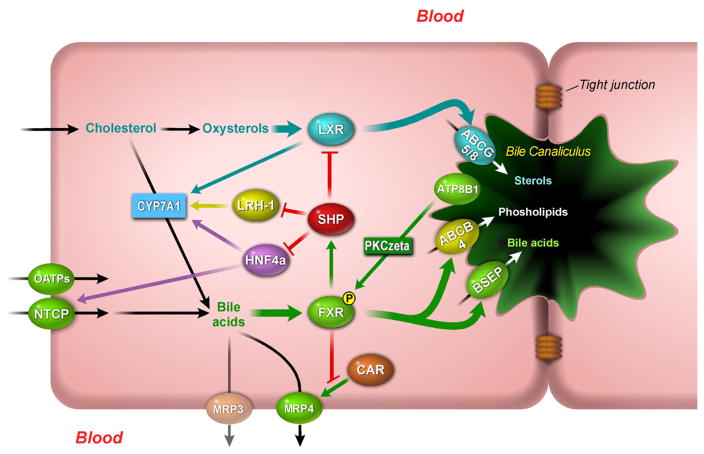
Fig. 2.
FXR-mediated bile acid transport and metabolism in the hepatocyte. Hepatic uptake of bile acids from the circulation takes place at the sinusoidal (basolateral) membrane of the hepatocyte and is mediated by NTCP and several members of the OATP family. Within the hepatocyte, bile acids can be converted to cholesterol or eliminated via bile. In addition, cholesterol can be converted to bile acids by CYP7A1. Bile acids can activate FXR which in turn induces the expression of the bile acid transporter ABCB11 (BSEP) and the phospholipid transporter ABCB4 (MDR2/3). Bile acid secretion is also stimulated by the phospholipid flippase ATP8B1. Loss of ATP8B1 results in reduced activity and nuclear translocation of FXR which is caused by impaired protein kinase C zeta (PKCzeta)-mediated phosphorylation of FXR. Negative feedback on bile acid metabolism and secretion is mediated by SHP, which is induced by FXR and inhibits the action of several NRs including LXR, HNF4α and LRH-1. Via an alternative pathway, cholesterol is converted to oxysterols that can activate LXR which in turn induces the expression of the sterol transporters ABCG5/8 at the canalicular membrane. During cholestasis bile acids can also be excreted back into the circulation via the sinusoidal ABC-transporters MRP3 and 4. MRP4 in turn is repressed by FXR through competition for binding to an overlapping binding site with CAR.
3.1. Regulation of bile formation
FXR coordinately controls secretion of bile acids and phospholipids, the main constituents of bile (72% and 24% of solutes by weight, respectively), which together form mixed micelles for the emulsification of dietary lipids and lipid-soluble vitamins. Hepatobiliary transport of bile acids and phospholipids is mediated by dedicated transporters expressed at the canalicular (apical) membrane of the hepatocyte. Impaired function of these transporters leads to impaired bile formation or cholestasis and mutations in these genes are associated with progressive familial intrahepatic cholestasis (PFIC) syndromes, a heterogeneous group of autosomal recessive disorders, which can be classified into three subtypes [26–28]. PFIC1 and PFIC2 usually appear in the first months of life, whereas onset of PFIC3 may also occur later in infancy, in childhood or even during early adulthood. The predominant clinical manifestations are cholestasis, pruritus and jaundice. PFIC patients usually develop fibrosis and end-stage liver disease before adulthood. Serum gamma-glutamyltransferase (GGT) activity is usually normal in PFIC1 and PFIC2 patients, but is elevated in PFIC3 [26–28].
PFIC1 or Byler disease is characterized by the appearance of intrahepatic cholestasis in the first months of life, with recurrent episodes of jaundice and pruritus, and progression to chronic liver failure in later stages [29]. PFIC1 is caused by mutations in the ATP8B1 (FIC1) gene, encoding a phospholipid flippase that transports aminophospholipids from the outer to the inner leaflet of the canalicular membrane [29,30]. Mutations in ATP8B1 are also associated with benign recurrent intrahepatic cholestasis (BRIC), a milder form of hereditary cholestasis [29]. PFIC1 is associated with decreased FXR activity and nuclear translocation [31,32], which is caused by impaired protein kinase C zeta (PKCzeta)-mediated phosphorylation of cytosolic FXR and subsequent absence of nuclear translocation [33,34]. Studies with ATP8B1 KO mice revealed that Atp8b1 deficiency affects the canalicular phospholipid membrane asymmetry, rendering the canalicular membrane less resistant toward hydrophobic bile salts and subsequently resulting in impaired BA transport and cholestasis [35].
PFIC2 (Byler Syndrome) is characterized by early onset intrahepatic cholestasis, jaundice, pruritus and progression to hepatic fibrosis, cirrhosis and endstage liver disease before adulthood. PFIC2 patients exhibit a 100-fold reduction in bile acid secretion into bile resulting in the accumulation of bile acids within hepatocytes, cholestatis and liver injury. Unlike patients with PFIC3, serum levels of cholesterol and GGT are usually normal or only mildly elevated. PFIC2 is caused by mutations in the ABCB11 gene (bile salt export protein (BSEP) or sister of P-glycoprotein (S-PGP)). Shortly after the identification of ABCB11 as a bile acid transporter, the human ortholog was cloned and found to be mutated in PFIC2 [36]. Mutations in ABCB11 have also been associated with two milder cholestatic syndromes: (1) benign recurrent intrahepatic cholestasis type 2 (BRIC2), which is characterized by intermittent episodes of cholestasis without progression to liver disease and (2) intrahepatic cholestasis of pregnancy (ICP), which typically presents with pruritus and abnormal liver tests in the third trimester of pregnancy and is associated with increased risk of intrauterine fetal death and prematurity [37,38]. Although the functional consequences of most mutations in ABCB11 are still unknown, several mutations have been demonstrated to result in impaired activity, stability or trafficking to the membrane [39,40]. The severity of the different cholestatic phenotypes has further been demonstrated to correlate with activity and levels of expression of ABCB11 [41]. In contrast to humans with PFIC2, targeted inactivation of Abcb11 in mice resulted only in a mild non-progressive cholestasis [42]. Surprisingly, although secretion of CA, the major bile acid in mice, was greatly reduced (to 6% of wild-type), total bile acid output in mutant mice was still about 30% of wild-type. Also, secretion of an unexpectedly large amount of tetrahydroxylated bile acids, which were not present in wild-type mice, was observed. These results suggested that hydroxylation and an alternative canalicular transport mechanism for bile acids could compensate for the absence of Abcb11 and protect the mutant mice from severe cholestatic liver injury [43]. Abcb11 KO mice fed with a CA supplemented diet displayed a more severe PFIC2 phenotype, indicating that with bile acid loading this compensatory transport was not sufficient [43]. Further analysis of the Abcb11 KO mice showed that expression of Abcb1 (Mdr1) was markedly increased, especially after CA feeding, while Abcb4 (Mdr2), Abcc2 (Mrp2), and Abcc3 (Mrp3) were increased only to a moderate extent [44]. Moreover, plasma membrane vesicles isolated from a cell line overexpressing ABCB1 exhibited ATP-dependent bile salt transport, albeit with a 5-fold lower affinity compared to ABCB11. These findings suggested that, in mice Abcb1 may act as a compensatory bile acid transporter, and could explain the relatively mild phenotype of Abcb11 KO mice [44]. In keeping with the more severe phenotype in humans, no upregulation of ABCB1 was found in PFIC2 patients [45]. In addition to its role in PFIC2, Abcb11 has been mapped to the Lith1 locus for gallstone susceptibility in mice [46]. Interestingly, the Lith1 locus also harbors the nuclear receptor LXRα, which is associated with gallstone formation through the regulation of cholesterol transport by ABCG5/8. The role of Abcb11 in the formation of gallstones was confirmed by the finding that gallstone-susceptible C57L/J mice (Lith1 mice) displayed increased levels of Abcb11 as compared to gallstone-resistant AKR/J mice [47,48] and further by the fact that transgenic mice overexpressing hepatic Abcb11 rapidly developed cholesterol gallstones [49]. FXR transcriptionally activates ABCB11 [50–52], and bile acids increase ABCB11 expression in primary hepatocytes or HepG2 cells with the same rank order of potency that activates FXR [23]. Conversely, the secondary bile acid LCA decreases ABCB11 expression by antagonizing FXR activation [10]. An important mechanism of drug-induced cholestasis is inhibition of ABCB11, with accumulation of bile acids in hepatocytes and subsequent liver injury. Examples of such drugs include cyclosporin A, rifampicin and glibenclamide. Reductions in ABCB11 have also been implicated in sepsis-induced cholestasis [53]. Recently, a liver receptor homologue-1 (LRH-1) response element (LRHRE) was identified in the human ABCB11 promoter and overexpression of LRH-1 was shown to induce expression of ABCB11, suggesting that the NR LRH-1 supports FXR in the regulation of bile acid levels [54].
