Abstract
The α-hemolysin, encoded by the hla gene, is a major virulence factor in S. aureus infections. Changes in key amino acid residues of α-hemolysin can result in reduction, or even loss, of toxicity. The aim of this study was to investigate the diversity of the hla gene sequence and the relationship of hla variants to the clonal background of S. aureus isolates. A total of 47 clinical isolates from China were used in this study, supplemented with in silico analysis of 318 well-characterized whole genome sequences from globally distributed isolates. A total of 28 hla genotypes were found, including three unique to isolates from China, 20 found only in the global genomes and five found in both. The hla genotype generally correlated with the clonal background, particularly the multilocus sequence type, but was not related to geographic origin, host source or methicillin-resistance phenotype. In addition, the hla gene showed greater diversity than the seven loci utilized in the MLST scheme for S. aureus. Our investigation has provided genetic data which may be useful for future studies of toxicity, immunogenicity and vaccine development.
Introduction
Staphylococcus aureus is one of the leading human pathogens worldwide. It causes a broad range of diseases from superficial infections to life-threatening invasive diseases. Antimicrobial therapy is sometimes ineffective, owing to the development of antimicrobial-resistant strains, such as methicillin-resistant S. aureus (MRSA) [1]. S. aureus expresses various virulence factors, including a broad range of exotoxins. Some of these toxins, such as most of the phenol-soluble modulins and α-hemolysin (also known as α-toxin), are encoded in the core-genome; while others, such as Panton-Valentine leucocidin, are encoded by acquired mobile genetic elements [2, 3].
The α-hemolysin, which belongs to a class of small β-barrel pore-forming cytotoxins, is a major virulence factor in S. aureus infections [2, 4]. It is encoded by the 960-bp hla gene, which is initially produced as a 319-residue precursor, then processed to a 293-residue (approximately 33 kDa) mature toxin [4, 5]. Previous studies have proven that changes in key amino acid residues of α-hemolysin, such as a histidine substitution at amino acid 35, can result in reduction or even loss of virulence [6, 7].
In this study, we determined the genotypes of hla in 47 S. aureus isolates collected in China, and compared this with the clonal background of the isolates. For comparison, hla genotype and clonal background were also determined for well-characterized and published whole genome sequences of 318 global strains by in silico analysis.
Methods
Ethics
The study was approved by the Human Research Ethics Committee of Peking Union Medical College Hospital (No. S-263). Written consent was obtained from patients, and the study was carried out in accordance with approved guidelines.
Staphylococcus aureus isolates
Isolates were collected from patients with S. aureus infections in Beijing Electric Power Hospital during January 2011 to June 2011. A total of 47 S. aureus isolates were collected consecutively, each from different individuals. The most common type of infection was pneumonia (n = 31, 66.0%), followed by soft tissue infection (n = 6, 12.8%), urinary tract infection (n = 6, 12.8%), bloodstream infection (n = 2, 4.3%), joint infection (n = 1, 2.1%), and gallbladder infection (n = 1, 2.1%).
Molecular identification of isolates and mecA gene detection
DNA extraction of S. aureus isolates was performed as previously described [8]. A multiplex PCR was used for simultaneous amplification of 16S rRNA, femA and mecA genes for identification and differentiation of methicillin-susceptible S. aureus (MSSA) and MRSA isolates [9].
Genotyping of the 960-bp hla gene
Primer pair of hlaF1 (5’- TTAGCCGAAAAACATCATTTC-3’) and hlaR2 (5’- TTATTCCCGACGAAATTCCAA-3’) was designed for amplification of the complete 960-bp hla gene encoding the α-hemolysin precursor, which was made up of a 78-bp nucleotide sequence encoding the 26 aa signal peptide, followed by the 882-bp nucleotide sequence encoding the 293 aa mature α-hemolysin.
