Abstract
Background
Our study investigated 2 common single-nucleotide polymorphisms (SNPs) of vascular endothelial growth factor (VEGF) for their influences on serum VEGF levels, disease activity, and synovial lesions in rheumatoid arthritis (RA).
Material/Methods
Clinical information and venous blood samples were collected from 98 RA patients and 100 healthy controls. Genotyping on samples from the subjects was performed using matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS). Serum VEGF levels were determined using the enzyme-linked immunosorbent assay (ELISA). The synovial thickness and joint effusion of 28 joints were measured in RA patients, and total sharp score (TSS) and disease activity score (DAS) of 28 joints were recorded.
Results
The genotype and allele frequencies of VEGF rs833070 (G>A) and rs3025030 (G>C) were significantly different between RA group and control group (all P<0.05). VEGF rs833070 and rs3025030 polymorphisms were associated with increasing VEGF serum levels in the RA group (all P<0.01). Statistically significant difference was observed in DAS28 between the different genotypes of VEGF rs833070 in RA patients (P<0.05). Importantly, significant differences in synovial thickening, joint effusion and synovial angiogenesis were observed between the different genotypes of VEGF rs833070 and rs3025030 polymorphisms (all P<0.05).
Conclusions
Our study provides evidence that VEGF polymorphisms might be important indicators of disease activity and synovial lesions, and prognostic factors in evaluating the treatment effectiveness in RA.
MeSH Keywords: Polymorphism, Genetic; Rheumatology; Sarcoma, Synovial
Background
Rheumatoid arthritis (RA) is a chronic, systemic and progressive inflammatory disorder primarily characterized by persistent chronic synovitis, progressive erosions, and cartilage destruction, which may cause deformed and painful joints, even resulting in loss of function [1]. RA affects 0.5–1% adults in the developed world, with 5–50 per 100,000 people being newly diagnosed with the condition, annually. Furthermore, the onset of RA is most frequent in females and the elderly [2]. RA, without intervention, may eventually cause joint damage, disability, reduced quality of life, cardiovascular events and other comorbidities [3,4]. Although a variety of environmental and behavioral factors confer a high risk of developing RA, genetic factors are suspected to account for up to 50% of the risk for developing RA [5].
Vascular endothelial growth factor (VEGF) is an important signaling protein and a secreted ligand released by cells to stimulate vasculogenesis and angiogenesis. VEGF plays an important role in regulating angiogenesis through promoting vascular endothelial cell growth, migration, and lumen formation [6]. In addition, VEGF is also capable of inducing proinflammatory change, seen in chronic inflammation, which involves leukocyte accumulation, collagen deposition, and blood vessel remodeling [7]. VEGF is also released by inflammatory cells and, in turn may represent the inflammatory component in many disease processes [8]. In this context, enhanced serum VEGF levels are associated with the duration and severity in many disorders, such as RA [9]. The human VEGF gene is localized on chromosome 6p12, and contains of 8 exons. The VEGF gene is also an independent risk factor for RA severity, and correlates with multiple disease parameters, such as disease activity, joint damage, and functional disability [10]. Two common single-nucleotide polymorphisms (SNPs), rs833070 (G>A) located in intron 2 and rs3025030 (G>C) located in intron 5, are suspected to result in altered protein expression of VEGF and have strong links to the onset of RA, although some studies dispute such a link [11]. Our present study evaluates the common SNPs in the VEGFA gene [rs833070 (G>A) and rs3025030 (G>C)] for their influences on the circulating levels of VEGF protein and their effects on disease activity and synovial lesions in RA.
Material and Methods
Ethics statement
The study was approved by the Ethics Committee of the Zhujiang Hospital, Southern Medical University. The written informed consent was provided by each eligible patient and the study conformed to the Declaration of Helsinki [12].
Patients
This study was conducted between October 2010 and May 2012 on a population of RA patients (n=98) from Zhujiang Hospital, Southern Medical University. Patients with RA who satisfied all aspects of the 2010 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) classification criteria for RA were recruited to the study [13]. There were 32 male and 66 female RA patients, with an age range of 18–80 years (mean age, 50.82±12.53 years) and mean disease duration of 3.0 years (range, 0.8–8.0 years). A group of 100 healthy volunteers (male, 35; female, 65; age range, 15–85 years; mean age, 48.67±13.41 years) were enrolled as the control group from the Medical Examination Center of the Shengjing Hospital of China Medical University. No statistical difference in age or sex existed between the RA group and the control group. Patients with systemic lupus erythematosus (SLE), Sjögren syndrome (SS), juvenile idiopathic arthritis (JIA), ankylosing spondylitis (AS), polymyositis (PM), dermatomyositis (DM), other autoimmune diseases, hereditary diseases, severe heart, lung, liver, or kidney dysfunction, benign or malignant tumors, or other related diseases were excluded from this study.