PFIC3 is characterized by reduced or absent phospholipid excretion into bile [28]. Phospholipids are an essential constituent of the bile and act to reduce the detergent activity of bile acid micelles, thereby protecting the membranes of cells lining the biliary tree from damage. In the absence of phospholipids, bile acid toxicity results in damage to cholangiocytes and progressive cholestatic liver injury accompanied by increased serum levels of GGT. PFIC3 is caused by mutations in the ABCB4 gene (MDR2/3 P-glycoprotein). ABCB4 was originally isolated based on homology with ABCB1 [55,56]. However, unlike ABCB1, ABCB4 was not involved in conferring the MDR phenotype and its function remained unknown for several years until KO mice were generated [57]. Abcb4 KO mice displayed progressive liver damage at an early age, which was accompanied by hyperbilirubinemia and increased liver enzymes in plasma. Further analysis revealed the absence of phospholipids and dramatically reduced levels of cholesterol and glutathione in bile, whereas bile flow itself was about 2-fold increased [57]. A link with a human disease was made when de Vree et al. showed that ABCB4 is mutated in patients with PFIC3 [58]. Later it was established that ABCB4 functions as a phospholipid floppase, promoting the ATP-dependent transfer of phosphatidylcholine from the inner to the outer leaflet of the plasma membrane lipid bilayer [59]. In humans, ABCB4 is induced in cholestasis [21], and is regulated by FXR [60]. Trans-activation of ABCB4 by FXR has been demonstrated through direct binding of FXR/RXRα heterodimers to a highly conserved inverted repeat element (FXR response element) in the distal promoter [60]. In rats, Abcb4 is induced by the FXR agonist GW4064 [61], but can still be induced in FXR KO mice fed a CA diet [19], suggesting that several bile acid-responsive regulatory mechanisms must be capable of inducing this gene.
3.2. SHP-mediated repression
FXR also downregulates many target genes indirectly via transcriptional induction of another NR, small heterodimer partner (SHP, NR0B2) [24,62]. SHP is an atypical member of the NR subfamily as it lacks a DNA-binding domain. SHP can interact with and negatively affect the transcriptional activation activity of several other members of the NR subfamily, including LXR, LRH-1, HNF4α as well as transcription factors belonging to the basic-helix-loop-helix family [63,64]. It has been suggested that SHP represses nuclear hormone receptor-mediated target gene trans-activation via two mechanisms; competition with nuclear receptor coactivator proteins as well as its direct transcriptional repressor function [65,66]. In addition, SHP-mediated gene repression is associated with chromatin remodelling through recruitment of histone deacetylases (HDACs), the Swi/Snf-Brm complex and G9a methyltransferase to the CYP7A1 promoter [67,68].
FXR represses transcription of CYP7A1, encoding the rate-limiting enzyme in the classic (neutral) bile acid synthesis pathway, and CYP8B1, which is required for synthesis of CA [6]. CYP7A1 expression is controlled by a variety of factors, including hormones, oxysterols, bile acids, drugs and diurnal rhythms [69]. CYP7A1 is positively regulated by the orphan nuclear receptors Hepatocyte Nuclear Factor 4α (HNF4α) and Liver Receptor Homolog 1 (LRH-1). HNF4α is a key regulator of hepatic gene expression and a major activator of HNF1α, which in turn activates the expression of a large number of liver-specific genes, including those involved in glucose, cholesterol, and fatty acid metabolism [70–72]. LRH-1 is highly expressed in liver and is required for the hepatic expression of CYP7A1 and CYP8B1 [73,74]. In rodents, CYP7A1 is also positively regulated by LXR which binds to a direct repeat NR motif (DR-4) in the Cyp7a1 promoter when activated by oxysterols, and strongly induces Cyp7a1 transcription [75]. This regulation is not present in other species and explains why humans develop hypercholesterolemia on a diet high in cholesterol whereas in rodents cholesterol can be converted to bile acids by LXR-mediated stimulation of CYP7A1 transcription [76].
Bile acids repress CYP7A1 through FXR-induced expression of SHP, which in turn negatively interacts with several other members of the NR subfamily, including LXR, HNF4α and LRH-1 [22,58,77]. The importance of SHP in the feedback regulation of bile acid synthesis was demonstrated in Shp KO mice, which have increased Cyp7a1 and Cyp8b1 expression and activity and a corresponding increase in the bile acid pool size [78,79], and in SHP transgenic mice, which have reduced expression of Cyp7a1 and a smaller hepatic bile acid pool size [80]. In addition to blocking cholesterol catabolism, FXR also promotes lipid clearance through inducing genes involved in lipoprotein metabolism and inhibiting hepatic lipogenesis via SHP-mediated repression of SREBP-1c [7,81,82].
The FXR-SHP axis also negatively regulates bile acid uptake systems in the gut and liver, including the reabsorption of bile acids in the ileal enterocytes by ASBT (apical sodium-dependent BA transporter, SLC10A2) [83], and in the hepatocytes by NTCP (Na+-taurocholate cotransporting polypeptide, SLC10A1) and OATP-C (SLC21A6) [25]. Additionally, FXR stimulates transcription of the ileal bile acid-binding protein (I-BABP) and the heterodimeric organic solute transporters OSTα/OSTβ, which are involved in trafficking bile acids across the enterocyte into the portal circulation [6,19,84] and it has also been implicated in the regulation of OATP8 (SLC21A8) in the liver [85].