Each PCR mix contained 12.5 μL of 2× EasyTaq PCR SuperMix (TransGen Biotech, Beijing, China), 2 μL of DNA template, 0.5 μM of each forward and reverse primer, and molecular biology grade water (TransGen Biotech) added to make a total volume of 25 μL. PCR was performed as follows: initial denaturation at 95°C for 15 min, followed by 30 cycles of 94°C for 2 min, 55°C for 2 min, 72°C for 2 min, with a final extension at 72°C for 10 min. The products were sequenced in both directions using the inner primer pair hlaF2 (5’- GAAGTTATCGGCTAAAGTTATAA-3’) and hlaR1 (5’- CATAATTAATACCCTTTTTCTC-3’) on the DNA analyzer ABI 3730XL system (Applied Biosystems, Foster City, CA).
The obtained 960-bp hla gene sequences were compared to a wild-type reference sequence from S. aureus strain WOOD 46 (GenBank accession no. X01645) [10], aligned using CLC sequence viewer (version 7, QIAGEN Aarhus, Denmark) to detect single nucleotide polymerases (SNPs), and designate genotypes (the hla DNA sequence was identical for all isolates belonging to a given genotype). Further, the α-hemolysin peptide sequences of isolates were deduced and aligned to determine the presence of amino acid substitutions.
Assignment of clonal background
All isolates were analyzed by multilocus sequence typing (MLST) and spa typing using previously established methods [11, 12]. Assignment of related sequence types (STs) into clonal complexes (CCs) was conducted using eBURST [13]. In addition, all MRSA isolates were characterized by staphylococcal cassette chromosome mec (SCCmec) typing as described by Chen et al. [14]. S. aureus clones were named in the format of ST-spa type or ST-SCCmec type-spa type (e.g. ST5-t002 for a MSSA clone, or ST239-III-t030 for a MRSA clone).
In silico analysis of published whole genome sequences
Because only limited number of S. aureus isolates was involved in the present study, to better define the hla gene diversity and association with clonal background amongst S. aureus with more different genetic background, 318 selected well-characterized published genomes derived from other geographic regions were further studied. The 318 selected published genomes included i) genetic information of all S. aureus isolates with complete assembled whole genome sequences as at July 8 2015 were obtained from the NCBI Genome database (70 isolates, S1 Table), and ii) S. aureus genome sequences from four previous publications (248 isolates, S2 Table) [15–18]. These genomes comprised examples from the major worldwide lineages of MRSA, e.g. CC8 (including USA-300), CC1 (including USA 400), CC22 (including EMRSA15), CC93 (including Queensland CA-MRSA), CC30 (including EMRSA16) and CC121 isolates.
The MLST STs and hla genotypes of the above isolates were determined by in silico mapping the paired-end reads of 318 isolates to seven gene loci sequences of S. aureus ST1 and wild-type hla reference sequence (GenBank accession no. X01645), respectively, using Burrows-Wheeler Alignment [19]. Base coverage of each position of genes was assessed using SAMtools mpileup packages (http://samtools.sourceforge.net/mpileup.shtml). The support number of reference base (ref) and alternative base (alt) are examined at each position in each strain. High quality SNPs were defined when SNPs satisfied the criteria of alt/(alt+ref)>0.8. According to these SNPs, the ST and hla genotype for each isolate was identified.
MLST STs and hla genotypes were entered into BioNumerics software v7.5 (Applied Maths, Austin, TX) for minimum-spanning-tree analysis. The diversity of the sequences of the hla gene and seven genes utilized in the MLST scheme for S. aureus was analyzed by DnaSP (version 5.1, University of Barcelona, Spain).
Results
Molecular identification and detection of the mecA gene
All 47 isolates were confirmed as S. aureus by molecular methods. Thirty-three isolates (70.2%) were determined to be MRSA by detection of the mecA gene.
Genotypes of the 960-bp hla gene
Amongst the 47 isolates from China, a total of eight hla genotypes (genotype 1 to 8) and six peptide sequence types were identified (Tables 1 and 2). Of the eight hla genotypes, genotype 1 was predominant (n = 27, 57% of 47 isolates), followed by genotype 3 (n = 7, 15%) and genotype 5 (n = 3, 6%). The remaining four genotypes were rare, with one or two isolates belonging to each (Table 1).
