Bone marrow (BM)-derived cell therapy for the damaged heart has now been under investigation for almost 2 decades. Right from the outset, both in vitro and in animal models of cardiac damage, early studies using BM-derived cells showed incredible promise and reported numerous broadly positive findings, driving enthusiasm for the clinical translation of this approach. To mention just a few of those early studies, findings from the late 1990’s included the successful differentiation of BM stromal cells into cardiomyocyte-like cells;1 increased angiogenesis and the formation of cardiomyocyte-like cells in vivo when BM-derived cells were injected into the scarred left ventricular (LV) wall;2 and the identification that the BM is a reservoir for circulating endothelial progenitor cells that contribute to new vessel formation in the adult.3 Then, in 2001, it was reported that the intravenous administration of human CD34+ BM-derived cells into immune-deficient rats following acute myocardial infarction (MI) led to the proliferation of preexisting vasculature and de novo new blood vessel formation in the infarct bed, which contributed to the salvage of viable myocardium and dramatic improvements in left ventricular ejection fraction (LVEF).4 Concurrently others reported that a specific fraction of BM cells which expressed c-kit, but not hematopoietic markers, could not only give rise to new vessels but also cardiomyocytes,5 and that the mobilization of BM cells using specific factors in the post-MI period leads to the homing of these cells to the MI region, reduced infarct size and improved survival in an animal model.6
With these and other remarkable pre-clinical studies appearing in rapid succession, clinician-scientists were swift to begin investigating BM-derived cells to ameliorate cardiovascular disease in humans.7 Indeed, still in 2001, what we believe to be the first human report of the use of BM-derived cells in patients suffering from ischemic heart disease (IHD) appeared in the literature.8 Even though only a pilot with 5 subjects, this study suggested safety and the potential for clinical benefit from BM-derived cells in humans with IHD, with improved coronary perfusion observed in 3 of the 5 patients.8 This publication signaled the beginning of what we consider the ‘first generation’ of cell therapy studies for cardiac disease (Figure), and from then onwards the field surged ahead at an even more rapid pace. Fast-forward over a decade, and literally hundreds of reports have emerged regarding the use of BM-derived cell therapy for patients with IHD. More prominent among these were the REPAIR-AMI study (reporting that BM-derived cell therapy was associated with improved LVEF and a reduction in the combined outcome of death, recurrence of MI or any revascularization procedure);9 the SWISS-AMI study (negative findings),10 two studies conducted by the National Institutes of Health (NIH)-sponsored Cardiovascular Cell Therapy Research Network (both reported negative findings),11,12 REGENT (overall negative study, but a trend to improved LVEF in patients with LVEF < 37% in the post-MI period), and the HEBE study (negative findings).13 Even as can be seen among the few studies mentioned here, the findings across these trials were quite mixed, which made overall interpretation challenging. However, reassuringly, there was no signal of adverse events.
Figure.
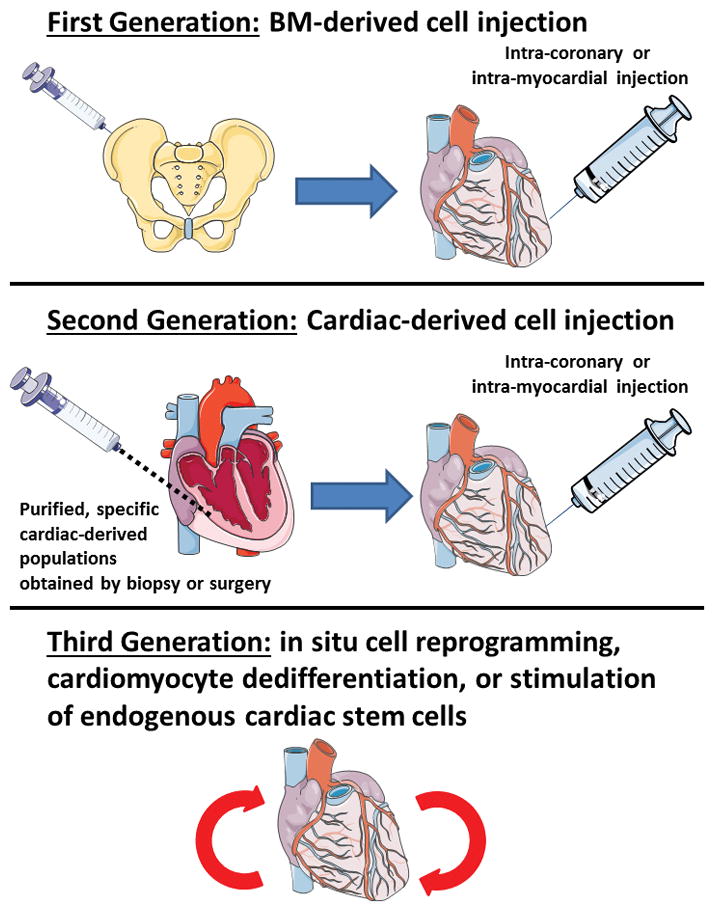
Author’s impression of current status of cell therapy studies to ameliorate cardiac damage and for cardiac regeneration.