3.3. Endocrine regulation of bile acid homeostasis
In addition to repression by SHP, an alternative SHP-independent pathway to repress CYP7A1, mediated by the c-Jun N-terminal kinase pathway (JNK), has been described [79,86] (Fig. 3). This pathway was linked to FXR activation by the finding that, in cultured hepatocytes, FXR directly induced the transcription of the fibroblast growth factor 19 (FGF-19), a secreted growth factor that signals through the FGFR4 cell-surface receptor tyrosine kinase and subsequent activation of the intracellular JNK pathway [87]. In vivo, FGF15 (the murine ortholog of FGF19) however, was found to be primarily induced in the ileum after administration of the FXR agonist GW4064 to mice [88]. FGF15 KO mice displayed increased hepatic CYP7A1 mRNA and protein levels and corresponding increases in CYP7A1 enzyme activity, suggesting that FGF15 functions as an enterohepatic signal to regulate bile acid homeostasis [88] (Fig. 3). A similar phenotype is observed in FGFR4 KO mice [10]. It was further found that the FXR agonist GW4064 could significantly repress CYP7A1 in liver specific FXR KO mice but not in intestinal specific FXR KO mice, demonstrating that activation of FXR in intestine but not liver is required for short-term repression of CYP7A1 in liver [89]. The existence of alternative pathways to repress CYP7A1 also explains observed differences in feedback repression between CYP7A1 and CYP8B1. In comparison to CYP7A1, FXR-mediated repression of CYP8B1 was more dependent on the presence of FXR in liver (through SHP) and less dependent on its presence in intestine (FGF15). Consistent with these findings, recombinant FGF15 repressed CYP7A1 mRNA levels without affecting CYP8B1 expression. FXR-mediated repression of bile acid synthesis thus requires the complementary actions of FXR in both liver and intestine [89]. Alternatively, FGF19 can also repress CYP7A1 by increasing the stability of SHP through inhibiting its proteasomal degradation in a mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK-ERK) dependent manner [90] (Fig. 3). FGF15/19 has also been demonstrated to stimulate gallbladder filling, suggesting a postprandial feedback loop opposing the actions of cholecystokinin (CCK), which stimulates gallbladder emptying [91]. In addition to its roles in bile acid metabolism, FGF15/19 has been demonstrated to lower serum glucose and triglycerides in diabetic mice by activation of the MAPK-ERK pathway and subsequent induction of hepatic protein and glycogen synthesis [92–94].
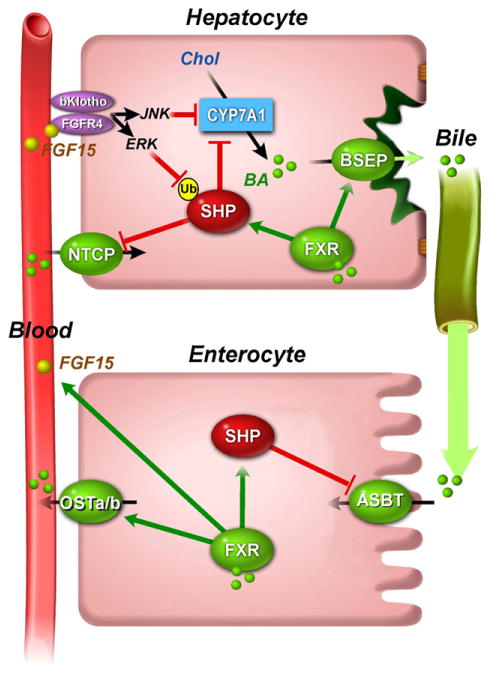
Fig. 3.
Regulation of enterohepatic circulation of bile acids by FXR. FXR regulates the enterohepatic circulation of bile acids in the hepatocyte and enterocyte. After a meal, bile is released from the gallbladder into the duodenum. Bile acid re-uptake in the ileum is mediated by ASBT in the brush border membrane. In the enterocyte, FXR induces the expression of OSTα/β, which mediate bile acid transport from the enterocyte into the portal circulation to hepatocytes where they are taken up via NTCP. In the hepatocyte, FXR induces the expression of BSEP, which mediates bile acid excretion into bile. Negative feedback on the enterohepatic circulation of bile acid is mediated by SHP, which is induced by FXR and inhibits CYP7A1 and NTCP in the hepatocyte, and ASBT in the enterocyte. Alternatively, in the endocrine pathway, FXR in the enterocyte induces the expression and secretion of FGF15/19, which binds in the liver to the FGFR4/βKlotho receptor complex and in turn activates JNK and ERK signaling pathways. Activation of the JNK and ERK pathways results in repression of CYP7A1 and stabilization of SHP by inhibition of its proteasomal degradation, respectively.
4. PXR (pregnane X receptor)
PXR (NR1I2, also known as the steroid and xenobiotic receptor (SXR) or the pregnane-activated receptor (PAR)), is a promiscuous nuclear receptor that is activated by structurally unrelated xenobiotics, steroids, drugs and bile acids [95,96]. In response to a diverse array of compounds, PXR coordinately regulates a suite of genes involved in the metabolism, transport, and ultimately, elimination of these molecules. PXR is highly expressed in the liver, small intestine, and colon [95,96]. Notably, these are the same tissues where cytochrome P450 3A (CYP3A) genes are most highly expressed. In rodents, lower levels of PXR mRNA have also been detected in the kidney, stomach, lung, ovary, and placenta [96]. In humans, PXR mRNA has been detected in both normal and neoplastic breast tissue [97]. PXR is most closely related to CAR (NR1I3, Constitutive Androstane Receptor); the two receptors share ~70% amino acid identity in their LBDs, and they also have an overlapping target gene pattern [98,99]. PXR has promiscuous, often low-affinity, ligand specificity. Orthologous receptors from human, rat, mouse and rabbit have been cloned and characterized and share approximately 95% identity in their DNA binding domains. In contrast, they share only 75–80% identity in the amino acid sequences in their LBDs [100]. This results in species-specific variations in PXR ligand specificity, which has an impact on the activation of target genes (particularly CYP3As) by different xenobiotics. For example, rifampicin is a potent activator of rabbit and human PXRs [95] while pregnenolone 16α-carbonitrile (PCN) activates mouse and rat PXRs [101]. The species-specific nature of PXR ligand specificity was further illustrated using mice where mouse PXR was replaced with human PXR. These mice displayed a human PXR-mediated xenobiotic response profile and represent a unique tool for the exploration of the impact of xenobiotics, including therapeutic drugs, on hepatic and intestinal function [102,103].
The spectrum of PXR ligands is large and includes xenobiotics such as rifampicin, natural and synthetic steroids, such as pregnenolone, progesterone, phytoestrogens, dexamethasone, and antiglucocorticoids, as well as drugs and plant products, such as hyperforin in St. John’s wort (Fig. 1). The crystal structure of the PXR ligand-binding domain has revealed several unique characteristics that account for its promiscuous ligand binding properties, including a large flexible, elliptical ligand binding pocket, and a relative lack of specific binding interactions, allowing PXR to bind ligands that are diverse in both their size and their structure [104,105].
5. Regulation of bile acid transport and metabolism by PXR
Although PXR was initially characterized as a xenosensor, the discovery that certain bile acids such as LCA can serve as ligands for both human and mouse PXR provided a link between PXR and bile acid regulation [106,107]. Below we will discuss the role of PXR in the detoxification of bile acids and the implications in cholestatic disorders (Fig. 4).
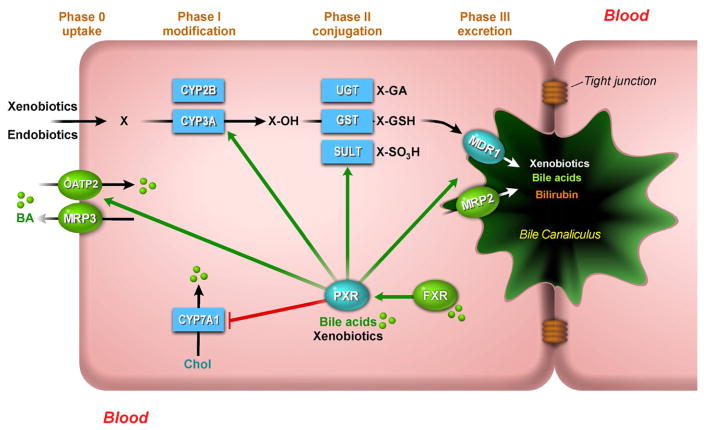
Fig. 4.