Table 1. Nucleotide mutations and corresponding amino acid substitutions of the eight S. aureus hla genotypes identified from 47 isolates from China.
| Nucleotide mutation position/corresponding amino acid substitutiona | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | -68 | -22 | -6&-5 | 144 | 177 | 399 | 438 | 453 | 479 | 606 | 624 | 669 | 708 | 765 | 777 | 824 | 864 | GenBank accession no. |
| Wild typea | G | G | G G | C | T | C | G | T | C | T | T | G | A | T | T | C | A | X01645.1 |
| Genotype 1 | A/ | A/ | AA/ | T/ | — | — | — | — | — | C/ | — | — | — | C/ | C/ | T/ | — | KT279554.1 |
| R(-23)H | Syn | G(-2)N | Syn | Syn | Syn | Syn | T275I | |||||||||||
| Genotype 2 | A/ | A/ | AA/ | T/ | — | — | — | — | — | C/ | — | — | G/ | C/ | C/ | T/ | — | KT279555.1 |
| R(-23)H | Syn | G(-2)N | Syn | Syn | Syn | Syn | Syn | T275I | ||||||||||
| Genotype 3 | — | A/ | AA/ | — | — | — | — | — | — | — | G/ | — | — | C/ | — | — | — | KT279558.1 |
| Syn | G(-2)N | D208E | Syn | |||||||||||||||
| Genotype 4 | — | A/ | AA/ | — | A/ | T/ | C/ | G/ | — | C/ | — | T/ | — | C/ | — | — | — | KT279556.1 |
| Syn | G(-2)N | Syn | Syn | Syn | Syn | Syn | Syn | Syn | ||||||||||
| Genotype 5 | — | A/ | AA/ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | KT279559.1 |
| Syn | G(-2)N | |||||||||||||||||
| Genotype 6 | — | A/ | AA/ | — | — | — | — | — | — | — | G/ | — | — | C/ | — | — | T/ | KT279557.1 |
| Syn | G(-2)N | D208E | Syn | Syn | ||||||||||||||
| Genotype 7 | — | A/ | AA/ | T/ | — | — | — | — | — | C/ | — | — | — | C/ | C/ | T/ | — | KT279561.1 |
| Syn | G(-2)N | Syn | Syn | Syn | Syn | T275I | ||||||||||||
| Genotype 8 | — | A/ | AA/ | T/ | — | — | — | — | Delb | C/ | —b | —b | —b | C/ | C/ | T/ | —b | KT279560.1 |
| Syn | G(-2)N | Syn | Synb | Synb | Synb | T275Ib | ||||||||||||
Abbreviations: Syn, synonymous mutation; Del, deletion mutation; N/A, not applicable; “—”, no mutation.
aNucleotide/peptide positions were designated relative to the first nucleotide/amino acid of the mature α-hemolysin (S. aureus strain WOOD 46, nucleotide sequence GenBank accession no. X01645).
bThe deletion at nucleotide position 479 of genotype 8 resulted in peptide termination at residue position 164.
Table 2. Relationship between hla genotypes and molecular clone backgrounds of 47 S. aureus isolates from China.
| hla genotype | Molecular clone | Clonal Complex (CC) | No. of isolates |
|---|---|---|---|
| Peptide sequence type 1 | |||
| Genotype 1 | ST239-MRSA-III-t030 | CC8 | 13 |
| Genotype 1 | ST239-MRSA-III-t037 | CC8 | 8 |
| Genotype 1 | ST239-MRSA-III-t2270 | CC8 | 5 |
| Genotype 1 | ST239-MRSA-III-t459 | CC8 | 1 |
| Genotype 2 | ST239-MRSA-III-t030 | CC8 | 2 |
| Peptide sequence type 2 | |||
| Genotype 3 | ST5-MRSA-II-t002 | CC5 | 3 |
| Genotype 3 | ST5-MSSA-t002 | CC5 | 3 |
| Genotype 3 | ST25-MSSA-t078 | CC25 | 1 |
| Peptide sequence type 3 | |||
| Genotype 4 | ST59-MRSA-IV-t437 | CC59 | 1 |
| Genotype 4 | ST59-MSSA-t163 | CC59 | 1 |
| Genotype 5 | ST1-MSSA-t127 | CC1 | 3 |
| Peptide sequence type 4 | |||
| Genotype 6 | ST6-MSSA-t2467 | CC6 | 1 |
| Genotype 6 | ST6-MSSA-t701 | CC6 | 1 |
| Genotype 6 | ST7-MSSA-t091 | CC7 | 1 |
| Genotype 6 | ST7-MSSA-t796 | CC7 | 1 |
| Peptide sequence type 5 | |||
| Genotype 7 | ST188-MSSA-t189 | CC1 | 1 |
| Peptide sequence type 6 | |||
| Genotype 8 | ST188-MSSA-t189 | CC1 | 1 |