Clinical data collection
General clinical data of all the study subjects such as age, sex, disease duration, present and past medical history, and the history of hereditary disease were collected and recorded. Common clinical laboratory parameters for RA including routine blood test, erythrocyte sedimentation rate (ESR), rheumatoid factor (RF) and acute C-reactive protein (CRP) were collected.
Detection of VEGF polymorphisms
Morning fasting venous blood samples (2 ml) were collected from all subjects. EDTA was used as an anticoagulant for blood collection. Genomic DNA was extracted from the white blood cells (WBCs) collected from venous blood by using a DNA Extraction Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocol. The SNP genotyping was conducted using the matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) (Sequenom Inc., San Diego, CA, USA) [14]. The primers (Table 1) were designed with MassARRAY® Assay design 3.1 software, available with the MassARRAY SNP genotyping system (Sequenom, Inc., San Diego, CA, USA). The PCR reaction condition was: 45 cycles of predenaturation at 94ºC for 15 s, denaturation at 94ºC for 20 s, annealing at 56ºC for 30 s, and extension at 72ºC for 1 min, followed by a final extension at 72ºC for 3 min, and stored at 4ºC. SAP reaction solution was prepared and added into a 384-well plate containing PCR-amplified products to degrade dNTP at 37ºC for 20 min, then placed at 85ºC for 5 min to inactivate SAP, and stored at 4ºC. Next, the iPlex reaction system was prepared and added into the 384-well plate, and the single-base extension reaction was conducted under the following conditions: at 94ºC for 30 s, at 94ºC for 5 s, at 52ºC for 5 s, at 80ºC for 5 s, at 72ºC for 3 min, and storing at 4ºC. The purification of PCR products was performed by demineralization with resin. The DNA samples were transferred from the 384-well plate onto the MassARRAY SpectroCHIP covered with matrix, and detected with the mass spectrometer. The genotyping analysis was performed by using the MassARRAY® TYPER software.
Table 1.
Primer sequence and primer length (bp) of rs833070 (G/A) and rs3025030 (G/C) in the vascular endothelial growth factor gene.
| SNP | Primers | Primer length (bp) |
|---|---|---|
| rs833070 (G/A) | 5′-ACGTTGGATGAAGTTCACAGCACCCGAACA-3′ | 86 |
| 5′-ACGTTGGATGCCCTGGTTTGCATTCCTTTG-3′ | ||
| rs3025030 (G/C) | 5′-ACGTTGGATGAAAATGTGTGGGCTGCTTGG-3′ | 111 |
| 5′-ACGTTGGATGACACACTGAAGGAGCTGTAG-3′ |
Enzyme-linked immunosorbent assay (ELISA)
The serum concentrations of VEGF were measured by the ELISA method using an ELISA kit provided by Wuhan Boshide Company (Wuhan, China) in accordance with the manufacturer’s instructions [15].
Disease activity score (DAS)
A total of 28 joints, including shoulders, elbows, wrists, metacarpophalangeal joints, proximal interphalangeal joints of hands, and knee joints, were scored by DAS scoring system [16]. The number of swollen and tender joints in each RA patient was examined and recorded, and the DAS28 score was calculated by combining the ESR and self-evaluation in patients with RA. [n1, the number of tender joints; n2, the number of swollen joints; n3, ESR (mm/h); n4, health evaluation in RA patients]. The DAS28 score of <2.6 is considered as remission, 2.6–3.2 as low mobility, 3.3–5.1 as moderate mobility, and >5.1 as frequent mobility.
Ultrasonography
The ultrasonic examination was performed using a Siemens ACUSON S2000™ color Doppler (Siemens, Erlangen, Germany), with the probe frequency of 8–13 MHZ. The 28 joints of each RA patient were examined using direct contact method. All patients adopted the supine or sitting position, adjusted based on the examined joints, with the examined joint fully exposed. An experienced physician with ultrasound expertise, who did not know the disease status and treatment status of the patients, monitored the ultrasound examinations.