PXR-mediated bile acid transport and metabolism in the hepatocyte. PXR can mitigate the harmful effects of toxic bile acids (BA) such as LCA by activation of hepatic detoxification pathways. Activation of PXR induces the uptake of xeno- and endobiotics (phase 0), their modification by members of the cytochrome P450 subfamily (phase I), conjugation by glutathione S-transferases (GSTs), UDP-glucuronosyl-transferases (UGTs) and sulfotransferases (SULTs) (phase II) and elimination (phase III) by MRP2 (excretion of bilirubin and some bile acids), and the multidrug transporter MDR1 (excretion of a wide variety of xenobiotics and endobiotics). PXR can be directly activated by certain bile acids or indirectly via transcriptional regulation by FXR. Negative feedback on bile acid metabolism is mediated by inhibition of CYP7A1. During cholestasis bile acids can also be excreted back into the circulation via the sinusoidal ABC-transporters MRP3 and 4.
5.1. Activation of PXR by bile acids
A function for PXR in bile acid homeostasis was first suggested by the demonstration that it can be activated by the secondary bile acid LCA [106,107]. PXR was found to be directly activated by bile acids as low affinity ligands, with LCA and its major metabolite in mice, 3-keto LCA, both being efficacious activators of human and mouse PXR at concentrations between 10 and 100 μM. The rank order of potency (3-keto-LCA > LCA > DCA = CA) differs from that of FXR [106,107]. However neither conjugated LCA, nor any of the other conjugated bile acids activate PXR. In addition to direct activation by bile acids, PXR itself is a transcriptional target of bile acid-activated FXR [108] (Fig. 4).
5.2. Regulation of bile acid metabolism (phases I and II)
PXR can mitigate the harmful effects of toxic bile acids such as LCA by activation of two hepatic detoxification pathways, namely hydroxylation by members of the cytochrome P450 subfamily (phase I) and conjugation by glutathione S-transferases (GSTs), UDP-glucuronosyltransferases (UGTs) and sulfotransferases (SULTs) (phase II) (Fig. 4). These reactions make bile acids more hydrophilic and facilitate their transporter-mediated elimination (phase III) through bile or urine. The potential importance of this function was supported by the observation that non-bile acid PXR activators, such as the steroid PCN, significantly decreased CYP7A1 expression [106], with competition between PXR and PGC-1α for binding to HNF4α, thereby blocking PGC-1α-stimulated activation of CYP7A1 by HNF4α [109].
It was proposed that PXR acts as a physiological sensor of LCA and its metabolites, inhibiting Cyp7a1, which blocks BA synthesis, inducing Oatp2, and presumably increasing uptake of LCA and other bile acids from sinusoidal blood into the hepatocyte where hydroxylation by Cyp3a11 or other Cyp3a subfamily members could take place. These more hydrophilic and less toxic hydroxylated bile acid metabolites could then be excreted in the urine or feces. However, as LCA is a secondary bile acid, formed in the intestine from bacterial 7-dehydroxylation of CDCA, it would not be expected to accumulate significantly in some forms of clinical cholestasis, such as biliary obstruction, where the enterohepatic circulation of bile acids is interrupted. Moreover, while bile duct ligation of mice markedly induced a human CYP3A4 reporter transgene, intraperitoneal injection of LCA had little effect on the reporter, despite causing hepatic necrosis [110]. Therefore the relevance of PXR as a pathophysiological sensor for LCA is uncertain in respect of CYP3A gene regulation.
5.3. Regulation of bile acid elimination (phase III)
Some toxic bile acids such as conjugates of LCA can also be eliminated by the canalicular ABC-transporter ABCC2 (previously known as MRP2 or canalicular multispecific organic anion transporter (cMOAT)). Deficiency in ABCC2 is associated with the Dubin–Johnson syndrome, a recessive disorder, characterized by conjugated hyperbilirubinemia [111]. ABCC2 can transport a variety of compounds including bilirubin diglucuronide, sulfates, some bile acids (e.g. conjugates of LCA), xenobiotics (e.g. cisplatin, anthracyclines, vinca alkaloids, methotrexate), as well as glutathione conjugates into bile, and is therefore a major determinant of bile acid-independent bile flow [112]. It has been suggested that bile acids can regulate ABCC2 expression, since CDCA, an FXR ligand, can induce the expression of ABCC2 mRNA in human and rat hepatocytes [113]. An atypical promoter everted repeat element (ER-8) has been identified within the rat Abcc2 promoter that is involved in the ligand-mediated induction of Abcc2 by FXR, PXR and CAR in cultured cells [114]. Subsequently, in vivo studies of mice with cholestasis induced by common bile duct ligation have found regulation of Abcc2 to be independent of FXR [115]. Induction of Abcc2 in the liver of WT but not PXR KO mice has been reported after administration of PCN or CA [116,117], implying a significant in vivo role for PXR in regulation of Abcc2.
PXR is also an important activator of the ABC-transporter ABCB1 (MDR1, P-glycoprotein). ABCB1 is best known for its role in multidrug resistance and is a key player in the defence of the body against xenotoxins [111]. The transcriptional regulation of ABCB1 is complex, and numerous transcription factors have been implicated in its regulation [118]. A large number of drugs have been identified as either substrates or inhibitors of ABCB1, and a range of exogenous stimuli can increase transcription of the Abcb1 promoter [119]. Bile acids and their conjugated metabolites are not substrates for ABCB1; however certain substrates for ABCB1, including drugs, may cause drug induced cholestasis by interacting with bile acid homeostasis such as inhibition of ABCB11 [113]. In vitro studies have implicated rifampicin- and paclitaxel-mediated activation of PXR in the induction of ABCB1 expression in human colon carcinoma cell lines, and induction of Abcb1b by a range of xenobiotics has also been shown to be dependent on PXR [99,120]. However, in a study involving administration of CA to mice, Abcb1a expression was induced independently of PXR and FXR [22], suggesting that induction by endobiotics differs from induction by xenobiotics. In vivo, Abcb1a and Abcb1b are induced by physiological concentrations of bile acids via FXR, independently of PXR [121], demonstrating the complexity of in vivo regulation of these transporters.
6. Implications for cholestatic liver disorders
Pharmacological therapy for cholestasis is limited, and ursodeoxycholic acid (UDCA) is the only disease-modifying drug therapy with evidence of efficacy, improving symptoms, hepatic enzyme abnormalities, and reducing death and liver transplantation in patients with primary biliary cirrhosis (PBC), when commenced sufficiently early in the course of the disease [122,123], and improving both maternal and fetal outcomes in cholestasis of pregnancy [124]. However, a majority of PBC patients are incomplete responders to UDCA [125], and UDCA has not been demonstrated to be efficacious in other forms of chronic cholestasis, such as primary sclerosing cholangitis (PSC). There is a pressing need for effective therapies for PSC, as no pharmacological agents have shown benefit in randomized controlled trials. Studies to date show that UDCA at the dose used to treat PBC (15 mg/kg/day) is ineffective, while high dose UDCA (30 mg/kg/day) is deleterious [126]. In addition, there are several potential problems with designing trials for novel therapies in PSC. Firstly, the course of the condition is frequently one of intermittent flares and remissions, making it difficult to separate out drug effects in short to medium term clinical trials. Secondly, dominant strictures of large bile ducts leading to significant biliary obstruction can occur, leading to a mixed picture of small and large duct cholestasis, so careful patient selection is crucial.
Thus, there is a need for novel therapies for treatment of cholestasis, both to delay progression of liver disease and relieve associated symptoms. The physiological response to cholestasis generally involves downregulation of the hepatocyte basolateral uptake transporters [21] and upregulation of the basolateral efflux transporters [127]. Interestingly, the apical transporter function is often preserved. Abcb11 expression is only modestly impaired, or preserved, both in animals with bile duct obstruction [115] and in humans with cholestasis [21,128]. ABCB4 and ABCB1 are both induced in humans with cholestasis [21], suggesting that bile acids that are specific ligands for FXR may help to maintain expression of these transporters during cholestatic injury.