Eighteen SNPs were found, including four on the signal peptide encoding portion (nucleotide positions -68, -22, -6 and -5 relative to the first nucleotide of mature α-hemolysin portion), and 14 on the mature α-hemolysin encoding sequence (nucleotide positions 144, 177, 399, 438, 453, 479, 606, 624, 669, 708, 765, 777, 824 and 864) (Table 1). The majority of the SNPs (12/18, 67%) were synonymous—only five (28%, nucleotide positions -68, -6, -5, 624 and 824) were nonsynonymous. Lastly, a deletion mutation was detected in nucleotide position 479 of genotype 8, presumably resulting in a prematurely terminating transcript (Table 1). Of the 18 SNPs identified, nucleotide position -68 was ST239-specific, positions 177, 399, 438,453 and 669 were ST59-specific, position 765 was ST1-specific, whilst the other 11 were not lineage specific.
Clonal background of S. aureus isolates
A total of six ST-SCCmec-spa types and nine ST-spa types were identified amongst 32 MRSA and 15 MSSA isolates from China, respectively (Table 2). 88% (29/33) of the MRSA isolates belonged to CC8, 9% (3/33) belonged to CC5, and 3% (1/33) belonged to CC59. The ST239-III-t030 clone comprised over half of the CC8 MRSA isolates (15/29, 52%), followed by ST239-III-t037 (8/29, 28%), ST239-III-t2270 (5/29, 17%) and ST239-MRSA-III-t459 (1/29, 4%). All three CC5 MRSA isolates were ST5-II-t002 (Table 2).
In comparison, the distribution of MSSA clones was more diverse, with no clone comprised of more than three isolates. ST5-t002 was represented by three MRSA and three MSSA isolates in this study, and ST59 was represented by one MRSA and one MSSA isolates with differing spa types. No other ST or CC was common to both MRSA and MSSA isolates (Table 2).
Relationship between hla genotypes and clonal background
A strong correlation was observed between both hla genotypes and α-hemolysin peptide sequence types, and the clonal background of isolates from China.
Of the eight hla genotypes, six were restricted to either MRSA (genotypes 1 and 2) or MSSA (genotypes 5, 6, 7 and 8) strains. The predominant MRSA clone, ST239-MRSA-III-t030, was represented by hla genotype 1 (13/15 isolates, 86.7%) or genotype 2 (2/15 isolates, 13.3%). All of the remaining ST239-MRSA-III isolates possessed hla genotype 1. Both genotype 1 and 2 hla genes encoded α-hemolysin of peptide sequence type 1 (Table 2). hla genotypes 5 and 6 were found in one and four MSSA clones, respectively. Of the two ST188-MSSA-t189 isolates, one possessed hla genotype 7 and the other genotype 8, which differed by one deletion mutation at nucleotide position 479 (Table 1).
The remaining two hla genotypes, genotypes 2 and 3, were represented by both MRSA and MSSA isolates. Genotype 2 was found in six isolates of ST5-t002 (including three isolates each of ST5-MRSA-II-t002 and ST5-MSSA-t002) and one isolate of ST25-MSSA-t078. Genotype 3 was found in two ST59 isolates (one isolate each of ST59-MRSA-IV-t437 and ST59-MSSA-t163), and one isolate of ST1-MSSA-t127.
The in silico analysis of 318 well-characterized genomes
Amongst 318 well-characterized S. aureus genomes, 25 hla genotypes were identified, including 20 genotypes not found in the 47 isolates from China (S1 and S2 Tables), for a total of 28 hla genotypes identified in this study. The SNPs identified for all clinical isolates and published genomes are summarized in S3 Table.