Ultrasonic examination
Based on the classification standard of Walther, the synovial thickness was classified into 4 grades: grade I, no synovial hyperplasia and thickness <2 mm; grade II, mild synovial hyperplasia and thickness=2–4 mm; grade III, moderate synovial hyperplasia and thickness=5–9 mm; grade IV, severe synovial hyperplasia and thickness >9 mm [17]. Joint effusion in joint scotoma (anteroposterior diameter) was determined based on different joint size and position: knee suprapatellar bursa scotoma >4 cm is considered as joint effusion; medial and lateral knee scotoma >2 cm as joint effusion; scotoma of shoulder joins, elbow joints, and wrist joints, metacarpophalangeal and proximal interphalangeal joints >2 cm as joint effusion.
Power Doppler evaluation
The semi-quantitative signal of each point of synovium in the 28 joints was observed and classified: grade 0, no signal or blood flow; grade 1, slight signal, and single or independent vascular signals less than 3; grade 2, moderate fusion vessel, and more than 3 independent signals, or fusion signals less than half the synovial region; grade 3, significant signal, and visible vascular signals in more than half the medial region of the joints.
Recording items
Recording items consisted of the followings: (1) US joint count SH: the number of joints with thickened synovium; (2) US joint count SF: the number of joints with effusion; (3) US joint count PD: the number of joints with energy signals; (4) US index SH: total synovial thickening score, which was the sum of the synovial thickening score of the 28 joints; (5) US index SF: total joint effusion score, which was the sum of joint effusion score of the 28 joints; (6) total sharp scores (TSS): the sum of the highest power Doppler score of each joint.
Statistical analysis
Statistical analysis was conducted using SPSS 18 software (SPSS Inc., Chicago, IL, USA), and the data are represented as means ± standard deviation (SD), median, or percentage. The statistical comparison between 2 groups was conducted using the t-test or the analysis of variance (ANOVA). Genotype distribution in the control group was tested by Hardy-Weinberg equilibrium (HWE). The differences in genotype and allele distribution between the RA group and the control group are represented as odds ratio (OR) and 95% confidence interval (CI). P values for all tests are 2-tailed, and <0.05 was considered as statistically significant.
Results
Characteristics of RA patients
Table 2 shows the demographic and clinical characteristics of 198 subjects, consisting of 98 RA patients and 100 controls, from a hospital based population at the time of recruitment. Comparisons between the RA group and the control group demonstrated that ESR, RF, acute CRP, and serum VEGF levels were significantly higher in patients with RA than in controls (all P<0.05). No statistical differences were seen in age, gender, WBC, red blood cell (RBC), hemoglobin (HGB) and platelet (PLT) count (all P>0.05).
Table 2.
Comparison between demographic and clinical characteristics of patients with rheumatoid arthritis and demographic features of healthy controls.
| Variable | Control group (n=100) | RA group (n=98) | P |
|---|---|---|---|
| Age (years) | 48.67±13.41 | 50.82±12.53 | 0.245 |
| Gender (male/female) | 35/65 | 32/66 | 0.727 |
| WBC (×109/L) | 6.31±1.88 | 6.81±2.88 | 0.114 |
| RBC (×10/L) | 4.69±0.31 | 4.64±0.66 | 0.498 |
| PLT (×10/L) | 276.6±64.5 | 282.55±65.9 | 0.521 |
| HGB (g/L) | 129.6±12.0 | 127.0±11.9 | 0.125 |
| ESR (mm/h) | 16.31±2.75 | 36.39±3.44 | <0.001 |
| CRP (mg/L) | 2.01±0.37 | 13.36±4.72 | <0.001 |
| RF (IU/mL) | 12.18±1.72 | 43.58±3.53 | <0.001 |
| VEGF (pg/mL) | 307.30±119.52 | 1436.44±423.41 | <0.001 |
Values are means ± standard deviation or number; RA – rheumatoid arthritis; WBC – white blood cell; RBC – red blood cell; PLT – platelet; HGB – hemoglobin; ESR – erythrocyte sedimentation rate; CRP – C-reactive protein; RF – rheumatoid factor; VEGF – vascular endothelial growth factor.