FXR KO mice display an altered bile acid and lipid homeostasis [19,20], and an altered response to various animal models of cholestasis. High concentrations of dietary CA cause a marked increase in serum, liver and urine bile acid concentrations, and severe hepatotoxicity in FXR KO mice, associated with the loss of expression of Abcb11, and reduced biliary elimination of bile acids [19–22]. However in a BDL model of complete biliary obstruction, FXR KO mice had a mortality and morbidity advantage, and were protected from developing hepatic bile infarcts, even with concurrent deletion of PXR. This protection is probably secondary to downregulation of the FXR-regulated apical transporters ABCB11, ABCB4 and ABCB1, reducing pressure in obstructed bile ducts, as well as other effects including upregulation of the sinusoidal ABC-transporter MRP4 (ABCC4) that mediates transport of bile acids back into the circulation [121,129]. Indeed, FXR was shown to repress expression of MRP4 through competition for binding to an overlapping binding site with CAR [130]. These findings suggest a role for targeted therapy for different cholestatic syndromes, and specifically, a clinical role for FXR antagonists in the treatment of obstructive cholestasis.
PXR-mediated regulation also has a marked impact on the development of hepatic damage in cholestasis as demonstrated in PXR KO mice subjected to various models of cholestasis and/or bile acid overload. Pxr KO mice display an increase in the areas of hepatic necrosis and bile infarcts after injection of LCA [106,107], or BDL [131] and increased sensitivity to lithogenic diet-induced cholesterol gallstone disease (CGD) [132]. Mechanisms for this include loss of PXR-mediated bile acid detoxification mechanisms, encompassing both metabolism and transport. Conversely, PXR activation by PCN or the herbal medicine St. John’s wort protected WT mouse livers against LCA-induced necrosis and CGD [106,107,132]. When PXR KO mice were fed with LCA, they had elevated urinary concentrations of LCA compared with controls, associated with failure to induce hepatic Cyp3a11 and Oatp2 [106,107], and increased liver damage. In contrast, PXR activation by PCN protected WT mouse livers against necrosis caused by LCA. These findings suggest that PXR can function as a receptor for LCA or one of its metabolites, and regulate LCA detoxification in vivo.
7. Current and novel FXR and PXR based drug therapies
7.1. FXR agonists
FXR agonists would be expected to provide a positive therapeutic effect where cholestasis is present in the absence of obstruction of large bile ducts. As covered in the preceding section, FXR-mediated up-regulation of apical hepatocyte bile acid transporters and increased bile production is counterproductive when bile cannot be delivered to the intestine. Recently, a potent selective FXR agonist has entered clinical phase II trials for PBC. 6-ethyl CDCA (6-ECDCA) is a modified bile acid with an EC50 for FXR activation of 99 nM [133] (Fig. 1). When 6-ECDCA was administered as an additional agent for 12 weeks to patients with PBC with an incomplete biochemical response to UDCA, significant improvements in serum alkaline phosphatase and GGT were observed [134]. At higher doses 6-ECDCA caused dose-limiting pruritus, consistent with it being a substituted bile acid, so future studies of small molecule non bile acid-based FXR agonists will be interesting. The efficacy of 6-ECDCA as monotherapy for PBC and its place in long-term management of this chronic condition remains to be explored and phase III trials designed to address these questions should commence soon.
7.2. FXR antagonists
As covered earlier in this review, FXR antagonists may have therapeutic value for attenuating liver injury where there is segmental or total biliary obstruction. While the focus of medical management should be aimed at alleviating the obstruction by surgery or biliary stenting, there may be an adjunctive role for a pharmacological therapy that diverts bile acids to the circulation for elimination via the kidney, as has been demonstrated to occur in BDL Fxr KO mice [121,129]. The natural compound guggulsterone (Fig. 1) has been identified as an FXR antagonist but its propensity to modulate other NRs (covered in Section 2) is a limiting factor for this molecule as a starting point for drug development. More selective natural [135] and synthetic [136] FXR antagonists have been reported but there have been no published in vivo studies to date.
7.3. PXR agonists
Given the pivotal role of PXR in the regulation of therapeutic drug metabolism and its propensity to mediate drug–drug interactions, it is not surprising that PXR has been largely neglected as a drug development target for cholestatic liver disorders. Long before the discovery of PXR, the macrocyclic antibiotic rifampicin, now recognized to be a human PXR agonist (Fig. 1), had been used to treat the intractable pruritus associated with severe cholestasis [137]. However, long-term therapy with rifampicin is not always well tolerated and drug-related hepatitis has been reported [138], a complication that is undesirable in a patient with severe existing liver disease.
The herbal remedy St. John’s wort is a widely used alternative therapy for anxiety and depression. It contains the high affinity PXR agonist hyperforin [139] (Fig. 1), which explains the propensity of St. John’s wort to cause herb–drug interactions. In a pilot study of St. John’s wort administered for 20 weeks to patients with PBC already receiving UDCA we found that this agent was well-tolerated and improved pruritus (C. Stedman, S. Coulter, C. Liddle; unpublished observations). Thus, while PXR agonists are unlikely to be used as first line treatment for cholestatic liver disorders, their role as an adjunctive therapy is worthy of further study.
8. Future perspectives
Cholestatic liver disorders cover a wide spectrum of diseases of diverse etiologies. However, they all share the consequences of retention of bile constituents, especially bile acids. As covered in this review, the nuclear receptors FXR and PXR are activated by bile acids and in turn regulate aspects of the enterohepatic cycling and metabolism of bile acids. It follows that any disease process in which either of these factors is important could potentially benefit from pharmacological manipulation of these nuclear receptors. As covered above, FXR agonists inhibit bile acid synthesis and promote bile acid excretion while PXR agonists promote bile acid detoxification, predominantly by the induction of CYP enzymes that mediate bile acid hydroxylation. Manipulation of both FXR and PXR, either by administration of pharmacological ligands or genetic abrogation, have been shown to influence cholestatic liver injury in animal models to the point of significantly impacting on liver injury and survival after complete bile duct ligation or other models of bile acid accumulation, such as bile acid feeding. Research priorities now include the development of additional pharmacologic tools, such as non-bile acid FXR agonists and FXR antagonists, for the manipulation of these receptors and exploration of their effects in a more diverse range of in vivo models, particularly in view of the observed differences between rodents and man in some aspects of transporter regulation. High affinity, non-bile acid FXR ligands such as GW6064 [11] and fexaramine [12] (Fig. 1) have been developed, but both compounds suffer from poor pharmacokinetic profiles, especially poor oral bioavailability. However, these molecules demonstrate the feasibility of this approach and compounds with suitable characteristics for human clinical trials are expected in the near future. Another related path of drug discovery could center on ligands for FGFR4, the receptor for FGF19, which also suppresses bile acid production through repression of CYP7A1 (Fig. 3), and could potentially be administered in combination with FXR agonists. This approach is worthy of exploration in animal models.
Early phase human studies of existing bile acid-derived FXR agonists are in progress in PBC and clinical trials in PSC, the PFIC syndromes and PFIC-associated syndromes, such as BRIC and BRIC2, are likely to follow. Therapeutic targeting of PXR is likely to remain as a second line therapy, given the profound effects of PXR activation on the disposition of co-administered drugs. Still, in an area of therapeutics where choices are either limited or non-existent, further evaluation of selective, well-tolerated, PXR agonists is worthwhile.