In an attempt to integrate and analyze these many studies of BM-derived cell therapy in patients with IHD, a series of meta-analyses were conducted over the last several years. The pattern of the results and conclusions of these meta-analyses has been not dis-similar from that seen in the original studies; in that the early and even many of the more recent meta-analyses were generally quite positive in their findings.14–19 The largest of these appeared in 2012 and included 50 studies with 2625 subjects, and reported that compared with controls, BM-derived cell therapy was associated with a 3.96% absolute improvement in LVEF, smaller infarct size, and reduced all-cause and cardiac mortality.16 These results warrant cautious interpretation, however, as this analysis combined randomized and cohort data, and documented substantial heterogeneity in the overall treatment effect. A subsequent meta-analysis that only included randomized controlled trials (RCTs) demonstrated a comparable effect with a 2.55% absolute improvement in LVEF, yet also reported significant heterogeneity across studies.17 These limitations notwithstanding, as recently as just ~18 months ago, for all practical purposes it appeared that “the jury was in” and that BM-derived cell therapy only required confirmation of efficacy in a phase III study before it could be widely implemented in the clinic.
However, at about the same time, a series of negative analyses also began to appear. The first was a publication by Francis et al, who reported a concerning number of ‘discrepancies and contradictions’ in the BM-derived cell therapy literature.20 This was followed by a weighted regression and meta-analysis from the same group.21 In this second paper it was again concluded that discrepancies and inconsistencies were common in the BM-derived cell therapy literature, with 600 discrepancies in 133 reports from 49 trials. Furthermore, there was a significant association between the number of discrepancies and the reported increment in LVEF with BM-derived cell therapy, and that only 5 trials had no discrepancies and among these trials the mean LVEF effect size was −0.4%.21 A third negative report appeared with the meta-analysis of de Jong et al.22 When de Jong et al focused strictly on RCTs of BM-derived cells in the post-MI period that had LV functional endpoints defined by magnetic resonance imaging, rather than the less sensitive measures of LV angiography or echocardiography as was used in many of the earlier studies, there was no effect on cardiac function, volumes, or infarct size. Moreover, de Jong et al found no beneficial effect of BM-derived cell therapy on major adverse cardiac and cerebrovascular event rates after a median follow-up duration of 6 months.22 Suddenly, the efficacy of BM-derived cell therapy had been thrown into question.
While it is possible to debate the relative merits of these many positive and negative meta-analyses and their findings, and while in no way belittling the significant efforts that went into these studies, they all suffer from the significant limitations inherent in study-level meta-analyses that are conducted using aggregate or statistical summary data. As a critical aspect of this statistical approach, individual patient data (IPD) are not obtained or used for a study-level meta-analysis, and all of these studies simply extracted the summary study data from the original individual publications, or occasionally sourced it directly from the original investigators,17 and then proceeded to estimate a pooled treatment effect using these aggregate data.