Distributions of VEGF SNPs
The genotype and allele frequencies of the VEGF genetic polymorphisms, rs833070 (G>A) and rs3025030 (G>C), in RA patients and controls are displayed in Table 3. Genotypes of VEGF rs833070 (G>A) and rs3025030 (G>C) in controls were distributed in accordance with HWE (all P>0.05). The distributions of allele and genotype frequencies of the VEGF rs833070 (G>A) in the RA group were significantly different from the control group (all P<0.05). The VEGF rs833070 AA genotype and A allele frequency were significant higher in the RA group than in the control group (AA genotype frequency: 59.2% vs. 43.0%, P<0.05; A allele frequency: 78.1% vs. 67.0%, P<0.05). We detected that the polymorphisms located in rs833070 of the VEGF gene were strongly linked with an increased risk of RA (AA vs. GG + AG, OR=1.922, 95%CI=1.093–3.382, P=0.023; A vs. G, OR=1.752, 95%CI=1.119–2.745, P=0.014). Similarly, statistical differences in the distribution of genotypic and allele frequencies of VEGF rs3025030 (G>C) were observed between the RA group and the control group (all P<0.05). VEGF rs3025030 CC genotype frequency was 50.0% in RA patients vs. 67.0% in controls (P<0.05). In addition, VEGF rs3025030 C allele frequency was 69.4% in RA patients and 81.0% in controls (P<0.01). VEGF rs3025030 CC genotype might be related to a reduced risk of RA (CC vs. GC + GG, OR=0.473, 95%CI=0.266–0.840, P=0.010).
Table 3.
Comparison of distribution of genotype and allele frequencies of vascular endothelial growth factor rs833070 (G>A) and rs3025030 (G>C) polymorphisms between rheumatoid arthritis patients (n=98) and controls (n=100).
| SNP | RA group (n=98) | Control group (n=100) | P | OR | 95%CI |
|---|---|---|---|---|---|
| rs833070 | |||||
| GG | 3 (3.1%) | 9 (9.0%) | |||
| AG | 37 (37.8%) | 48 (48.0%) | 0.036 | ||
| AA | 58 (59.2%) | 43 (43.0%) | |||
| GG + AG | 40 (40.8%) | 57 (57.0%) | Ref | ||
| AA | 58 (59.2%) | 43 (3.0%) | 0.023 | 1.922 | 1.093–3.382 |
| AG + AA | 95 (96.9%) | 91 (91.0%) | Ref | ||
| GG | 3 (3.1%) | 9 (9.0%) | 0.080 | 0.319 | 0.084–1.217 |
| G allele | 43 (21.9%) | 66 (33.0%) | 1.000 | ||
| A allele | 153 (78.1%) | 134 (67.0%) | 0.014 | 1.753 | 1.119–2.745 |
| rs3025030 | |||||
| CC | 48 (50.0%) | 67 (67.0%) | |||
| GC | 40 (40.8%) | 28 (28.0%) | 0.032 | ||
| GG | 10 (10.2%) | 5 (50.0%) | |||
| GC + CC | 88 (89.8%) | 95 (95.0%) | Ref | ||
| GG | 40 (40.8%) | 28 (28.0%) | 0.167 | 2.159 | 0.710–6.566 |
| GC + GG | 50 (51.0%) | 33 (33.0%) | Ref | ||
| CC | 40 (40.8%) | 28 (28.0%) | 0.010 | 0.473 | 0.266–0.840 |
| C | 136 (69.4%) | 162 (81.0%) | 1.00 | ||
| G | 60 (30.6%) | 38 (19.0%) | 0.007 | 1.881 | 1.180–2.997 |
SNP – single-nucleotide polymorphism; RA – rheumatoid arthritis; CI – confidence interval; Ref – reference.
Comparison of serum VEGF levels
Figures 1 and 2 show the comparison of VEGF serum levels between different genotypes of rs833070 and rs3025030 in RA patients. The comparisons of serum levels of VEGF based on genotypes of rs833070 showed that the serum level of VEGF was significantly higher in RA patients with AA genotype (1737.5±250.3 pg/mL) compared to RA patients with GG genotype (623.7±183.6 pg/mL) and AG genotype (1030.2±107.9 pg/mL) (P<0.01) (Figure 1). Additionally, serum VEGF levels in RA patients with rs3025030 CC genotype were lower than in patients carrying the rs3025030 GC genotype and GG genotype (1232.94±358.39 vs. 1586.67±398.39 vs. 1812.37±309.07, P<0.01) (Figure 2).