Acknowledgments
We would like to thank Ann Atkins for critically reading the manuscript and Jamie Simon for help with the artwork. This work was supported by the Human Frontier Science Program (HFSP) (JWJ), Marie Curie Reintegration grant under the 7th Framework Programme of the European Commission (JWJ), National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Nuclear Receptor Signaling Atlas orphan receptor Grant U19DK62434, the Environmental Protection Agency Superfund Program Grant (P42 ES10337), the Helmsley Charitable Trust, and National Health and Medical Research Council of Australia (NHMRC) project grants 402493 and 512354.
Abbreviations
- BDL
bile duct ligation
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- FXR
Farnesoid X Receptor
- LCA
lithocholic acid
- LXR
liver X receptor
- NR
nuclear receptor
- PBC
primary biliary cirrhosis
- PCN
pregnenolone 16α-carbonitrile
- PFIC
progressive familial intrahepatic cholestasis
- PSC
primary sclerosing cholangitis
- PXR
pregnane X receptor
- UDCA
ursodeoxycholic acid
Footnotes
Article from the special issue orphan receptors.
Contributor Information
Johan W. Jonker, Email: j.w.jonker@med.umcg.nl.
Michael Downes, Email: downes@salk.edu.
References
- 1.Chitturi S, Farrell GC. Drug-induced cholestasis. Semin Gastrointest Dis. 2001;12:113–124. [PubMed] [Google Scholar]
- 2.Trauner M, Boyer JL. Cholestatic syndromes. Curr Opin Gastroenterol. 2004;20:220–230. doi: 10.1097/00001574-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Alvelius G, Hjalmarson O, Griffiths WJ, Björkhem I, Sjövall J. Identification of unusual 7-oxygenated bile acid sulfates in a patient with Niemann-Pick disease, type C. J Lipid Res. 2001;42:1571–1577. [PubMed] [Google Scholar]
- 4.Hofmann AF. Cholestatic liver disease: pathophysiology and therapeutic options. Liver. 2002;22:14–19. doi: 10.1034/j.1600-0676.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- 5.Higuchi H, Miyoshi H, Bronk SF, Zhang H, Dean N, Gores GJ. Bid anti-sense attenuates bile acid-induced apoptosis and cholestatic liver injury. J Pharmacol Exp Ther. 2001;299:866–873. [PubMed] [Google Scholar]
- 6.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 7.Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 8.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamp WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 9.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Lo JL, Huang L, Zhao A, Metzger E, Adams A, Meinke PT, Wright SD, Cui J. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem. 2002;277:31441–31447. doi: 10.1074/jbc.M200474200. [DOI] [PubMed] [Google Scholar]
- 11.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, Jones SA, Willson TM. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 12.Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer JL, Anisfeld AM, Edwards PA, Rosenfeld JM, Alvarez JG, Noel JP, Nicolaou KC, Evans RM. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–1092. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30:277–294. doi: 10.2165/00002018-200730040-00001. [DOI] [PubMed] [Google Scholar]
- 14.Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–1706. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- 15.Owsley E, Chiang JY. Guggulsterone antagonizes farnesoid X receptor induction of bile salt export pump but activates pregnane X receptor to inhibit cholesterol 7alpha-hydroxylase gene. Biochem Biophys Res Commun. 2003;304:191–195. doi: 10.1016/s0006-291x(03)00551-5. [DOI] [PubMed] [Google Scholar]
- 16.Brobst DE, Ding X, Creech KL, Goodwin B, Kelley B, Staudinger JL. Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J Pharmacol Exp Ther. 2004;310:528–535. doi: 10.1124/jpet.103.064329. [DOI] [PubMed] [Google Scholar]
- 17.Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275:10638–10647. doi: 10.1074/jbc.275.14.10638. [DOI] [PubMed] [Google Scholar]
- 18.Shen H, Zhang Y, Ding H, Wang X, Chen L, Jiang H, Shen X. Farnesoid X receptor induces GLUT4 expression through FXR response element in the GLUT4 promoter. Cell Physiol Biochem. 2008:1–14. doi: 10.1159/000149779. [DOI] [PubMed] [Google Scholar]
- 19.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 20.Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, Stellaard F, Shan B, Schwarz M, Kuipers F. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem. 2003;278:41930–41937. doi: 10.1074/jbc.M306309200. [DOI] [PubMed] [Google Scholar]
- 21.Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H, Trauner M. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol. 2003;39:480–488. doi: 10.1016/s0168-8278(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 22.Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Kliewer SA, Gonzalez FJ, Sinal CJ. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. The small heterodimer partner interacts with the pregnane X receptor and represses its transcriptional activity. J Biol Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- 23.Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ, Venkataramanan R, Cai H, Sinal CJ, Gonzalez FJ, Schuetz JD. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411–39418. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 25.Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Jacquemin E. Progressive familial intrahepatic cholestasis. Genetic basis and treatment. Clin Liver Dis. 2000:753–763. doi: 10.1016/s1089-3261(05)70139-2. [DOI] [PubMed] [Google Scholar]
- 27.Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 2009;4:1. doi: 10.1186/1750-1172-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis. 2010;30:134–146. doi: 10.1055/s-0030-1253223. [DOI] [PubMed] [Google Scholar]
- 29.Paulusma CC, Elferink RP, Jansen PL. Progressive familial intrahepatic cholestasis type 1. Semin Liver Dis. 2010;30:117–124. doi: 10.1055/s-0030-1253221. [DOI] [PubMed] [Google Scholar]
- 30.Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, Lomri N, Berger R, Scharschmidt BF, Knisely AS, Houwen RH, Freimer NB. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1999;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 31.Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126:756–764. doi: 10.1053/j.gastro.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Cai SY, Gautam S, Nguyen T, Soroka CJ, Rahner C, Boyer JL. ATP8B1 deficiency disrupts the bile canalicular membrane bilayer structure in hepatocytes, but FXR expression and activity are maintained. Gastroenterology. 2009;136:1060–1069. doi: 10.1053/j.gastro.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankenberg T, Miloh T, Chen FY, Ananthanarayanan M, Sun AQ, Balasubramaniyan N, Arias I, Setchell KD, Suchy FJ, Shneider BL. The membrane protein ATPase class I type 8B member 1 signals through protein kinase C zeta to activate the farnesoid X receptor. Hepatology. 2008;48:1896–1905. doi: 10.1002/hep.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen F, Ellis E, Strom SC, Shneider BL. ATPase Class I Type 8B Member 1 and protein kinase C zeta induce the expression of the canalicular bile salt export pump in human hepatocytes. Pediatr Res. 2010;67:183–187. doi: 10.1203/PDR.0b013e3181c2df16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulusma CC, Groen A, Kunne C, Ho-Mok KS, Spijkerboer AL, de Waart R, Hoek FJ, Vreeling H, Hoeben KA, van Marle J, Pawlikowska L, Bull LN, Hofmann AF, Knisely AS, Oude Elferink RP. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195–204. doi: 10.1002/hep.21212. [DOI] [PubMed] [Google Scholar]