The limitations of study-level meta-analyses are significant. Indeed, one of the major concerns with study-level meta-analyses is that in trials using repeated measures (i.e. LVEF at baseline and follow-up), it is critical to account for the relationship within each subject of these repeated measures. In other words, there is a relationship for each subject between their baseline and follow-up LVEF (and other functional measures) and it is important to account for these intra-subject correlations between timepoints. However, in a study-level meta-analysis, these intra-subject relationships are ignored.23 Furthermore, the amount of missing data at the patient level can further affect these analyses,23 and in a study-level meta-analysis this aspect is again ignored. Moreover, the absence of patient-specific covariates (e.g. age) makes standard analytic techniques such as multivariable regression or examining treatment effects in subgroups challenging, if not impossible at times.24 This again is of specific relevance to BM-derived cell therapy, as several claims were made in these study-level meta-analyses of benefits in certain sub-groups, such as those with low baseline LVEF.14–19 Finally, all of these problems are amplified when a high level of heterogeneity is present among the individual trials – a fact that has been widely acknowledged in the BM-derived cell therapy literature.15–18
Until the ACCRUE study of Gyöngyösi et al25 published in this issue of Circulation Research, there has never been a patient-level meta-analysis of cell therapy (performed using IPD). In brief, for a patient-level meta-analysis, the original data for each individual trial subject is sourced from the primary investigators, harmonized in a common dataset and then analyzed. While not a substitute for a phase III RCT, a patient-level meta-analysis overcomes many of the limitations of a study-level approach and is acknowledged as the gold standard methodology for meta-analyses.24 However, the challenge with patient-level meta-analyses is that the time, effort, resources and cost involved is increased exponentially over that of study-level analyses. To their great credit, in 2007 the ACCRUE consortium of investigators was formed to set about the task of gathering and harmonizing IPD from cardiovascular cell therapy studies to perform patient-level meta-analyses.25 The criteria for participation in ACCRUE were that the data must be from randomized or cohort clinical studies, and that the study involved percutaneous administration of cells or cell-based products, or cytokine mobilization of BM cells, administered with the aim of ameliorating cardiac disease. Importantly, their definitions, endpoints and rigorous statistical analysis plans were established prospectively. According to defined terms and conditions, centers and principal investigators were invited to contribute their study data, and the ACCRUE dataset now includes 1871 IPD sets from 28 studies.25
Using this gold standard patient-level meta-analysis approach, for the present analysis, the ACCRUE investigators focused on IPD from 12 RCTs of intracoronary cell therapy after acute MI, with a combined total of 767 patients who received cell therapy and 485 controls. The authors appropriately considered study-level effects in their analytic approach by stratifying their analyses by trial, thereby generating a singular pooled treatment effect. Unlike prior study-level meta-analyses, Gyöngyösi et al found, in summary, no significant effect of cell therapy on either: 1) the combined primary endpoint of freedom from major adverse cardiac and cerebrovascular events (MACCE) and death; 2) another combined endpoint of freedom from death, re-MI, or stroke; 3) freedom from death alone; 4) freedom from MACCE alone, and; 5) cardiac functional outcomes (LVEF, end-diastolic volume, end-systolic volume). Furthermore, no predictive factors, sub-groups, patient characteristics or other factors were identified that were associated with a positive effect of cell therapy.25 It is important to note that the overall treatment effect on the endpoint of MACCE was in a direction of benefit (HR 0.88) and imprecise with a 95% CI between 0.63 and 1.18. As a result, the possibility of more modest, yet clinically meaningful benefit from such therapy cannot be completely excluded based on these findings.
Like any landmark study, this patient-level meta-analysis has raised more questions than it answered. What it has clearly demonstrated is that, using the most rigorous statistical methods available, when the bulk of the prior data from well conducted randomized cell therapy studies are combined, we may conclude that intracoronary cell therapy is safe but that it offers no functional or clinical benefits. While disappointing, the consistency of the findings across a range of endpoints, subgroups and patient characteristics is compelling. We believe it would be quite unlikely that another group would now attempt to perform another patient-level meta-analysis of these data, and therefore the question of the utility of intracoronary cell therapy based collectively on the heterogeneous studies conducted to date has now been put to rest: negative.