Figure 1.
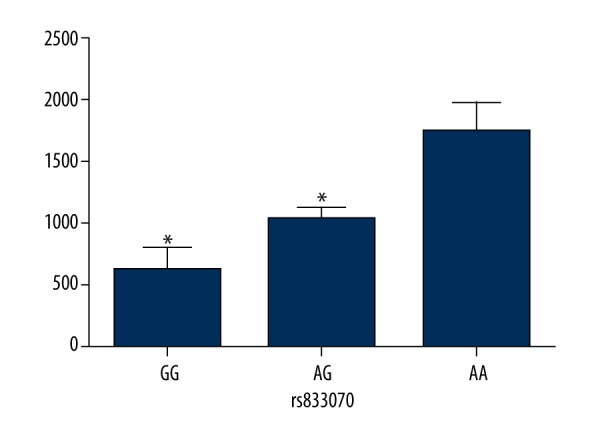
Comparison of serum vascular endothelial growth factor (VEGF) levels between GG, AG, and AA genotype of VEGF rs833070 polymorphisms in rheumatoid arthritis patients. The serum levels of VEGF were determined in 98 rheumatoid arthritis patients. * Compared with AA genotype, P<0.01.
Figure 2.
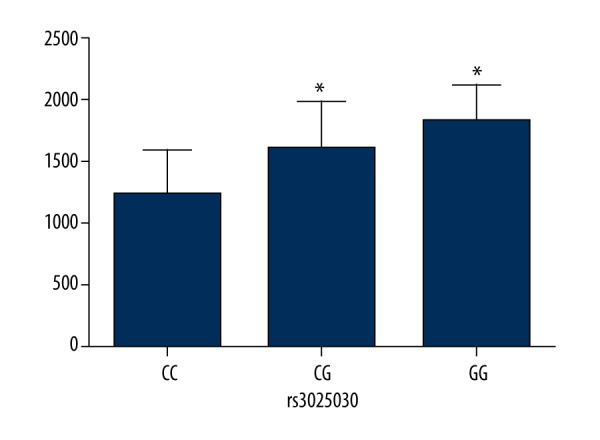
Comparison of serum vascular endothelial growth factor (VEGF) levels between CC, GC, and GG genotype of VEGF rs3025030 polymorphisms in rheumatoid arthritis patients. The serum levels of VEGF were determined in 98 rheumatoid arthritis patients. * Compared with CC genotype, P<0.01.
Association of VEGF SNPs with disease severity in RA
Table 4 shows DAS28 stratified by the genotypes of the VEGF rs833070 and rs3025030 polymorphisms in all patients with RA. The mean DAS28 of all RA patients was 4.68±0.76, and RA patients were classified into low-activity RA patients (DAS28 ≤5.1) and high-activity RA patients (DAS28 >5.1) according to DAS28 score system. Statistical difference was observed in DAS28 between different genotypes of VEGF rs833070 in RA patients (P<0.05). RA patients carrying rs833070 AA genotype had a higher proportion of DAS28 >5.1 compared with patients carrying rs833070 GG and AG genotype (100% vs. 33.3% vs. 56.8%). No statistical difference was detected in DAS28 between different genotypes of VEGF rs3025030 in RA patients (P>0.05).
Table 4.
Comparison of disease activity in rheumatoid arthritis between GG, AG, and AA genotype of rs833070, as well as between CC, GC, and GG genotype of rs3025030 in the vascular endothelial growth factor gene showing the relationship of vascular endothelial growth factor polymorphisms with disease activity in patients with rheumatoid arthritis.
| DAS28 (n,%) | P | ||
|---|---|---|---|
| ≤5.1 | >5.1 | ||
| rs833070 | <0.0001 | ||
| GG | 2 (66.7%) | 1 (33.3%) | |
| AG | 16 (43.2%) | 21 (56.8%) | |
| AA | 0 (0.0%) | 58 (100%) | |
| rs3025030 | 0.773 | ||
| CC | 10 (100%) | 38 (0.0%) | |
| GC | 6 (72.5%) | 34 (27.5%) | |
| GG | 2 (10.0%) | 8 (90.0%) | |
DAS28 – 28-joint disease activity score.