Substantial diversity amongst S. aureus hla gene was found. Compared to the seven loci utilized in the MLST, the hla gene had higher nucleotide diversity (0.0256 vs. 0.0042–0.0119), more haplotypes identified (28 vs. 10–19), greater haplotype diversity (0.899 vs. 0.517–0.804) and higher non-synonymous polymorphisms/ synonymous sites ratio (3.598 vs. 3.059–3.569) (Table 3). Of note, 79 of 107 ST22 S. aureus isolates and one ST188 isolate (hla genotype 8) had one to 16 deletion mutations in their hla sequences (S3 Table). In addition, all 20 ST36 isolates had a SNP C→T at sequence position 259, which resulted in a premature stop codon (S3 Table). These mutations would presumably inhibit production of the toxin protein.
Table 3. Comparison of genetic diversity between the hla gene and seven gene loci utilized in the multilocus sequence typing scheme.
| Multilocus sequence typing loci | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characters | hla | arcC | aroE | glpF | gmk | pta | tpi | yqiL |
| Nucleotide diversity | 0.0256 | 0.0083 | 0.0093 | 0.0042 | 0.0083 | 0.0079 | 0.0119 | 0.0073 |
| No. of haplotypes | 28 | 13 | 15 | 12 | 10 | 18 | 19 | 19 |
| Haplotype diversity | 0.899 | 0.760 | 0.796 | 0.517 | 0.726 | 0.804 | 0.800 | 0.799 |
| No. of non-synonymous sites | 730.09 | 350.45 | 356.18 | 350.45 | 327.30 | 362.89 | 312.13 | 400.19 |
| No. of synonymous sites | 202.91 | 105.55 | 99.82 | 114.55 | 91.70 | 111.11 | 89.87 | 115.81 |
| No. of non-synonymous sites/ no. of synonymous sites | 3.598 | 3.320 | 3.568 | 3.059 | 3.569 | 3.266 | 3.473 | 3.455574 |
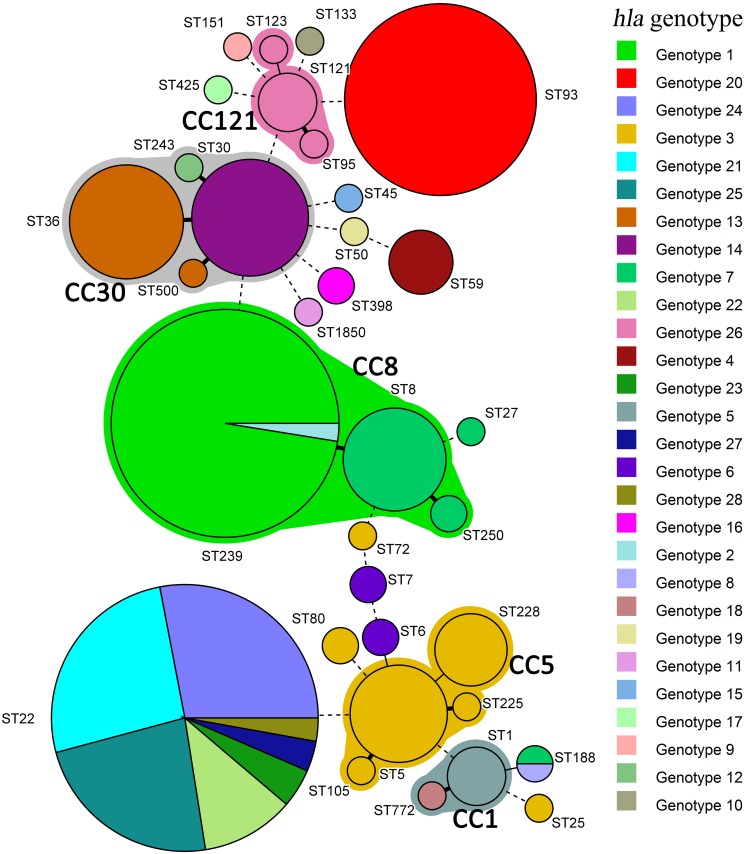
In combining the 47 isolates from China and the 318 globally distributed genomes for composite analysis, it was again noted that hla genotype was closely related to the clonal background of the isolate, in particular the ST, with little association with the geographic origin, host source or methicillin-resistance phenotype (Table 2, S1 and S2 Tables and Fig 1). The minimum-spanning tree analysis of MLST data shown that for 30 of 33 STs identified in the present study, isolates belonging to the same ST shared a unique hla genotype, and ST22 was the only sequence type that comprised more than three (seven in all) hla genotypes (Fig 1). In addition, STs belonging to the same CC frequently shared the same or close-related hla genotype, e.g. ST5, ST105, ST225 and ST228 of CC5 was comprised of 24 isolates belonging to hla genotype 3, ST8 and ST250 of CC8 was comprised of 18 isolates belonging to hla genotype 7, and ST95, ST121 and ST123 of CC121 was comprised of seven isolates belonging to hla genotype 26 (Fig 1).