As for the questions raised by Gyöngyösi et al,25 there are many. To first critique the study itself and as a limitation of all meta-analyses, it must be acknowledged that unique design aspects of individual original studies that were aiming to tease out potential beneficial effects in certain patient or cell subgroups may have been lost in the larger analysis. On this note and while we unreservedly applaud the efforts of the ACCRUE investigators, we question the rationale for the inclusion of the CADUCEUS study26 in this meta-analysis. CADUCEUS reported positive findings and was the only study in this meta-analysis to inject cardiosphere-derived cells.26 Cardiosphere-derived cells are unique in that they are derived from the heart, and not the BM as with all other studies in this meta-analysis. Therefore, it could be said that including CADUCEUS in this meta-analysis is somewhat akin to grouping together “apples and oranges”. Nevertheless, CADUCEUS was the smallest of all studies included in this meta-analysis (25 subjects out of the 1252 analyzed; or only 2%)26 and it is unlikely that it would have significantly impacted the overall results. Therefore, for practical purposes, we view the results of this ACCRUE meta-analysis as applying to BM-derived cell therapy. Further possible limitations include the potential for selective inclusion of studies in this analysis. Of the 55 studies deemed eligible for inclusion, only 28 ultimately provided IPD. Thus, only about half of the potentially eligible datasets were included. One is left to wonder if there may have been any biases at play in the decision to submit, or decline to submit, data to the ACCRUE database. However, the ACCRUE database currently represents over 70% of all clinical cardiac cell therapy studies and approximately 60% of all intracoronary cell studies. Moreover, the majority of the potentially eligible studies that were not included were relatively small, with a mean of 27 patients in the cell therapy arm and 19 controls, as compared to a mean of 64 active study patients and 40 controls for the studies included in ACCRUE. Therefore it certainly seems that the ACCRUE investigators have made every effort to obtain as much meaningful IPD as possible, and that their dataset represents the most comprehensive set of cell therapy studies that are likely to be collected, harmonized and analyzed in this way.
Some will also ask “why did intracoronary cell therapy not show a benefit?” We believe there are many possible reasons. To begin with, there are a myriad of potential explanations related to cell type, cell handling and preparation, cell dose, recipient milieu (e.g. inflammatory status, timing after MI, cell homing signals), recipient age, mode of cell delivery, need for multiple cell deliveries, selection of endpoints and more. However, there are also many examples of therapies that appeared promising in animal studies, but which failed in the clinic. Perhaps, based on these initial studies, we must be at least prepared to conclude that this ‘first generation’ BM-derived cell therapy approach may offer no benefit.
Another critical question raised and perhaps the most pressing, is: “where does this leave the cell therapy field?” As for BM-derived cell therapy, all eyes will now turn to the BAMI study. BAMI is a multinational, multicenter, phase III RCT that has been underway for over a year and which aims to demonstrate that the intracoronary administration of autologous BM-derived mononuclear cells is safe and reduces all-cause mortality in patients with reduced LVEF after successful reperfusion for acute MI.27 BAMI aims to recruit 3000 subjects which, notably, is almost 2.5 times the total number of subjects in the present Gyöngyösi et al25 meta-analysis. The BAMI design is based on the original REPAIR-AMI study,9 which showed a beneficial effect in just over 200 subjects (and which was included in this ACCRUE meta-analysis). BAMI is expected to complete enrolment in 2017 and to report findings in 2018.27 BAMI will certainly be a pivotal clinical study and, positive or negative, will have a major impact on the field. Beyond BAMI, there remains much to be excited about in the cell therapy arena. Already, what we consider to be second generation cell therapies are being tested in the clinic. As we mentioned, cardiosphere-derived cells have shown promise,26 as too have other novel populations such as c-kit+ cardiac progenitor cells.28 Furthermore, researchers are already working to refine what we believe will be the third generation of studies, based on cell reprogramming, cardiomyocyte dedifferentiation, or insitu stimulation of endogenous cardiac stem cells (Figure). While only time will tell, we believe the findings of this laudable meta-analysis by Gyöngyösi et al represent the inevitable one step back on the way to taking two steps forward.
Acknowledgments
Jason Kovacic acknowledges research support from National Institutes of Health (K08HL111330), AstraZeneca, the American Heart Association (14SFRN20490315; 14SFRN20840000) and The Leducq Foundation (Transatlantic Network of Excellence Award). Valentin Fuster acknowledges research support from AstraZeneca and the American Heart Association (14SFRN20490315).
We thank Dr. Usman Baber for his statistical input regarding the relative merits and limitations of study- and patient-level meta-analyses.
The figure was created using Servier medical art.