Synovial lesions in RA
As seen in Table 5, among the 98 RA patients, synovial thickening was detected in 82 patients and in a total of 1,065 joints, with a positive rate of 38.8% (1065/2744). Joint effusion was observed in 90 patients and 747 joints, with a positive rate of 27.2% (747/2744). Doppler signal in 63 patients and 661 joints (excessive blood vessel formation), showed a positive rate of 24.1% (661/2744).
Table 5.
Positive rate of synovial thickening, joint effusion and synovial angiogenesis [number of joints (%)] detected by high-resolution ultrasound and Doppler ultrasound.
| Joint | No. of examined joints | Joints with synovial thickening | Joints with joint effusion | Joints with excessive synovial angiogenesis |
|---|---|---|---|---|
| Shoulders | 196 | 82 (41.8) | 65 (33.2) | 73 (37.2) |
| Elbows | 196 | 102 (52.0) | 58 (29.6) | 79 (40.3) |
| Wrists | 196 | 108 (55.1) | 69 (35.2) | 67 (34.2) |
| Metacarpophalangeal joints | 980 | 274 (30.0) | 209 (21.3) | 154 (15.7) |
| Proximal interphalangeal joints of the hands | 980 | 351 (35.8) | 225 (33.0) | 175 (17.9) |
| Knees | 196 | 148 (75.5) | 121 (61.7) | 113 (57.7) |
| Total | 2744 | 1065 (38.8) | 747 (27.2) | 661 (24.1) |
No. – number.
Association of VEGF SNPs with synovial lesions in RA
As illustrated in Table 6, there was statistical significance in the positive rate of synovial thickening, joint effusion and synovial angiogenesis between different genotypes of VEGF rs833070 and rs3025030 (P<0.05). The positive rate of synovial thickening, joint effusion and synovial angiogenesis in RA patients carrying rs833070 AG and AA genotype was evidently higher than patients carrying rs833070 GG genotype (P<0.05). Similarly, the positive rate of synovial thickening, joint effusion and synovial angiogenesis in RA patients with rs3025030 GC and GG genotype was higher compared to RA patients with rs3025030 CC genotype (P<0.05).
Table 6.
Comparison of synovial lesions in rheumatoid arthritis between GG, AG, and AA genotype of rs833070, as well as between CC, GC, and GG genotype of rs3025030 in the vascular endothelial growth factor gene showing the relationship of vascular endothelial growth factor polymorphisms with synovial lesions in patients with rheumatoid arthritis.
| Joints with synovial thickening | Joints with joint effusion | Joints with excessive synovial angiogenesis | |
|---|---|---|---|
| rs833070 | |||
| GG | 20 (23.8) | 12 (14.3) | 5 (6.0) |
| AG | 385 (37.2)* | 250 (24.1)* | 214 (20.7)* |
| AA | 653 (40.2)* | 489 (30.1)* | 442 (27.2)* |
| rs3025030 | |||
| CC | 479 (35.6) | 253 (18.6) | 114 (6.7) |
| GC | 460 (41.1)# | 310 (30.7)# | 413 (42.2)# |
| GG | 126 (45.0)# | 184 (72.5)# | 134 (57.5)# |
Compared with GG genotype, P<0.05;
compared with CC genotype, P<0.05.
Discussion
In the current study, we investigated the significance of 2 SNPs in VEGF gene, rs833070 and rs3025030, which alter VEGF protein levels in the serum. The association of these VEGF gene variations with circulating serum levels of VEGF, disease activity, and synovial lesions was systematically measured in RA patients. Additionally, we carried out genotyping in a hospital-based case-control study in a cohort of 98 RA patients and 100 controls to obtain a comprehensive view of the disease parameters in relation to the genotypes under study.