- 36.Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, Tanner MS, Kagalwalla AF, Németh A, Pawlowska J, Baker A, Mieli-Vergani G, Freimer NB, Gardiner RM, Thompson RJ. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 37.van Mil SW, van der Woerd WL, van der Brugge G, Sturm E, Jansen PL, Bull LN, van den Berg IE, Berger R, Houwen RH, Klomp LW. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127:379–384. doi: 10.1053/j.gastro.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 38.Pauli-Magnus C, Lang T, Meier Y, Zodan-Marin T, Jung D, Breymann C, Zimmermann R, Kenngott S, Beuers U, Reichel C, Kerb R, Penger A, Meier PJ, Kullak-Ublick GA. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14:91–102. doi: 10.1097/00008571-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Soroka CJ, Boyer JL. The role of bile salt export pump mutations in progressive familial intrahepatic cholestasis type II. J Clin Invest. 2002;110:965–972. doi: 10.1172/JCI15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plass JR, Mol O, Heegsma J, Geuken M, de Bruin J, Elling G, Müller M, Faber KN, Jansen PL. A progressive familial intrahepatic cholestasis type 2 mutation causes an unstable, temperature-sensitive bile salt export pump. J Hepatol. 2004;40:24–30. doi: 10.1016/s0168-8278(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 41.Lam P, Pearson CL, Soroka C, Xu S, Mennone A, Boyer JL. The level of plasma membrane expression in progressive and benign mutations of the bile salt export pump (Bsep/Abcb11) correlate with severity of cholestatic diseases. Am J Physiol Cell Physiol. 2007;293:C1709–C1716. doi: 10.1152/ajpcell.00327.2007. [DOI] [PubMed] [Google Scholar]
- 42.Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci USA. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R, Lam P, Liu L, Forrest D, Yousef IM, Mignault D, Phillips MJ, Ling V. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38:1489–1499. doi: 10.1016/j.hep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Lam P, Wang R, Ling V. Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry. 2005;44:12598–12605. doi: 10.1021/bi050943e. [DOI] [PubMed] [Google Scholar]
- 45.Keitel V, Burdelski M, Warskulat U, Kühlkamp T, Keppler D, Häussinger D, Kubitz R. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41:1160–1172. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- 46.Bouchard G, Nelson HM, Lammert F, Rowe LB, Carey MC, Paigen B. High-resolution maps of the murine Chromosome 2 region containing the cholesterol gallstone locus. Lith Mamm Genome. 1999;10:1070–1074. doi: 10.1007/s003359901163. [DOI] [PubMed] [Google Scholar]
- 47.Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–1411. [PubMed] [Google Scholar]
- 48.Hoda F, Green RM. Hepatic canalicular membrane transport of bile salt in C57L/J and AKR/J mice: implications for cholesterol gallstone formation. J Membr Biol. 2003;196:9–14. doi: 10.1007/s00232-003-0620-4. [DOI] [PubMed] [Google Scholar]
- 49.Henkel A, Wei Z, Cohen DE, Green RM. Mice overexpressing hepatic Abcb11 rapidly develop cholesterol gallstones. Mamm Genome. 2005;16:903–908. doi: 10.1007/s00335-004-2465-2. [DOI] [PubMed] [Google Scholar]
- 50.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 51.Gerloff T, Geier A, Roots I, Meier PJ, Gartung C. Functional analysis of the rat bile salt export pump gene promoter. Eur J Biochem. 2002;269:3495–3503. doi: 10.1046/j.1432-1033.2002.03030.x. [DOI] [PubMed] [Google Scholar]
- 52.Plass JR, Mol O, Heegsma J, Geuken M, Faber KN, Jansen PL, Müller M. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology. 2002;35:589–596. doi: 10.1053/jhep.2002.31724. [DOI] [PubMed] [Google Scholar]
- 53.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Song X, Kaimal R, Yan B, Deng R. Liver receptor homolog 1 transcriptionally regulates human bile salt export pump expression. J Lipid Res. 2008;49:973–984. doi: 10.1194/jlr.M700417-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roninson IB, Chin JE, Choi KG, Gros P, Housman DE, Fojo A, Shen DW, Gottesman MM, Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcino ma cells. Proc Natl Acad Sci USA. 1986;83:4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Bliek AM, Baas F, ten Houte de Lange T, Kooiman PM, van der Velde-Koerts T, Borst P. The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J. 1987;6:3325–3331. doi: 10.1002/j.1460-2075.1987.tb02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, Van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 58.de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Oude Elferink RP, Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AJ, Timmermans-Hereijgers JL, Roelofsen B, Wirtz KW, van Blitterswijk WJ, Smit JJ, Schinkel AH, Borst P. The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett. 1994;354:263–266. doi: 10.1016/0014-5793(94)01135-4. [DOI] [PubMed] [Google Scholar]
- 60.Huang L, Zhao A, Lew JL, Zhang T, Hrywna Y, Thompson JR, de Pedro N, Royo I, Blevins RA, Peláez F, Wright SD, Cui J. Farnesoid X receptor activates transcription of the phospholipid pump MDR3. J Biol Chem. 2003;278:51085–51090. doi: 10.1074/jbc.M308321200. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 63.Båvner A, Sanyal S, Gustafsson JA, Treuter E. Transcriptional corepression by SHP: molecular mechanisms and physiological consequences. Trends Endocrinol Metab. 2005;16:478–488. doi: 10.1016/j.tem.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Hagedorn CH, Wang L. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta. 2011;1812:893–908. doi: 10.1016/j.bbadis.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol Cell Biol. 2000;20:187–195. doi: 10.1128/mcb.20.1.187-195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee YK, Moore DD. Dual mechanisms for repression of the monomeric orphan receptor liver receptor homologous protein-1 by the orphan small heterodimer partner. J Biol Chem. 2002;277:2463–2467. doi: 10.1074/jbc.M105161200. [DOI] [PubMed] [Google Scholar]
- 67.Kemper JK, Kim H, Miao J, Bhalla S, Bae Y. Role of an mSin3A-Swi/Snf chromatin remodeling complex in the feedback repression of bile acid biosynthesis by SHP. Mol Cell Biol. 2004;24:7707–7719. doi: 10.1128/MCB.24.17.7707-7719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang S, Miao J, Xiang L, Ponugoti B, Treuter E, Kemper JK. Coordinated recruitment of histone methyltransferase G9a and other chromatin-modifying enzymes in SHP-mediated regulation of hepatic bile acid metabolism. Mol Cell Biol. 2007;27:1407–1424. doi: 10.1128/MCB.00944-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Handschin C, Meyer UA. Regulatory network of lipid-sensing nuclear receptors: roles for CAR, PXR, LXR, and FXR. Arch Biochem Biophys. 2005;433:387–396. doi: 10.1016/j.abb.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 70.Gupta RK, Kaestner KH. HNF-4alpha: from MODY to late-onset type 2 diabetes. Trends Mol Med. 2004;10:521–524. doi: 10.1016/j.molmed.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115:1006–1015. doi: 10.1172/JCI200522365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miura A, Yamagata K, Kakei M, Hatakeyama H, Takahashi N, Fukui K, Nammo T, Yoneda K, Inoue Y, Sladek FM, Magnuson MA, Kasai H, Miyagawa J, Gonzalez FJ, Shimomura I. Hepatocyte nuclear factor-4alpha is essential for glucose-stimulated insulin secretion by pancreatic beta-cells. J Biol Chem. 2006;281:5246–5257. doi: 10.1074/jbc.M507496200. [DOI] [PubMed] [Google Scholar]
- 73.Nitta M, Ku S, Brown C, Okamoto AY, Shan B. CPF: an orphan nuclear receptor that regulates liver-specific expression of the human cholesterol 7alpha-hydroxylase gene. Proc Natl Acad Sci USA. 1999;96:6660–6665. doi: 10.1073/pnas.96.12.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee YK, Moore DD. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci. 2008;13:5950–5958. doi: 10.2741/3128. [DOI] [PubMed] [Google Scholar]
- 75.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 76.Chiang JY, Kimmel R, Stroup D. Regulation of cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRalpha) Gene. 2001;262:257–265. doi: 10.1016/s0378-1119(00)00518-7. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Choi M, Suino K, Kovach A, Daugherty J, Kliewer SA, Xu HE. Structural and biochemical basis for selective repression of the orphan nuclear receptor liver receptor homolog 1 by small heterodimer partner. Proc Natl Acad Sci USA. 2005;102:9505–9510. doi: 10.1073/pnas.0501204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerr TA, Saeki S, Schneider M, Schaefer K, Berdy S, Redder T, Shan B, Russell DW, Schwarz M. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev Cell. 2002;2:713–720. doi: 10.1016/s1534-5807(02)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M, Moore DD. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721–731. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 80.Boulias K, Katrakili N, Bamberg K, Underhill P, Greenfield A, Talianidis I. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. EMBO J. 2005;24:2624–2633. doi: 10.1038/sj.emboj.7600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28:220–224. doi: 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- 83.Neimark E, Chen F, Li X, Shneider BL. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 2004;40:149–156. doi: 10.1002/hep.20295. [DOI] [PubMed] [Google Scholar]