Fig 1. Minimum-spanning tree of MLST data from 47 S. aureus clinical isolates collected in China and 318 whole genome sequences.
Circle sizes represent the number of isolates; circles are color coded by hla genotypes and labeled with the ST. Each circle represents a unique ST. Major clonal complexes (CCs), ie. CC8, CC5, CC30, CC121 and CC1, were labeled by green, brown, gray, pink and celadon colors, respectively. Thick solid lines, single-locus variant; Thin solid lines, double-locus variant; dashed lines, ≥3-locus variant.
Although the same hla genotype may be shared by unrelated STs, this was observed uncommonly. For instance, one isolate each that belonged to ST72 and ST25 isolates were hla genotype 3, which was mostly associated with CC5 S. aureus isolates. Likewise, one ST188 isolate was identified as hla genotype 25, which was mostly associated with non-ST239 CC8 isolates.
Discussion
Infections due to antimicrobial-resistant pathogens are a growing problem all over the world. In developing countries like India and China, antimicrobial resistance is particularly prevalent, owing to previous unregulated overuse of antimicrobials [20, 21]. S. aureus is one of the commonest Gram-positive bacterial pathogens, and in many places, the majority of S. aureus infections are now caused by multidrug-resistant strains, including MRSA and vancomycin-resistant S. aureus (VRSA). Immunotherapies are now being investigated as an alternative therapeutic options for staphylococcal infections in the hope that these may avoid the selection pressure associated with the use of antimicrobials [3, 22].
The S. aureus α-hemolysin was the first described of a family of bacterial pore-forming β-barrel toxins, which play an important role in the pathogenesis of staphylococcal disease [4, 23]. As such, it was chosen as a potential target for the development of vaccines to combat S. aureus infections, and positive results have been obtained in some preclinical trials targeting pneumonia and skin and soft tissue infections [23–26]. It has been noted that substitutions in amino acid residues may reduce the activity of α-hemolysin. For instance, a α-hemolysin mutant with a H35L substitution was found to have no hemolytic or lethal activity, despite retaining the ability to bind to target cells [6, 7]. The EMRSA-16 CC30 S. aureus isolates were another example. As observed in the present study, and as reported elsewhere, CC30-ST36 isolates had a SNP C→T at nucleotide sequence position 259, which resulted in a premature stop codon [27, 28]. It has been proven that CC30 isolates possessed this SNP had significantly reduced toxin production and decreased lethality in a mouse model [27]. These α-hemolysin mutants could be considered as candidate immunogens in prototypic S. aureus vaccines [23–26, 29].
Despite this work, little has been described regarding the genetic polymorphism of the hla gene in S. aureus. This is an important consideration, since variation in α-hemolysin peptide sequences could potentially lead to failure in antigen-antibody binding and thus compromise vaccine efficacy. In this study, we have illustrated the diversity of the hla gene in S. aureus and the relationship of hla sequence with clonal background, using 47 S. aureus clinical isolates from China supplemented with 318 well-characterized and globally distributed isolates with published whole genome sequences.