The results of the SNP genotyping and ELISA based measurement of serum VEGF revealed that VEGF rs833070 AA genotype is related to higher serum VEGF level, and the same genotype and haplotype also occurred more frequently in RA patients. The GG genotype of VEGF rs3025030 was associated with a lower VEGF serum level, and the same genotype and haplotype showed a lower frequency in patients with RA, suggesting that VEGF rs833070 genetic polymorphisms is a significant risk factor in RA, while rs3025030 mutation in the VEGF gene might play a protective role in RA through lowering VEGF levels in serum. Previous studies have also reported enhanced serum VEGF levels in RA patients; however, the links to VEGF genetic variations as a mechanism to regulate serum VEGF levels, and leading to an increased or decreased risk of RA, are still unclear [10,18]. It is hypothesized that VEGF polymorphisms influence VEGF gene transcription, resulting in altered serum levels of VEGF [5]. Although only a small change is observed in the absolute values of serum VEGF levels due to VEGF genetic polymorphisms, the disease outcomes show large differences and probably reflect a sustained activation of mechanisms promoting RA [19]. RA mainly results from pannus formation caused by synovial angiogenesis [20]. Furthermore, VEGF protein, one of the most potent inducers of angiogenesis, plays a vital role in activating angiogenesis in RA through up-regulation of the VEGF pathway [6]. Therefore, it is plausible to conclude that VEGF rs833070 polymorphism increases serum VEGF levels, and the higher VEGF serum levels confer increased risk of RA. On the other hand, VEGF rs3025030 polymorphism reduces VEGF protein levels in serum and decreases the onset of RA. Consistent with our observation, Ozgonenel et al. also demonstrated that higher VEGF levels are correlated to late phase and high risk of RA, independent of age and sex [10]. Moreover, Chen et al., who studied a sample of 413 hospital-based RA patients of Caucasian origin, reported that the T allele at VEGF rs3025039 could influence the onset of RA [21]. Additionally, a previous meta-analysis, which studied a total of 24 independent studies associated with autoimmune disease, revealed that VEGF polymorphisms were related to the susceptibility to RA [22].
In addition, the findings from our study show that rs833070 AA genotype in the VEGF gene was closely associated with high disease activity in RA, and both rs833070 and rs3025030 variants in the VEGF gene were positively associated with synovial thickening, joint effusion, and synovial angiogenesis, indicating that VEGF genetic polymorphisms might play roles in promoting disease activity, synovial thickening, joint effusion, and synovial angiogenesis in RA. Serum VEGF levels were positively correlated to ESR, RF, and CRP, which are independent variables for disease activity [23]. In addition, higher VEGF in serum might increase vascular permeability, which in turn contributes to joint effusion and joint swelling, which is related to the synovial thickening seen in RA [24]. As serum VEGF level rises, the number of swollen and sensitive joints, which is one of the DAS28 calculation parameters, rises as expected, contributing to high DASs [25]. The synovial angiogenesis in RA might be explained by the role of VEGF in angiogenesis [26]. A previous study showed that VEGF plays a central role in RA-related joint destruction, as evidenced by the observed radiologic changes and increased DAS in patients with high serum VEGF levels [27]. As well, Ozgonenel et al. reported that higher VEGF levels are associated with late phase and high disease activity in RA, independent of age and sex [9]. Chen et al. also have documented that genetic variation in the VEGF gene is associated with serum VEGF levels in RA, and shows an association with disease activity in RA patients who have never smoked, independent of serum VEGF levels [10]. One limitations of our study is that this was a hospital-based case-control study, hence the subjects were not representative of general population; nevertheless, this case-control study still had the ability to contribute to relatively reliable results. Another limitation is that the polymorphisms were evaluated based on their function, and may not supply a comprehensive view of the genetic variability of VEGF; therefore, further fine-mapping studies are needed. In addition, the sample size of this study was too small to investigate the low penetrance effect of the SNPs.
Conclusions
Our study shows that variants in the VEGF gene might serve as biomarkers for disease activity and synovial lesions in RA, and are a useful biochemical parameter to assess the treatment effectiveness in RA.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Footnotes
Competing interests
The authors have declared that they have no competing interests.