References
- 1.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 5.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 6.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacic JC, Muller DW, Harvey R, Graham RM. Update on the use of stem cells for cardiac disease. Intern Med J. 2005;35:348–356. doi: 10.1111/j.1445-5994.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamano K, Nishida M, Hirata K, Mikamo A, Li TS, Harada M, Miura T, Matsuzaki M, Esato K. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: Clinical trial and preliminary results. Japanese circulation journal. 2001;65:845–847. doi: 10.1253/jcj.65.845. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM, Investigators R-A. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 10.Surder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, Turchetto L, Radrizzani M, Astori G, Schwitter J, Erne P, Zuber M, Auf der Maur C, Jamshidi P, Gaemperli O, Windecker S, Moschovitis A, Wahl A, Buhler I, Wyss C, Kozerke S, Landmesser U, Luscher TF, Corti R. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: Effects on global left ventricular function. Circulation. 2013;127:1968–1979. doi: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- 11.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy R. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The latetime randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy Research N. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The time randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch A, Nijveldt R, van der Vleuten PA, Tijssen JG, van der Giessen WJ, Tio RA, Waltenberger J, ten Berg JM, Doevendans PA, Aengevaeren WR, Zwaginga JJ, Biemond BJ, van Rossum AC, Piek JJ, Zijlstra F, Investigators H. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: Results of the randomized controlled hebe trial. Eur Heart J. 2011;32:1736–1747. doi: 10.1093/eurheartj/ehq449. [DOI] [PubMed] [Google Scholar]
- 14.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Clarke MJ, Watt SM, Martin-Rendon E. Long-term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: Factors that may influence outcomes. PLoS One. 2012;7:e37373. doi: 10.1371/journal.pone.0037373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: A systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delewi R, Hirsch A, Tijssen JG, Schachinger V, Wojakowski W, Roncalli J, Aakhus S, Erbs S, Assmus B, Tendera M, Goekmen Turan R, Corti R, Henry T, Lemarchand P, Lunde K, Cao F, Huikuri HV, Surder D, Simari RD, Janssens S, Wollert KC, Plewka M, Grajek S, Traverse JH, Zijlstra F, Piek JJ. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of st-segment elevation myocardial infarction: A collaborative meta-analysis. Eur Heart J. 2014;35:989–998. doi: 10.1093/eurheartj/eht372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delewi R, Andriessen A, Tijssen JG, Zijlstra F, Piek JJ, Hirsch A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A meta-analysis of randomised controlled clinical trials. Heart. 2013;99:225–232. doi: 10.1136/heartjnl-2012-302230. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 20.Francis DP, Mielewczik M, Zargaran D, Cole GD. Autologous bone marrow-derived stem cell therapy in heart disease: Discrepancies and contradictions. Int J Cardiol. 2013;168:3381–3403. doi: 10.1016/j.ijcard.2013.04.152. [DOI] [PubMed] [Google Scholar]
- 21.Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, Howard JP, Cole GD, Francis DP group Dw. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (damascene): Weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: A meta-analysis and update on clinical trials. Circulation. Cardiovascular interventions. 2014;7:156–167. doi: 10.1161/CIRCINTERVENTIONS.113.001009. [DOI] [PubMed] [Google Scholar]
- 23.Jones AP, Riley RD, Williamson PR, Whitehead A. Meta-analysis of individual patient data versus aggregate data from longitudinal clinical trials. Clin Trials. 2009;6:16–27. doi: 10.1177/1740774508100984. [DOI] [PubMed] [Google Scholar]
- 24.Donegan S, Williamson P, D’Alessandro U, Garner P, Smith CT. Combining individual patient data and aggregate data in mixed treatment comparison meta-analysis: Individual patient data may be beneficial if only for a subset of trials. Statistics in medicine. 2013;32:914–930. doi: 10.1002/sim.5584. [DOI] [PubMed] [Google Scholar]
- 25.Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moyé LA, Sürder D, Corti R, Huikuri H, Miettinen J, Wöhrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma DE, Diederichsen A, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer G. Meta-analysis of cell-based cardiac studies (accrue) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [Accessed march 9th 2015]; Source: Https://clinicaltrials.Gov/ct2/show/nct01569178.
- 28.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