- 84.Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–G485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- 85.Jung D, Podvinec M, Meyer UA, Mangelsdorf DJ, Fried M, Meier PJ, Kullak-Ublick GA. Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology. 2002;122:1954–1966. doi: 10.1053/gast.2002.33583. [DOI] [PubMed] [Google Scholar]
- 86.Gupta S, Stravitz RT, Dent P, Hylemon PB. Down-regulation of cholesterol 7alpha-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J Biol Chem. 2001;276:15816–15822. doi: 10.1074/jbc.M010878200. [DOI] [PubMed] [Google Scholar]
- 87.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 90.Miao J, Xiao Z, Kanamaluru D, Min G, Yau PM, Veenstra TD, Ellis E, Strom S, Suino-Powell K, Xu HE, Kemper JK. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 2009;23:986–996. doi: 10.1101/gad.1773909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 92.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 93.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 94.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blumberg B, Sabbagh W, Juguilon H, Bolado J, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 97.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 98.Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 99.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 100.LeCluyse EL. Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact. 2001;134:283–289. doi: 10.1016/s0009-2797(01)00163-6. [DOI] [PubMed] [Google Scholar]
- 101.Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, Kliewer SA, Moore JT. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 102.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 103.Xie W, Evans RM. Pharmaceutical use of mouse models humanized for the xenobiotic receptor. Drug Discov Today. 2002;7:509–515. doi: 10.1016/s1359-6446(02)02251-1. [DOI] [PubMed] [Google Scholar]
- 104.Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292:2329–2333. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- 105.Watkins RE, Maglich JM, Moore LB, Wisely GB, Noble SM, Davis-Searles PR, Lambert MH, Kliewer SA, Redinbo MR. A crystal structure of human PXR in complex with the St. John’s wort compound hyperforin. Biochemistry. 2003;42:1430–1438. doi: 10.1021/bi0268753. [DOI] [PubMed] [Google Scholar]
- 106.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281:19081–19091. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- 109.Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2005;288:G74–G84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- 110.Stedman C, Robertson G, Coulter S, Liddle C. Feed-forward regulation of bile acid detoxification by CYP3A4: studies in humanized transgenic mice. J Biol Chem. 2004;19:11336–11343. doi: 10.1074/jbc.M310258200. [DOI] [PubMed] [Google Scholar]
- 111.Jonker JW, Schinkel AH. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 112.Akita H, Suzuki H, Ito K, Kinoshita S, Sato N, Takikawa H, Sugiyama Y. Characterization of bile acid transport mediated by multidrug resistance associated protein 2 and bile salt export pump. Biochim Biophys Acta. 2001;1511:7–16. doi: 10.1016/s0005-2736(00)00355-2. [DOI] [PubMed] [Google Scholar]
- 113.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 114.Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 115.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H, Trauner M. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 116.Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312:841–848. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- 117.Teng S, Piquette-Miller M. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 2007;151:367–376. doi: 10.1038/sj.bjp.0707235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–7511. doi: 10.1038/sj.onc.1206950. [DOI] [PubMed] [Google Scholar]
- 119.Sun J, He ZG, Cheng G, Wang SJ, Hao XH, Zhou MJ. Multidrug resistance P-glycoprotein: crucial significance in drug disposition and interaction. Med Sci Monit. 2004;10:RA5–RA14. [PubMed] [Google Scholar]
- 120.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 121.Stedman C, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci USA. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poupon RE. Ursodeoxycholic acid for primary biliary cirrhosis: lessons from the past—issues for the future. J Hepatol. 2000;32:685–688. doi: 10.1016/s0168-8278(00)80232-9. [DOI] [PubMed] [Google Scholar]
- 123.Corpechot C, Carrat F, Bahr A, Chretien Y, Poupon RE. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297–303. doi: 10.1053/j.gastro.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 124.Mullally BA, Hansen WF. Intrahepatic cholestasis of pregnancy: review of the literature. Obstet Gynecol Surv. 2002;57:47–52. doi: 10.1097/00006254-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 125.Leuschner M, Dietrich CF, You T, Seidl C, Raedle J, Herrmann G, Ackermann H, Leuschner U. Characterisation of patients with primary biliary cirrhosis responding to long term ursodeoxycholic acid treatment. Gut. 2000;46:121–126. doi: 10.1136/gut.46.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology. 2001;34:351–359. doi: 10.1053/jhep.2001.26213. [DOI] [PubMed] [Google Scholar]
- 128.Zollner G, Fickert P, Zenz R, Fuchsbichler A, Stumptner C, Kenner L, Ferenci P, Stauber RE, Krejs GJ, Denk H, Zatloukal K, Trauner M. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33:633–646. doi: 10.1053/jhep.2001.22646. [DOI] [PubMed] [Google Scholar]
- 129.Marschall HU, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, Silbert D, Fuchsbichler A, Sjovall J, Trauner M. Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- 130.Renga B, Migliorati M, Mencarelli A, Cipriani S, D’Amore C, Distrutti E, Fiorucci S. Farnesoid X receptor suppresses constitutive androstane receptor activity at the multidrug resistance protein-4 promoter. Biochim Biophys Acta. 2011;1809:157–165. doi: 10.1016/j.bbagrm.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 131.Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 2005;102:2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.He J, Nishida S, Xu M, Makishima M, Xie W. PXR prevents cholesterol gallstone disease by regulating biosynthesis and transport of bile salts. Gastroenterology. 2011;140:2095–2106. doi: 10.1053/j.gastro.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;15:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 134.Mason AL, Luketic VA, Lindor KD, Hirschfield GM, Gordon SC, Mayo MJ, Kowdley KV, Pares A, Trauner M, Sciacca CI, Beecher Jones T, Pruzanski M, Shapiro DA. Farnesoid-X receptor agonists—a new class of drugs for the treatment of PBC? An international study evaluating the addition of obeticholic acid (INT-747) to Ursodeoxycholic acid. Hepatology. 2010;52(S1):375A. [Google Scholar]
- 135.Sepe V, Bifulco G, Renga B, D’Amore C, Fiorucci S, Zampella A. Discovery of sulfated sterols from marine invertebrates as a new class of marine natural antagonists of farnesoid-x-receptor. J Med Chem. 2011;54:1314–1320. doi: 10.1021/jm101336m. [DOI] [PubMed] [Google Scholar]
- 136.Kainuma M, Makishima M, Hashimoto Y, Miyachi H. Design, synthesis, and evaluation of non-steroidal farnesoid X receptor (FXR) antagonist. Bioorg Med Chem. 2007;15:2587–2600. doi: 10.1016/j.bmc.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 137.Cynamon HA, Andres JM, Iafrate RP. Rifampin relieves pruritus in children with cholestatic liver disease. Gastroenterology. 1990;98:1013–1016. doi: 10.1016/0016-5085(90)90027-x. [DOI] [PubMed] [Google Scholar]
- 138.Prince MI, Burt AD, Jones DE. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut. 2002;50:436–439. doi: 10.1136/gut.50.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]