Source of support: This study was funded by National Key Basic Research Program (No. 2010CB529102)
References
- 1.Wang CH, Yao H, Chen LN, et al. CD147 induces angiogenesis through a vascular endothelial growth factor and hypoxia-inducible transcription factor 1alpha-mediated pathway in rheumatoid arthritis. Arthritis Rheum. 2012;64:1818–27. doi: 10.1002/art.34341. [DOI] [PubMed] [Google Scholar]
- 2.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 3.Kingsley G, Scott IC, Scott DL. Quality of life and the outcome of established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25:585–606. doi: 10.1016/j.berh.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Kerola AM, Kauppi MJ, Nieminen T, et al. Psychiatric and cardiovascular comorbidities as causes of long-term work disability among individuals with recent-onset rheumatoid arthritis. Scand J Rheumatol. 2015;44:87–92. doi: 10.3109/03009742.2014.929174. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Qiu H, Zhang H, et al. Vascular endothelial growth factor A (VEGFA) polymorphisms in Chinese patients with rheumatoid arthritis. Scand J Rheumatol. 2013;42:344–48. doi: 10.3109/03009742.2013.787454. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34:1785–88. doi: 10.1248/bpb.34.1785. [DOI] [PubMed] [Google Scholar]
- 7.Kamoun M, Houman MH, Hamzaoui A, Hamzaoui K. Vascular endothelial growth factor gene polymorphisms and serum levels in Behcet’s disease. Tissue Antigens. 2008;72:581–87. doi: 10.1111/j.1399-0039.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozgonenel L, Cetin E, Tutun S, et al. The relation of serum vascular endothelial growth factor level with disease duration and activity in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29:473–77. doi: 10.1007/s10067-009-1343-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Dawes PT, Mattey DL. Polymorphism in the vascular endothelial growth factor A (VEGFA) gene is associated with serum VEGF-A level and disease activity in rheumatoid arthritis: differential effect of cigarette smoking. Cytokine. 2012;58:390–97. doi: 10.1016/j.cyto.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Lv HZ, Lin T, Xia LP, et al. Vascular endothelial growth factor gene polymorphisms and rheumatoid arthritis. J Investig Med. 2011;59:593–98. doi: 10.2310/JIM.0b013e31820c9e21. [DOI] [PubMed] [Google Scholar]
- 12.M PN. World Medical Association publishes the Revised Declaration of Helsinki. Natl Med J India. 2014;27:56. [PubMed] [Google Scholar]
- 13.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 14.Pieles U, Zurcher W, Schar M, Moser HE. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: a powerful tool for the mass and sequence analysis of natural and modified oligonucleotides. Nucleic Acids Res. 1993;21:3191–96. doi: 10.1093/nar/21.14.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972;109:129–35. [PubMed] [Google Scholar]
- 16.van der Heijde DM, van ‘t Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579–81. [PubMed] [Google Scholar]
- 17.Walther M, Harms H, Krenn V, et al. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–38. doi: 10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Fan J, Lin H, et al. The anti-malaria agent artesunate inhibits expression of vascular endothelial growth factor and hypoxia-inducible factor-1alpha in human rheumatoid arthritis fibroblast-like synoviocyte. Rheumatol Int. 2011;31:53–60. doi: 10.1007/s00296-009-1218-7. [DOI] [PubMed] [Google Scholar]
- 19.Wu FT, Stefanini MO, Mac Gabhann F, et al. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14:528–52. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semerano L, Clavel G, Assier E, Denys A, Boissier MC. Blood vessels, a potential therapeutic target in rheumatoid arthritis? Joint Bone Spine. 2011;78:118–23. doi: 10.1016/j.jbspin.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Mattey DL. Age at onset of rheumatoid arthritis: association with polymorphisms in the vascular endothelial growth factor A(VEGFA) gene and an intergenic locus between matrix metalloproteinase (MMP) 1 and 3 genes. Clin Exp Rheumatol. 2012;30:894–98. [PubMed] [Google Scholar]
- 22.Chen H, Zhang T, Gong B, et al. Association between VEGF -634G/C polymorphism and susceptibility to autoimmune diseases: a meta-analysis. Gene. 2015;558:181–86. doi: 10.1016/j.gene.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Kurosaka D, Hirai K, Nishioka M, et al. Clinical significance of serum levels of vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in patients with rheumatoid arthritis. J Rheumatol. 2010;37:1121–28. doi: 10.3899/jrheum.090941. [DOI] [PubMed] [Google Scholar]
- 24.Hoeppner LH, Phoenix KN, Clark KJ, et al. Revealing the role of phospholipase Cbeta3 in the regulation of VEGF-induced vascular permeability. Blood. 2012;120:2167–73. doi: 10.1182/blood-2012-03-417824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 2012;64:1794–803. doi: 10.1002/acr.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188–93. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Q, Guo R, Wood R, et al. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis Rheum. 2011;63:2318–28. doi: 10.1002/art.30421. [DOI] [PMC free article] [PubMed] [Google Scholar]