All ST239 from China were MRSA, and carried either genotype 1 or genotype 2 hla. These two genotypes differed by just one synonymous nucleotide mutation, (peptide sequence type 1). Amongst the 70 global S. aureus isolates with published genomes, seven isolates were ST239-MRSA-III, all of which also carried genotype 1 hla, regardless of the isolates’ geographic origins. The ST239-MRSA-III clone has been reported largely to be hospital-acquired and widely disseminated in Brazil, Australia, New Zealand and many Asian countries in the past decade, although the prevalence different spa types within this clone (e.g. spa type t030 and t037) vary in different regions [8, 30, 31]. In a previous genome-based phylogeographic analysis, it was determined that human movement played an important role in the global dissemination of ST239-MRSA-III [32]. Therefore, the consistent hla genotype of this clone across different regions is not surprising.
Interestingly, all of the 14 ST5 S. aureus isolates (six clinical isolates from China and eight global strains), including nine ST5-MRSA-II and five ST5-MSSA strains, possessed genotype 3 hla. Genotype 3 hla was also found in other CC5 S. aureus clones, including ST228-MRSA-I-t041 (n = 8), ST105-MRSA-II-t002 (n = 1) and ST225-MRSA-II-t003 (n = 1). Likewise all six ST59 isolates analyzed in this study, despite diverse methicillin-resistance phenotypes, SCCmec and spa types, carried genotype 4 hla. The ST59 lineage is primarily a community-acquired MRSA clone predominant in China and several other Asian countries [33]. These results again indicate that the hla genotype correlated closely with the ST.
Meanwhile, ST22 isolates shown significantly higher hla genotype diversity (comprised seven hla genotypes in all) than other S. aureus clones. Only occasional discrepancies between hla genotype and ST were observed. Future vaccine development will need to account for the influence of this diversity on vaccine effect.
Conclusion
We have found substantial diversity amongst S. aureus hla gene and amino acid sequences. Strong correlations between hla genotypes and clonal background were found in S. aureus, regardless of the isolates’ geographic origins and methicillin-resistance phenotype. Although the relative virulence of different hla genotypes remain undetermined, our investigation has provided some preliminary epidemiologic data which will be essential for future vaccine development.
Supporting Information
(DOCX)
(DOCX)
(XLSX)
Data Availability
All nucleotide sequence files are available from the NCBI GenBank database (accession numbers KT279554 to KT279561).
Funding Statement
YCX recieved a National Research Special Fund for Public Welfare Industry of Health of China (grant number 201402001, http://www.moh.gov.cn/qjjys/s3577/201401/e9f3635e7acb47778225ccb729ffec62.shtml) from the Ministry of Health of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10–9. 10.1093/cid/cit613 . [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Yeh AJ, Cheung GY, Villaruz AE, Tan VY, Joo HS, et al. Basis of virulence in a Panton-Valentine leukocidin-negative community-associated methicillin-resistant Staphylococcus aureus strain. J Infect Dis. 2015;211(3):472–80. 10.1093/infdis/jiu462 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12(1):49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel). 2013;5(6):1140–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker B, Krishnasastry M, Zorn L, Kasianowicz J, Bayley H. Functional expression of the alpha-hemolysin of Staphylococcus aureus in intact Escherichia coli and in cell lysates. J Biol Chem. 1992;267(15):10902–9. . [PubMed] [Google Scholar]
- 6.Menzies BE, Kernodle DS. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun. 1994;62(5):1843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker B, Bayley H. Key residues for membrane binding, oligomerization, and pore forming activity of staphylococcal alpha-hemolysin identified by cysteine scanning mutagenesis and targeted chemical modification. J Biol Chem. 1995;270(39):23065–71. . [DOI] [PubMed] [Google Scholar]
- 8.Xiao M, Wang H, Zhao Y, Mao LL, Brown M, Yu YS, et al. National surveillance of methicillin-resistant Staphylococcus aureus in China highlights a still-evolving epidemiology with 15 novel emerging multilocus sequence types. J Clin Microbiol. 2013;51(11):3638–44. 10.1128/JCM.01375-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Talib H, Yean CY, Al-Khateeb A, Hassan H, Singh KK, Al-Jashamy K, et al. A pentaplex PCR assay for the rapid detection of methicillin-resistant Staphylococcus aureus and Panton-Valentine Leucocidin. BMC Microbiol. 2009;9:113 10.1186/1471-2180-9-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray GS, Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus wood 46. Infect Immun. 1984;46(2):615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38(3):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koreen L, Ramaswamy SV, Graviss EA, Naidich S, Musser JM, Kreiswirth BN. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol. 2004;42(2):792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186(5):1518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Mediavilla JR, Oliveira DC, Willey BM, de Lencastre H, Kreiswirth BN. Multiplex real-time PCR for rapid Staphylococcal cassette chromosome mec typing. J Clin Microbiol. 2009;47(11):3692–706. 10.1128/JCM.00766-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stinear TP, Holt KE, Chua K, Stepnell J, Tuck KL, Coombs G, et al. Adaptive change inferred from genomic population analysis of the ST93 epidemic clone of community-associated methicillin-resistant Staphylococcus aureus. Genome Biol Evol. 2014;6(2):366–78. 10.1093/gbe/evu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holden MT, Hsu LY, Kurt K, Weinert LA, Mather AE, Harris SR, et al. A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res. 2013;23(4):653–64. 10.1101/gr.147710.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdam PR, Templeton KE, Edwards GF, Holden MT, Feil EJ, Aanensen DM, et al. Molecular tracing of the emergence, adaptation, and transmission of hospital-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2012;109(23):9107–12. 10.1073/pnas.1202869109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurt K, Rasigade JP, Laurent F, Goering RV, Zemlickova H, Machova I, et al. Subpopulations of Staphylococcus aureus clonal complex 121 are associated with distinct clinical entities. PLoS One. 2013;8(3):e58155 10.1371/journal.pone.0058155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathur P, Singh S. Multidrug resistance in bacteria: a serious patient safety challenge for India. J Lab Physicians. 2013;5(1):5–10. 10.4103/0974-2727.115898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao YH, Giske CG, Wei ZQ, Shen P, Heddini A, Li LJ. Epidemiology and characteristics of antimicrobial resistance in China. Drug Resist Updat. 2011;14(4–5):236–50. 10.1016/j.drup.2011.07.001 . [DOI] [PubMed] [Google Scholar]
- 22.Ohlsen K, Lorenz U. Immunotherapeutic strategies to combat staphylococcal infections. Int J Med Microbiol. 2010;300(6):402–10. 10.1016/j.ijmm.2010.04.015 . [DOI] [PubMed] [Google Scholar]
- 23.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12 10.3389/fcimb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202(7):1050–8. 10.1086/656043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mocca CP, Brady RA, Burns DL. Role of antibodies in protection elicited by active vaccination with genetically inactivated alpha hemolysin in a mouse model of Staphylococcus aureus skin and soft tissue infections. Clin Vaccine Immunol. 2014;21(5):622–7. 10.1128/CVI.00051-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun. 2009;77(7):2712–8. 10.1128/IAI.00115-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLeo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, Mathema B, et al. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A. 2011;108(44):18091–6. 10.1073/pnas.1111084108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGavin MJ, Arsic B, Nickerson NN. Evolutionary blueprint for host- and niche-adaptation in Staphylococcus aureus clonal complex CC30. Front Cell Infect Microbiol. 2012;2:48 10.3389/fcimb.2012.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menzies BE, Kernodle DS. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun. 1996;64(5):1839–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho KS, Mamizuka EM, Gontijo Filho PP. Methicillin/Oxacillin-resistant Staphylococcus aureus as a hospital and public health threat in Brazil. Braz J Infect Dis. 2010;14(1):71–6. . [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Liu Y, Jiang X, Chen M, Wang H. Rapid change of methicillin-resistant Staphylococcus aureus clones in a Chinese tertiary care hospital over a 15-year period. Antimicrob Agents Chemother. 2010;54(5):1842–7. 10.1128/AAC.01563-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray RR, Tatem AJ, Johnson JA, Alekseyenko AV, Pybus OG, Suchard MA, et al. Testing spatiotemporal hypothesis of bacterial evolution using methicillin-resistant Staphylococcus aureus ST239 genome-wide data within a bayesian framework. Mol Biol Evol. 2011;28(5):1593–603. 10.1093/molbev/msq319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr Opin Microbiol. 2012;15(5):588–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All nucleotide sequence files are available from the NCBI GenBank database (accession numbers KT279554 to KT279561).